Abstract
Objective
Evaluate impact of screening stress testing for coronary artery disease (CAD) in asymptomatic patients with diabetes in a community-based population.
Patients and Methods
Observational study of 3,146 patients from Olmsted County, Minnesota, with new diabetes diagnosis from 1/1/1992 through 12/31/2008, without history of CAD or cardiac symptoms. With combined all-cause mortality and myocardial infarction as primary outcome, weighted Cox proportional hazards regression was performed with screening stress testing within 2 years of diabetes diagnosis as time-dependent covariate. For descriptive analysis, participants were classified per their clinical experience during the first 2 years post-diagnosis as screened (asymptomatic, underwent stress test), unscreened (asymptomatic, no stress test), or symptomatic (experienced symptoms or event).
Results
Participants included 53% men; mean (SD) age at diabetes diagnosis was 55 (13.8) years, and 97% had type 2 diabetes. In event-free survival analysis, 292 patients comprised screened cohort and 2,246 patients unscreened cohort. Death or myocardial infarction occurred in 454 patients (32 patients in screened cohort and 422 in unscreened cohort [5-year rate, 1.9% and 5.3%, respectively]) during median (interquartile range) follow-up 9.1 (5.3–12.5) years. Screening stress test was associated with improved event– free survival (hazard ratio, 0.61; P=.004), independent of cardiac risk factors. However, while stress tests were abnormal in 47 (16.1%) of screened patients, only 6 (2%) underwent coronary revascularization.
Conclusion
Although screening cardiac stress testing in asymptomatic patients with diabetes in community-based population was associated with improvement in long-term event-free survival, this does not appear to occur by coronary revascularization alone.
Keywords: coronary artery disease, diabetes mellitus, stress echocardiography, stress testing
Currently, 9.3% of the United States population has diabetes mellitus1 and coronary artery disease (CAD) is the leading cause of morbidity and death in these persons.2 Among patients with diabetes, however, symptoms of CAD are often absent even with advanced disease.3 Despite intuitive appeal and prior guideline support, screening for CAD in asymptomatic diabetes remains controversial4. The 2013 European Society of Cardiology acknowledges that routine screening could be considered for patients at particularly high risk5. Nevertheless, the 2016 American Diabetes Association6 guidelines do not recommend screening of asymptomatic patients with diabetes, stating “it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated.” These recommendations cite the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study7 that had no clinical benefit to routine screening of asymptomatic persons with type 2 diabetes and normal electrocardiography. However, cardiac event rates were low in all patients in DIAD (5-year cardiac event rate, 2.9%), limiting the study’s power to identify small differences. Additionally, a healthier cohort may have been selected, because prior stress testing was an exclusion criterion.
To our knowledge, a community-based estimate of the effect of cardiac stress testing in asymptomatic patients with diabetes is not available in the literature. Community-based research has many advantages8 and is facilitated in Olmsted County, Minnesota, by the Rochester Epidemiology Project (REP), which allows nearly complete enumeration of the population and linkage of persons with their lifetime inpatient and outpatient medical records.9 Our study evaluated the impact of screening stress testing for CAD in asymptomatic patients with diabetes in a community-based cohort.
PATIENTS AND METHODS
Using REP resources, we compared outcomes of asymptomatic diabetic patients who did or did not undergo a screening cardiac stress test within 2 years of their diabetes diagnoses, according to the judgment of their physicians. This retrospective cohort study was approved by both the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participating patients had provided previous written permission approving the use of their medical records for research.
Study Population
Olmsted County has an estimated population of 144,248 persons, of whom 85.7% are white (10); sociodemographically, the community is similar to the United States white population, except for higher education and income levels.9,11 Medical care is virtually self-contained within the community and all major health care providers (Mayo Clinic and its affiliated sites, Olmsted Medical Center, and Rochester Family Practice) participate in the REP, which serves to link persons with their lifetime medical records across these institutions. Each year, 80% of Olmsted County residents are seen at least once by a health care professional and 93% of residents have at least 1 health encounter in any given 3 years.12,13 Death data are collected through Mayo Clinic records, Olmsted County and Minnesota State death certificates, and the National Death Index. Cause of death is captured with International Classification of Diseases, Tenth Revision, codes.
