Abstract
Spontaneous intracranial hemorrhage (ICH) remains a devastating stroke subtype, affecting as many as 80,000 people annually in the United States and associated with extremely high mortality. In the absence of any pharmacological interventions demonstrated to improve outcome, care for patients with ICH remains largely supportive. Thus, despite advances in the understanding of ICH and brain injury, there remains an unmet need for interventions that improve neurologic recovery and outcomes. Recent research suggesting inflammation and APOE genotype play a role in modifying neurologic outcome after brain injury has led to the development of an APOE-derived peptide agent (CN-105). Preclinical studies have demonstrated that CN-105 effectively down regulates the inflammatory response in acute brain injury, including ICH. Following Investigational New Drug (IND) enabling studies in murine models, this first in-human single escalating dose and multiple dose placebo-controlled clinical trial was performed to define the safety and pharmacokinetics (PK) of CN-105. A total of 48 subjects (12 control, 36 active) were randomized in this study; all subjects completed the study. No significant safety issues were identified with both dosing regimens, and PK analysis revealed linearity without significant drug accumulation. The median half-life in the terminal elimination phase of CN-105 following a single or repeated dosing regimen did not change (approximately 3.6 hr). With the PK and preliminary safety of CN-105 established, the drug is now poised to begin first in disease phase 2 clinical trials in patients with ICH who urgently need new therapeutic options.
Keywords: intracerebral hemorrhage (ICH), brain injury, neuroinflammation, neuroprotection, apolipoprotein E (apoE), pharmacokinetics
INTRODUCTION
Acute brain injury resulting from cerebrovascular diseases and trauma is associated with extremely high mortality and morbidity 1,2. Despite advances in our understanding of basic cellular and molecular mechanisms associated with primary and secondary neuronal injury, no effective neuro-protective pharmacological interventions have improved functional outcomes 3,4. Supportive medical care remains the mainstay of management for patients with acute brain injury and mortality rates for many of these injuries have not improved in the last 2 decades 2,5,6. There is a clear and urgent unmet clinical need for neuroprotective therapeutics for acute brain injury.
Traditionally, new therapeutic strategies have focused on our increased mechanistic understanding of disease-specific pathophysiology. However, despite these important disease-specific differences, the neuroinflammatory response, characterized by glial activation and release of mediators of inflammation, neuronal excitotoxicity, and oxidative stress serve as a common denominator that exacerbates secondary neuronal injury in a variety of acute and chronic neuropathology 7. Moreover, in the setting of acute brain injury, neuroinflammation plays an important role in mediating tissue injury for days after the initial insult 8–10; this has the potential to lengthen the therapeutic window as compared to strategies that solely target excitotoxicity. Thus, a therapeutic strategy that targets maladaptive neuroinflammatory responses holds promise in the treatment of diverse forms of brain injury. Apolipoprotein E (apoE) is an endogenous brain protein synthesized in response to brain injury and exists in humans as 3 common protein isoforms, designated apoE2, apoE3, and apoE4, which differ by single amino acid substitutions at residues 112 and 158 11. Isoform-specific protective effects on endogenous neuroinflammatory responses have been observed in humans 12,13 and demonstrated in preclinical models of brain injury 14–16. Mechanisms by which apoE reduces neurologic inflammation have been demonstrated through specific receptor interactions 17–19 and down-regulation of microglial activation 20,21.
Previous studies from our lab and others have demonstrated that apolipoprotein E (apoE), a 299 amino acid protein produced within the brain modifies neuroinflammatory responses by downregulating glial activation and release of inflammatory mediators. However, apoE holoprotein’s therapeutic potential is limited, as it does not cross the blood brain barrier (BBB). To address this limitation, we have developed apoE mimetic peptides derived from the helical receptor binding region of apoE. These peptides have been demonstrated to improve histological and long term functional outcome in preclinical models of intracerebral hemorrhage (ICH) 22,23, traumatic brain injury 24–27, ischemic stroke 28,29, subarachnoid hemorrhage 30,31 and spinal cord injury 32,33.
Recently, we have selected CN-105, an apoE-mimetic pentapeptide derived from the receptor binding face of apoE (Figure 1), as the lead candidate for further development based on its profile in in vitro cell culture models of neuroinflammation and neuroprotection, in vivo efficacy in preclinical models of intracranial hemorrhage 34, stroke, and acute brain injury and preclinical safety profile34. Results from the rodent and beagle Good Laboratory Practice safety and toxicology studies demonstrated that repeated intravenous administration of CN-105 at 5 mg/kg/dose (100-fold the therapeutic dose identified in preclinical efficacy studies), four times per day (20 mg/kg/day) for 14 days was well-tolerated, and 20 mg/kg/day was identified as the no observed-adverse effect-level (NOAEL) in these studies. Based upon these promising preclinical results, the decision was made to further develop CN-105 for the treatment of human acute brain injuries.
Figure 1.
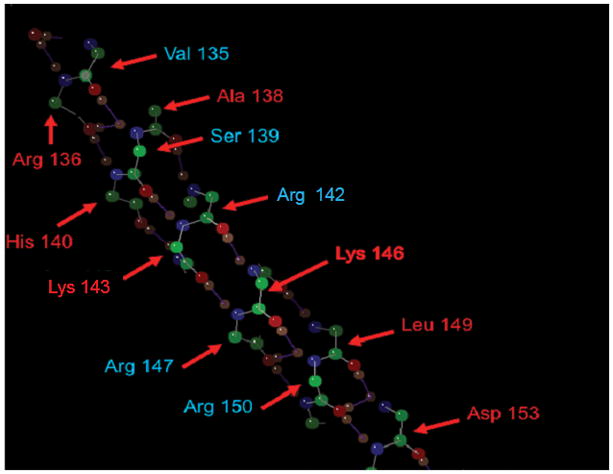
CN-105 has never been evaluated in humans and the safety and pharmacokinetics (PK) have not been defined, and the current first-in-human (FIH) study represents the first clinical translation of this molecule to a clinical population.
METHODS
The protocol was approved by the FDA under an Investigational New Drug Application and by the Duke University Institutional Review Board. Written informed consent was obtained for each volunteer prior to performing study-related procedures.
Eligibility Criteria
Healthy male and female volunteers aged 18–50, with BMI ranging 18–33 kg/m2, and weight of at least 50 kg were eligible for the study. Volunteers were required to have adequate peripheral vein access, no exposure to prescription medication (except contraception) or over the counter (OTC) medications or herbal/vitamin supplements (except acetaminophen ≤ 1 g/day and stable, non-glucocorticoid treatment of seasonal allergies) in the 7 days prior to study entry, no exposure to nicotine-containing products for ≥ 6 months, no current or recent (within 2 years) history of alcohol or drug abuse, caffeine consumption ≤ 3 cups of coffee per day, ability to comply with medically acceptable contraception or prior history of surgical sterilization, and no history of recent (30 days cellular and 90 days acellular) blood donation. Pregnant or lactating females and volunteers with significant medical or psychiatric illness by history or examination that would influence study results or preclude informed consent and study compliance were excluded.
Study Design
This was a Phase I, single center, randomized, double-blind, placebo controlled study to determine the safety, tolerability and pharmacokinetics (PK) of a single ascending dose (SAD) and repeated doses of intravenous CN-105 in healthy adults. In the SAD portion of this FIH study, 8 participants were randomized to CN-105 or saline control (6 active: 2 control) at 0.01, 0.03, 0.1, 0.3 and 1.0 mg/kg administered over 30 minutes. Intermittent weight based dosing was supported by preclinical animal models and was felt more practical in a clinical setting than a continuous intravenous dose34. The maximum dose in the study was the highest dose allowed by the U.S. Food and Drug Administration (FDA). Since this was a FIH study, an interim PK analysis was planned during the SAD portion of the study to determine the optimal PK sampling timepoints. In the repeat dose cohort 8 participants (6 active: 2 control) were randomized to receive repeated infusions of CN-105 over 30 minutes every 6 hours for 72 hours.