Identification of Study Cohort
Using REP resources, we identified 10,079 Olmsted County residents older than 18 years who received a new diagnosis of type 1 or type 2 diabetes from January 1, 1992, through December 31, 2008. The index date was defined as the date of diabetes diagnosis cited in the medical record. The codes used to identify these patients included the International Classification of Diseases, Ninth Revision, codes for diabetes and its complications and the comparable Hospital International Classification of Disease Adaptation system codes.14 Excluded from the study were patients with clinical diagnosis of heart failure or evidence of CAD before the diabetes diagnosis. Two experienced, trained data abstractors (R.E.B. and M.O.) reviewed medical records to ascertain eligibility and symptom status of patients at baseline and during follow-up.
The Olmsted County residents with diabetes who had undergone stress testing (exercise ECG, nuclear perfusion, or stress echocardiography) were identified through cross-reference of the previous codes with Current Procedural Terminology codes 78452, 78465, 93015, 93016, 93017, 93018, 93024, and 93350. The first stress test performed after diabetes diagnosis was considered for analysis. Of the 3,950 potential cases identified, 2,014 met the above criteria. For these patients, stress test datasets were interrogated to identify which patients had testing for screening purposes and which had testing prompted by dyspnea or chest pain. Among the 6,129 patients with diabetes who did not undergo stress testing, a random sample of 2,265 patients was selected for review. From this sample, 1,133 patients were excluded according to the study criteria; the remaining 1,132 were included. The final study population included 3,146 persons.
Clinical Data
Clinical characteristics at diabetes diagnosis, including cardiac risk factors, cardiovascular medications, and laboratory data, were collected. Cardiac risk factors included current smoking; hypertension (systolic blood pressure [BP] >140 mm Hg, diastolic BP >90 mm Hg, or use of antihypertensive therapy15); clinical diagnosis of peripheral arterial disease; dyslipidemia (total cholesterol >210 mg/dL, low-density lipoprotein cholesterol >130 mg/dL, high-density lipoprotein cholesterol <35 mg/dL, or use of lipid-lowering medication); and obesity (body mass index≥30 kg/m2). Baseline laboratory and BP measurements were collected as close as possible to the date of diabetes diagnosis, but data up to 2 years before or after diabetes diagnosis were included. Ten-year risk of cardiovascular disease was calculated according to the Framingham Risk Score (FRS).16 Stress testing was performed according to the usual clinical protocols and was considered abnormal—indicative of CAD—if there was 1) a fixed perfusion defect or fixed wall motion abnormality for nuclear perfusion and stress echocardiography, respectively, or 2) evidence of stress-induced ischemia with any test (ie, exercise electrocardiography, nuclear perfusion, or stress echocardiography).17–19
Follow-up
Medical response to stress testing result (Cardiology consultation, changes in medical therapy) was determined for those patients with fixed or inducible abnormality by manual chart review of all medical records within 6 months of stress testing.
Follow-up and mortality data were obtained to the end of 2012, with review of patient medical records linked through REP. The primary outcome was combined all-cause mortality rate and MI. Cardiac death was defined as fatal myocardial infarction (MI), death due to heart failure or arrhythmia, and sudden cardiac death. Surgical or percutaneous coronary artery revascularization was also recorded.
Statistical Analysis
The study was designed with an anticipated 5% to 10% event rate over a 10-year follow-up and was powered to detect a hazard ratio (HR) of 0.77. The 2-sided significance level was .05. Descriptive statistics were computed as mean (standard deviation [SD]) for continuous variables and as number and percentage for categorical variables. Baseline characteristics and stress test variables were compared in the 2 cohorts with 2-sided t tests for continuous variables and χ2 test for categorical variables.
Analyses were performed with the Statistical Package SAS statistical analysis software (SAS Institute Inc). Follow-up extended from the index date to the date of death or cardiovascular event. Cox proportional hazards regression was performed for the 3,146 patients, with screening stress testing within 2 years of diabetes diagnosis as a time-dependent covariate. The 1,132 patients without stress testing were weighted in this analysis according to the sampling fraction: The fraction of the included non–stress test patients divided by the total number expected to be included (ie, 1/[1,132/3,064]=2.71). Sensitivity analyses were performed without weighting and in the subset of patients with complete baseline laboratory and BP data. Patient participation was censored if patients had chest pain or dyspnea within 2 years and thus were not eligible to have received a screening stress test at 2 years. The 2-year time point was chosen because it maximized the screening events (screening stress testing done within that time frame) and the primary outcome events (all-cause mortality and MI after that time point). Results of additional analysis using 1-, 3-, and 4-year time points were consistent and thus not included. The final multivariable model was chosen with a stepwise selection to choose a subset of baseline variables that was associated with outcome at P<.05.