Data Monitoring Committee
A Data Monitoring Committee was used to evaluate safety and tolerability with the dose escalation cohorts and to select the final dose for the repeated dose cohort. Doses were escalated in successive cohorts unless one participant experienced an adverse effect of Severe Grade or two participants reported the same adverse effect of Moderate Intensity that was considered probably related to the study drug.
Safety evaluations
During and following dosing, safety and tolerability endpoints included reported adverse events (AEs), changes in vital signs, physical examination findings, ECG, and clinical laboratory tests (hematologic, chemistry, urinalysis).
Pharmacokinetic Sampling
During the SAD cohorts, blood samples were taken at 15 minutes prior to start of dosing and at 0.083, 0.167, 0.5, 1, 2, 4, 8, 12 and 24 hours from the start of dosing. Urine samples were obtained prior to dose administration and then pooled samples (0–3, 3–6, 6–8, 8–12, 12–24 hours) were collected from the start of dosing. Interim PK analysis after the second (0.03mg/kg) cohort resulted in removal of a 168 hour blood sample and addition of a urine sample from 8–12 hours. For repeat dose participants, blood samples were taken 15 minutes hour prior to start of dosing and then at 0.083, 0.167, 0.5, 1, 2, 4, 5-<6 hours for the first 2 doses and within 1 hour prior to each dose thereafter. Blood samples were also collected at 0.083, 0.167, 0.5, 1, 2, 4, 5-<6 and 12 hours after the last dose.
Plasma and urine drug concentrations were determined by MPI (Mattawan, MI). Plasma were stored in 1% HALT with K2EDTA at −70° Celsius before they were ready to be analyzed. Liquid chromatography-tandem mass spectrometry analysis was performed using a positive Turbo IonSpray® interface on a Sciex API-3000 (Applied Biosystems, Foster City, CA) and multiple reaction monitoring. The analytical range was 1.00 ng/mL to 1,000 ng/mL. The assay precision in quality-control samples was < 20% for the lowest limit of quantitation and < 15% for all other concentrations.
Pharmacokinetics and Safety Analysis
Individual plasma concentration versus time profiles of CN-105 for SAD and repeat doses were used to generate PK parameters using non-compartmental analysis. The noncompartmental PK analysis was performed in Phoenix WinNonLin (version 6.3, Pharsight Corporation) using concentration versus time data obtained for CN-105. All plasma concentrations below the quantitation limit were assigned a value of zero. For both SAD and repeat dose cohorts, the peak drug concentration (Cmax) and time of peak concentration (Tmax) were obtained from the observed data. For the SAD cohort, AUC from zero to last measurable concentration (AUC0-last) and AUC from 0 to infinity (AUC0-∞) and elimination half-life (t1/2) were calculated. For the repeat dose cohort, AUC from zero to 6 hours (AUCTAU) was assessed for CN-105. AUC0-last and AUCTAU were calculated using the trapezoidal rule. In addition, the AUC0-∞ was calculated from AUC0-last + Ct/λz where Ct is the last measurable concentration, and λz is the terminal elimination rate constant calculated by fitting 3 points to a linear regression. The half-life (t1/2) was calculated as 0.693/λz. At least 3 time points with measurable plasma concentrations were required for the calculation of AUClast and at least 3 time points (of which the first time point must be greater than Tmax) with measurable plasma concentrations were required for the calculation of λZ. Total body clearance (CL) was calculated as Dose/AUC∞. The volume of distribution (V) was calculated as V = CL/λz.
Primary analysis of safety and tolerability using vital signs, ECG, clinical laboratory results and AEs was performed using descriptive statistics. Categorical variables were analyzed as counts and percentages, while continuous variables were presented as means and standard deviations or medians and interquartile ranges. Statistical analyses were performed using Version 9.4 (or newer) of SAS® (Cary, NC) on an Unix operating system or Stata 13.1 (College Station, TX).
RESULTS
Demographics
Sixty-six adults were enrolled, and 48 completed the clinical trial. The study population was predominantly male (79%) and African American (69%) (Table 1). These demographics were similar between the placebo and treatment groups.