A landmark analysis20 was used to illustrate the time-dependent effect of screening stress test within 2 years of diagnosis. Patients were excluded from this analysis if they had symptoms, MI, or died within 2 years (ie, symptomatic cohort). At the 2-year point, those patients who remained were divided into 2 groups—those who had received a screening stress test up to that point (screened cohort) and those who had not undergone any stress testing (unscreened cohort). Kaplan-Meier methods were used to illustrate event-free survival beyond 2 years and tested with the log-rank test.
As a secondary analysis, patients in the screening stress test group were propensity-matched 1:2 to patients without a screening stress test. Propensity score was calculated with all factors listed in Table 1 (area under the curve, 0.71). The association of screening stress test with outcome was evaluated in the propensity-matched cohort using Cox regression and landmark analysis.
Table 1.
Baseline Clinical Characteristics for Unscreened and Screened Cohorts
| Variablea,b | Unscreened Cohort (n=2,246) |
Screened Cohort (n=292) |
P Value |
|---|---|---|---|
| Age at diabetes diagnosis, y | 54.7 (14.1) | 55.5 (10.7) | .31 |
| Male sex | 1,170 (52.1) | 188 (64.4) | <.001 |
| Body mass index, kg/m2 | 33.5 (7.5) | 34.4 (7.5) | .11 |
| Diabetes mellitus type | .01 | ||
| 1 | 76 (3.4) | 2 (0.7) | |
| 2 | 2,157 (96.6) | 285 (99.3) | |
| Diabetes treatment | .01 | ||
| Diet | 1,170 (53.5) | 178 (62.9) | |
| Oral medication | 786 (35.9) | 81 (28.6) | |
| Insulin | 232 (10.6) | 24 (8.5) | |
| Family history of CAD | 151 (6.8) | 34 (11.9) | .002 |
| Hypertension | 1,083 (49.0) | 154 (53.8) | .13 |
| Peripheral arterial disease | 41 (1.9) | 8 (2.8) | .28 |
| Current smoking | 319 (14.5) | 50 (17.5) | .17 |
| Statin use | 486 (22.6) | 71 (25.5) | .26 |
| Aspirin use | 524 (24.0) | 93 (32.9) | .001 |
| ACE-I or ARB use | 504 (24.1) | 68 (24.8) | .79 |
| β-Blocker use | 306 (18.0) | 51 (20.5) | .35 |
| Systolic BP, mm Hg | 136 (19) | 136 (18) | .62 |
| Diastolic BP, mm Hg | 79 (12) | 80 (11) | .25 |
| HDL-C, mg/dL | 44 (13) | 42 (12) | .01 |
| LDL-C, mg/dL | 120 (37) | 125 (40) | .06 |
| Total cholesterol, mg/dL | 212 (51) | 218 (69) | .06 |
| Triglycerides, mg/dL | 244 (212) | 272 (338) | .07 |
| Framingham Risk Scorec | 14.5 (9.5) | 16.7 (9.2) | <.001 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data are presented as number and percentage of patients for categorical and as mean (SD) for continuous variables.
Laboratory data were 71% complete; BP data, 81% complete; and comorbidity data, 98% complete.
Ten-year risk estimate of CAD.
RESULTS
Study Participants
A total of 10,079 patients in Olmsted County received a diagnosis of diabetes between January 1, 1992, and December 31, 2008. From these patients, those without a history of CAD or heart failure were identified: 292 who had a screening stress test within 2 years of diabetes diagnosis (screened cohort); 2,246 who were asymptomatic at 2 years and without stress testing within that time period (unscreened cohort); and 608 who became symptomatic, died, or had MI within 2 years (symptomatic cohort); thus, 3,146 patients were included (Figure 1). Of the 292 patients who underwent screening stress testing, clinical indications were as follows: 154/292 (53%) for cardiac risk factors, 45/292 (15%) for pre-operative evaluations, 5/292 (2%) for elevated coronary calcium scores, 31/292 (11%) for abnormal ECG findings, and 57/292 (19%) for other reasons.
Figure 1.