Table 1.
Characteristics of Enrolled Subjects
| Placebo (N=12) | CN-105 (N=36) | Total (N=48) | |
|---|---|---|---|
| Age (Median) (Q1, Q3) | 32.0 (28.9, 41.0) | 32.4 (28.3, 37.2) | 32.4 (28.3, 37.6) |
| Sex | |||
| Male | 9 | 29 | 38 |
| Female | 3 | 7 | 10 |
| Race | |||
| Black or African American | 7 | 26 | 33 |
| Caucasian | 3 | 7 | 10 |
| Other | 2 | 3 | 5 |
| BMI (Median) (Q1, Q3) | 27.6 (25.1, 30.7) | 26.3 (24.0, 28.4) | 26.6 (24.0, 29.2) |
N, number; Q, quartile; BMI, body mass index
Safety and Tolerability
Among the 48 subjects in this study, 23 (47%; 18 active: 5 placebo) experienced an AE. A total of 18 subjects (37.5%) experienced a treatment-emergent AE, 4 (33.3%) in the placebo group and 14 (38.9%) in the CN105 group. Bradycardia and headache were the most common treatment-emergent AEs. A total of 6 (12.5%) subjects experienced bradycardia, 2 (17%) in the placebo group and 4 (11%) in the CN-105 group; a total of 2 (4%) subjects reported headache, 0 (0%) in the placebo group and 2 (6%) in the CN-105 group. The bradycardia in most cases was not treatment emergent and likely related to the study population of young healthy volunteers. No Serious Adverse Events or deaths occurred. No concerning changes were observed in serial ECG, vital signs or clinical laboratory tests.
Pharmacokinetic summary
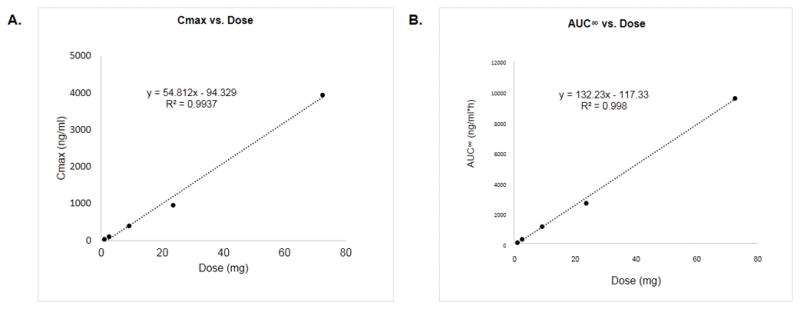
All subjects receiving CN-105 (n = 36) had evaluable PK data and there were no missing data. For the single ascending doses, exposure and PK parameters are summarized in Table 2. Following i.v. infusions, concentrations of CN-105 in plasma declined in a polyphasic manner, as shown in the mean concentration of drug vs. time plot (Figure 2). A short distribution phase was seen immediately post-infusion. A dominant β phase characterized much of the profile and exhibited log-linear behavior. At higher doses, an additional longer γ phase was present post-dose at low concentrations. PK parameters for the single ascending dose regimens are summarized in Table 2. The volume, clearance and half-life remained relatively constant over the range of doses evaluated. The mean of Cmax and AUC parameters plotted versus dose in Figure 3A and 3B were well represented by linear regression lines (r2 >0.99) and consistent with dose proportionality.
Table 2.