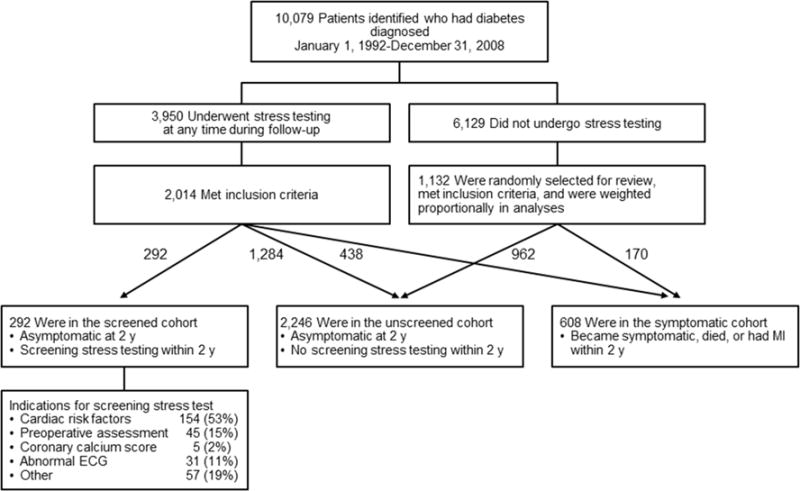
Cohort Selection Among Adult Patients of Olmsted County, Minnesota, With a Diagnosis of Diabetes Mellitus. Total included in analysis was 3,146 patients. MI indicates myocardial infarction.
Participants included 53% men; mean (SD) age at diabetes diagnosis was 55 (13.8) years, and 97% had type 2 diabetes. Baseline clinical characteristics for the screened cohort and unscreened cohort are shown in Table 1. The screened cohort had higher FRSs at baseline than the unscreened cohort (16.7 vs 14.5, P<.001). The symptomatic cohort showed similar baseline characteristics with FRS intermediate (15.6 (9.3)) between the unscreened and screened cohorts. Screening stress tests included exercise electrocardiography in 158 patients, nuclear perfusion in 71, and stress echocardiography in 63. Stress test results were abnormal in 47 (16.1%) and 36 (12.3%) of screened patients had inducible ischemia (Table 2).
Table 2.
Results of Screening Stress Testing Within 2 Years of Diabetes Mellitus Diagnosis
| Type of Stress Testa | No. of Patients (n=292) |
Normal Result | Inducible Ischemia | Fixed Abnormalityb | Nondiagnostic Findings |
|---|---|---|---|---|---|
| Stress echocardiography | 63 (22) | 47 (75) | 10 (16)c | 6 (10) | 0 (0) |
| Nuclear perfusion | 71 (24) | 40 (56) | 23 (32)d | 5 (7) | 3 (4) |
| Stress electrocardiography | 158 (54) | 149 (94) | 3 (2) | NA | 6 (4) |
| Evidence of occult CADe | 47 (16) |
Abbreviations: CAD, coronary artery disease; NA, not applicable.
Values are presented as number and percentage of patients. Percentages were determined after excluding missing results and nondiagnostic findings.
Fixed abnormality refers to either a fixed perfusion defect on nuclear perfusion study or resting regional wall motion abnormality on stress echocardiography.
Four patients had both fixed abnormality and inducible ischemia.
Four patients had both fixed abnormality and inducible ischemia.
Occult CAD refers to presence of inducible ischemia or fixed abnormality, or both.
Outcomes
Median (interquartile range) follow-up was 9.1 (5.3–12.5) years. During follow-up, death or MI occurred in 454 patients (32 in the screened cohort and 422 patients in the unscreened cohort [5-year rate, 1.9% and 5.3%, respectively]), and 167 in the symptomatic group. Death occurred in 474 patients (26 in the screened cohort and 325 in the unscreened cohort [5-year rate, 1.1% and 2.8%, respectively]). Of all patients not known to have died, 2,628 (98.4%) had follow-up greater than 1 year. Rates of events at 10 years, including coronary artery revascularization and cardiac death, are given in Figure 2.
Figure 2.
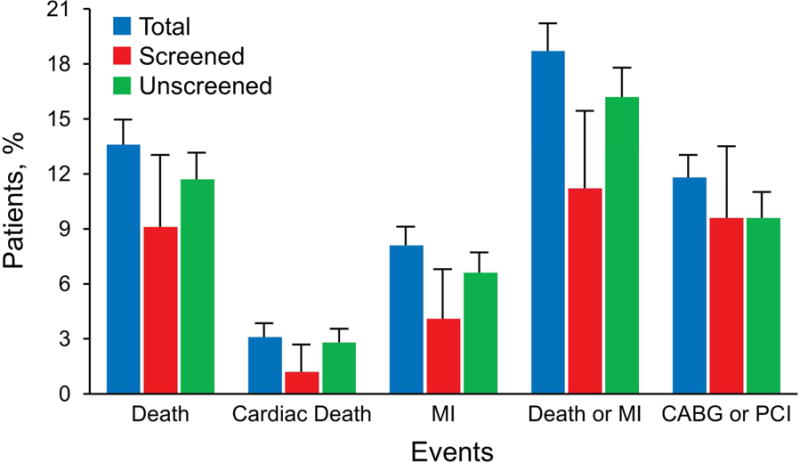
Event Rates for Entire Population. Data include the total group. CABG indicates coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention. Error lines represent 95% CIs.
Of the 47 patients with abnormal screening stress test consistent with CAD, information regarding clinical follow up was available in 45. Of these, 27 (60%) were referred to Cardiology, 25 (53%) were prescribed additional medical therapy, 17 (38%) were encouraged to make lifestyle modifications, and 5 (11%) were prescribed medication therapy intensification with increased doses.
Outcomes for the 47 patients with a screening stress test positive for inducible ischemia or fixed defect, were as follows: 6 had percutaneous coronary intervention or coronary artery bypass grafting (4 days, 22 days, 22 days, 273 days, 5.2 years, and 5.4 years post–stress test), 10 died (2.0 years [lung cancer], 5.1 years, 5.3 years, and 6.4 years [chronic obstructive pulmonary disease], 6.9 years [pneumonia], 7.1 years [ischemic heart disease], 8.4 years [congestive heart failure], 9.3 years [colon cancer], 10.8 years [lung cancer], and 15.4 years [sepsis] post–stress test); 2 patients had MI (5.2, 7.3 years post–stress test).
Analysis of the combined end point of death and MI, censoring for symptoms within 2 years, showed FRS, peripheral arterial disease, and aspirin use at the time of diabetes diagnosis to be correlated with an increased risk of events (HR, 1.05 [95% CI, 1.04–1.06], P<.001; HR, 1.72 [95% CI, 1.15–2.57], P=.008; and HR, 1.44 [95% CI, 1.22–1.69], P<.001, respectively). Statin use at diabetes diagnosis (HR, 0.67 [95% CI, 0.55–0.81], P<.001) and the time-dependent variable of having a screening cardiac stress test within 2 years of diabetes diagnosis (HR, 0.61 [95% CI, 0.44–0.85], P=.004) were associated with decreased risk for the combined end point (Table 3). Analyses without weighting (HR, 0.65) and in the patient subset with complete baseline laboratory and blood pressure data (HR, 0.71) showed similar benefit of a screening stress test. When the primary analysis was repeated with the endpoint of cardiac death, the estimated benefit of the screening stress test persisted, HR, 0.46 (95% CI 0.20–1.05); results were similar for the combined endpoint of cardiac death/MI, HR, 0.44 (95% CI 0.24–0.78).
Table 3.
Univariable and Multivariable Proportional Hazards Regression Analysis for Death or Myocardial Infarction
| Variablea | Univariable Analysis
|
Multivariable Analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (per 10 y) | 1.90 (1.80–2.00) | <.001 | ||
| Male sex | 1.02 (0.89–1.18) | .73 | ||
| Body mass index (per 1 unit) | 0.97 (0.95–0.98) | <.001 | ||
| Family history of CAD | 1.00 (0.75–1.33) | .99 | ||
| Hypertensionb | 1.44 (1.25–1.66) | <.001 | ||
| Peripheral arterial disease | 1.97 (1.32–2.94) | <.001 | 1.72 (1.15–2.57) | .008 |
| Current smoker | 1.04 (0.86–1.26) | .69 | ||
| Statin use | 0.83 (0.68–0.99) | .05 | 0.67 (0.55–0.81) | <.001 |
| Aspirin use | 1.56 (1.34–1.82) | <.001 | 1.44 (1.22–1.69) | <.001 |
| ACE-I or ARB use | 1.16 (0.99–1.37) | .07 | ||
| β-Blocker use | 1.56 (1.29–1.88) | <.001 | ||
| Framingham Risk Score | 1.05 (1.04–1.06) | <.001 | 1.05 (1.04–1.06) | <.001 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; HR, hazard ratio.
With censoring for symptoms within 2 years (n=520 events).
Defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or antihypertensive therapy.
Figure 3 presents Kaplan-Meier landmark analysis of the MI-free survival of the screened cohort vs the unscreened cohort. Median follow-up time was 9.1 (interquartile range 5.3–12.5) years overall, 8.9 (interquartile range, 5.5–11.9) years for the screened and 9.8 (interquartile range, 6.1–13.2) years for the unscreened cohort. Despite greater FRSs, the screened cohort showed better event-free survival during follow-up, with a difference persisting throughout 15 years of follow-up.
Figure 3.
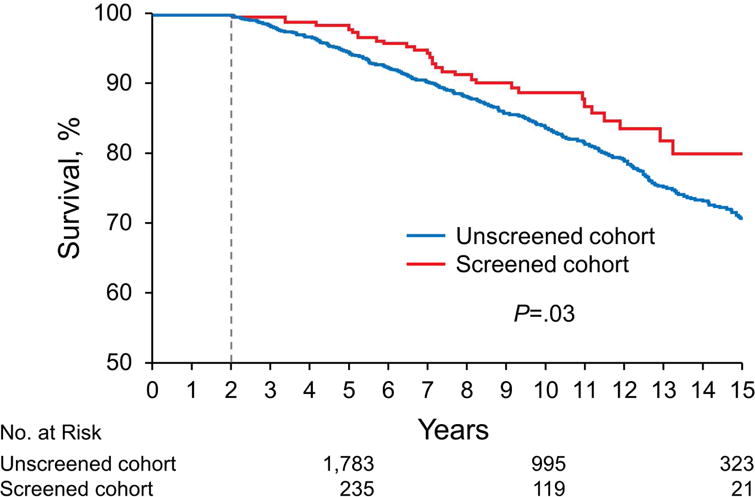
Kaplan-Meier Survival Curves Presenting Survival Free of All-Cause Mortality and Myocardial Infarction. Period spans from 2 through 15 years of follow-up for the screening cohort compared with the unscreened cohort. P value is reported from the unadjusted model. Numbers below plot represent number at risk at each time point. Vertical line shows the beginning of at-risk period.
In propensity-matched analysis, the screened and propensity-matched unscreened cohorts had very similar baseline characteristics and cardiac risk (Supplemental Table). Results of Cox proportional hazards regression with time-dependent variable of having a screening cardiac stress test within 2 years of diabetes diagnosis were similar (HR, 0.66 [95% CI, 0.44–0.99], P=.04). Kaplan-Meier landmark analysis of death and MI-free survival of the screened cohort and propensity-matched unscreened patients again showed better event-free survival for the screened cohort during follow-up (Supplemental Figure).
DISCUSSION
This is the first large-scale, community-based study designed to analyze the impact of early screening cardiac stress testing in asymptomatic patients with diabetes. The unique methodology allowed inclusion of large numbers of patients and reduced referral bias. Screening cardiac stress testing was associated with improved long-term event-free survival (HR, 0.63; P=.005), independent of other cardiac risk factors. While stress testing was abnormal in 16.1% of patients, only 2% of patients who were screened underwent coronary revascularization. This suggests that factors other than coronary revascularization alone may have improved outcomes in patients who underwent screening.
The success of primary screening recommendations rests on the prevalence of undiagnosed disease and the impact of early diagnosis on the natural history of the disease. Data from several studies highlight the prevalence of occult CAD among asymptomatic diabetic patients, although estimates vary widely. The DIAD study7 showed that 22% of asymptomatic patients with type 2 diabetes had abnormal stress myocardial perfusion imaging. This finding is consistent with estimates from Do You Need to Assess Myocardial Ischemia in Type-2 Diabetes21 which randomly assigned 631 diabetic patients to screening vs usual care, and the Basal Asymptomatic High-Risk Diabetics’ Outcome Trial,22 which screened 400 asymptomatic diabetic patients at baseline and after 2 years. The Milan Study on Atherosclerosis and Diabetes,23 in which 925 diabetic patients aged <65 years without known CAD, severe hypertension, or renal insufficiency underwent exercise electrocardiography followed with exercise thallium scintigraphy if the exercise electrocardiogram was abnormal, reported 12.1% with abnormal exercise electrocardiogram and 6.4% with abnormal exercise electrocardiography and scintigraphy. Other researchers have reported prevalence as high as 58% in asymptomatic diabetic patients.24 The large variation in prevalence of occult CAD likely reflects differences in study populations (eg, more male patients; more patients with dyslipidemia, hypertension, or peripheral artery disease).
Our study has somewhat greater generalizability because of its community-based approach. Among Olmsted County patients with diabetes, the mean 10-year risk of CAD by FRS was 14.9% and the rate of detected occult CAD was 16.1%. Stress tests were abnormal slightly less often than in the DIAD study, at least in part because we included screening stress tests up to 2 years after diabetes diagnosis; stress testing in the DIAD study was typically 8 to 9 years postdiagnosis.
The clinical effect of screening on the natural history of occult CAD is controversial, given the extensive medical optimization now recommended for all patients with diabetes. Despite the complexity of retrospective community–based data, our analysis was designed to create as clean a comparison as possible and to isolate screening stress testing as a variable. By censoring for symptoms at 2 years and defining screening stress testing within 2 years as the time-dependent variable, we eliminated a potential source of selection bias: Patients who qualify for screening stress testing many years after diabetes diagnosis may be healthier than those who have symptoms early. In our analysis, patients who underwent screening stress testing within 2 years of diabetes diagnosis had higher cardiac risk at baseline than the unscreened cohort (FRS, 16.8 vs 14.5; P<.001), yet had improved long-term outcomes.
Recent prospective trials7,21,25 have suggested a lack of clinical benefit associated with CAD screening. However, the rate of cardiac events in these studies was unexpectedly low, likely reflecting the exclusion of patients with prior stress testing or abnormal resting ECG from the DIAD study7 and the exclusion of patients with any known atherosclerotic cardiovascular disease from the FACTOR-64 trial,25 which explored the role of screening with coronary computer tomography in 900 patients with diabetes. Do You Need to Assess Myocardial Ischemia in Type-2 Diabetes21 was discontinued prematurely because of a lower-than-expected event rate and difficulties in enrollment.
Our community-based approach reduces potential for referral and selection bias. The event rates in our study are consistent with the 10-year mortality rate of 18.9% reported in the United Kingdom Prospective Diabetes Study26 that enrolled relatively healthy patients who had a recent diagnosis of type 2 diabetes and the 1.14% to 1.41% annual mortality rate in the Action to Control Cardiovascular Risk in Diabetes Study.27 These recent large studies in patients with type 2 diabetes did not involve recruitment of patients to a CAD screening or nonscreening strategy. It is possible that some clinicians are reluctant to enroll any but the healthiest patients in screening vs nonscreening study protocols.
The explanation for the relation between improved outcomes and stress testing remains uncertain. Too few patients underwent coronary revascularization to draw conclusions regarding early revascularization as a source of improved outcomes. Clinical benefit could be based on intensification of or improved adherence to medical management by those who undergo screening; however, this hypothesis has not been proven.28
The community-based approach in our study not only reduced referral bias but also allowed extended, relatively complete follow-up. Our retrospective cohort included incident diabetic cases and spanned almost 15 years. The study sample size (N=3,146) allowed for greater statistical power than prior studies.7,21,25
Some limitations should be acknowledged. Despite its growing diversity, the Olmsted County population is largely white, potentially limiting the generalizability. In addition, the retrospective nature of our observational study was necessarily associated with some degree of missing or incomplete data availability, especially given the multiple sources and large time-frame over which data were collected. However, results were similar in patients with and without partial missing baseline data. Guideline recommendations for management of diabetes also evolved over the time period of this study. Since stress testing was performed according to the judgment of the patients’ physicians, rather than according to specific criteria, and patients were not randomized, we cannot exclude the possibility that patients with diabetes who received screening stress testing differed by some socioeconomic or behavioral characteristic correlating with better health. However, baseline mean FRS was higher in screened vs unscreened cohorts, suggesting that this factor was unlikely to be a source of major bias.
CONCLUSION
Screening cardiac stress testing in asymptomatic patients in a community-based cohort within 2 years of the diabetes diagnosis was associated with improved long-term event-free survival, despite a mean higher FRS in those who underwent screening. CAD was suggested by stress test results in 16.1% of screened patients and most received intensified medical therapy. Only 2% of screened patients underwent coronary revascularization, suggesting that improved outcomes may have been secondary to factors other than coronary revascularization alone. Further study is indicated to understand the benefit of screening cardiac stress testing. The unique methodology employed in this observational study could be applied to the study of other conditions in which the optimal treatment strategy is uncertain and prospective enrollment of patients may be difficult and may introduce bias.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Mayo Clinic Department of Cardiology which supported biostatistical analysis. This study was made possible using the resources of the REP, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BP
blood pressure
- CAD
coronary artery disease
- CI
confidence interval
- DIAD
Detection of Ischemia in Asymptomatic Diabetics
- ECG
electrocardiogram
- FRS
Framingham Risk Score
- HR
hazard ratio
- MI
myocardial infarction
- REP
Rochester Epidemiology Project
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta(GA): U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999 Sep 7;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. Erratum in: Circulation 2000;101(13):1629–1631. [DOI] [PubMed] [Google Scholar]
- 3.Koistinen MJ. Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ. 1990;301(6743):92–95. doi: 10.1136/bmj.301.6743.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. Diabetes Care. 1998;21(9):1551–1559. doi: 10.2337/diacare.21.9.1551. [DOI] [PubMed] [Google Scholar]
- 5.Ryden L, Grant PJ, Anker SD, et al. ESC Committee for Practice Guidelines (CPG) ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34(39):3035–87. doi: 10.1093/eurheartj/eht108. Erratum in: Eur Heart J. 2014;35(27):1824. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2016;39(Suppl):S60–S71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 7.Young LH, Wackers FJ, Chyun DA, et al. DIAD Investigators Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorlie P, Wei GS. Population-based cohort studies: still relevant? J Am Coll Cardiol. 2011;58(19):2010–2013. doi: 10.1016/j.jacc.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Census Bureau. Census Redistricting Data (Public Law 94–171) Summary file—technical documentation. 2010 c2011 (Tables P1, P2 and H1) [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;1624(6):1614–24. doi: 10.1093/ije/dys195. Epub 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. Epub 2011 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567–1573. doi: 10.1038/ajg.2010.18. Epub 2010 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014 May 7;311(17):1809. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D’Agostino RB, Levy D, et al., editors. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20(9):1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Strauss HW, Miller DD, Wittry MD, et al. Society of Nuclear Medicine. Procedure guideline for myocardial perfusion imaging. J Nucl Med. 1998;39(5):918–923. [PubMed] [Google Scholar]
- 19.Gibbons RJ, Balady GJ, Bricker JT, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Committee to Update the 1997 Exercise Testing Guidelines ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40(8):1531–1540. doi: 10.1016/s0735-1097(02)02164-2. Erratum in: J Am Coll Cardiol. 2006; 48(8):1731. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 21.Lievre MM, Moulin P, Thivolet C, et al. DYNAMIT investigators Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials. 2011;12:23. doi: 10.1186/1745-6215-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zellweger MJ, Maraun M, Osterhues HH, et al. Progression to overt or silent CAD in asymptomatic patients with diabetes mellitus at high coronary risk: main findings of the prospective multicenter BARDOT trial with a pilot randomized treatment substudy. JACC Cardiovasc Imaging. 2014;7(10):1001–1010. doi: 10.1016/j.jcmg.2014.07.010. Epub 2014 Sep 17. [DOI] [PubMed] [Google Scholar]
- 23.Milan Study on Atherosclerosis and Diabetes (MiSAD) Group. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1997;79(2):134–139. doi: 10.1016/s0002-9149(96)00699-6. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan N, Miller TD, Hodge DO, et al. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45(1):43–49. doi: 10.1016/j.jacc.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 25.Muhlestein JB, Lappe DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312(21):2234–2243. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
- 26.Davis TM, Coleman RL, Holman RR, UKPDS Group Prognostic significance of silent myocardial infarction in newly diagnosed type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS) 79. Circulation. 2013;127(9):980–987. doi: 10.1161/CIRCULATIONAHA.112.000908. Epub 2013 Jan 29. [DOI] [PubMed] [Google Scholar]
- 27.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. Epub 2008 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galper BZ, Moran A, Coxson PG, et al. Using stress testing to guide primary prevention of coronary heart disease among intermediate-risk patients: a cost-effectiveness analysis. Circulation. 2012;125(2):260–270. doi: 10.1161/CIRCULATIONAHA.111.041293. Epub 2011 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