Pharmacokinetic values for single dose CN-105
| Parametera | Dose
|
||||
|---|---|---|---|---|---|
| 0.01 mg/kg | 0.03 mg/kg | 0.1 mg/kg | 0.3 mg/kg | 1.0 mg/kg | |
| Cmax (ng/ml) | 35.7 ± 10.9 | 107.5 ± 25.5 | 407.8 ± 128.9 | 965.8 ± 209.3 | 3943.3 ± 507.0 |
|
| |||||
| AUC0-∞ (ng/ml*hr) | 95.9 ±20.2 | 280.8 ± 39.6 | 1126.1 ± 194.5 | 2672.3 ± 272.3 | 9548 ± 1281.2 |
|
| |||||
| Volume (L) | 29.8 ± 3.2 | 30.4 ± 8.0 | 38.9 ± 12.0 | 45.6 ± 11.2 | 38.4 ± 8.1 |
|
| |||||
| Clearance (L/h) | 9.0 ± 1.1 | 9.0 ± 1.1 | 8.2 ± 1.9 | 8.8 ± 1.5 | 7.7 ± 1.7 |
|
| |||||
| T1/2 | 2.3 ± 0.5 | 2.4 ± 0.5 | 3.3 ± 0.6 | 3.6 ± .4 | 3.5 ± 0.3 |
AUC 0-∞ area under the plasma concentration–time curve from zero to infinity, CL clearance, Cmax maximum plasma drug concentration, t1/2 half-life
Data provided as mean ± SD
Figure 2.
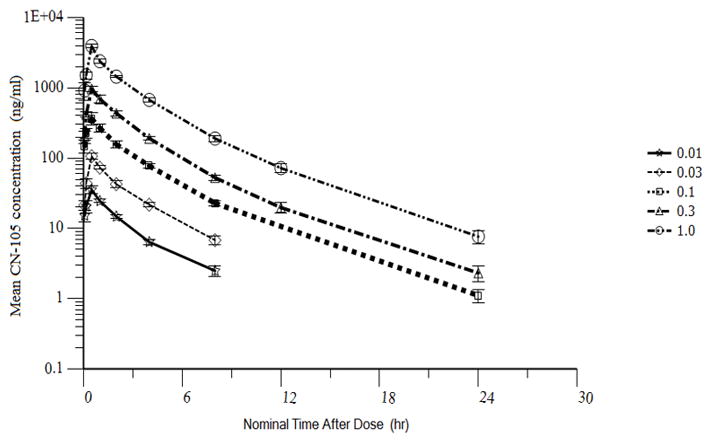
Figure 3.
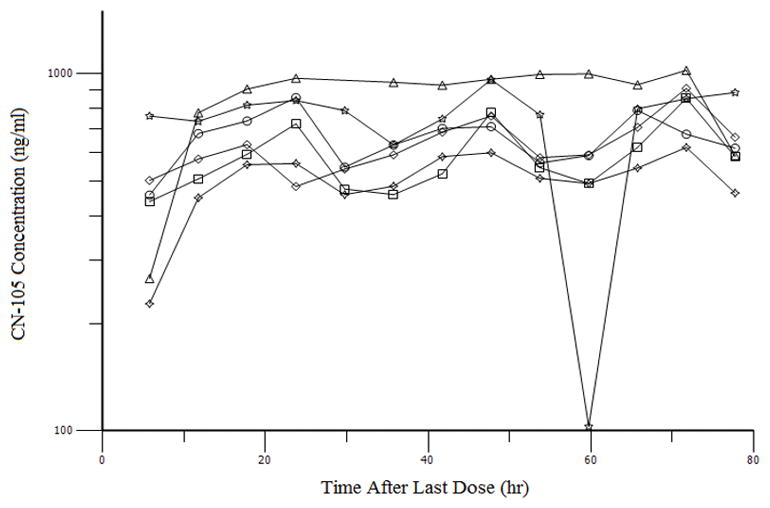
PK parameters for the multiple dosing regimen are summarized in Table 3. The median (range) terminal elimination half-life after the 13th dose was 3.6 hours (3.4 – 7.1). There was no significant accumulation of CN-105 as evidenced by the relatively constant AUCTAU ratios between the time points (Table 4), which is shown graphically in Figure 4. Trough concentrations reached a stable plateau by 20 hrs (Figure 4), and a steady state was achieved within the first 24 hours.
Table 3.
Pharmacokinetic values for repeated dose CN-105 (1 mg/kg)
| Dose Number | Cmax (ng/ml) | AUCTAU (0–6) (ng/ml*hr) |
|---|---|---|
| 1 (0 hr) | 5011.67 (814.01) | 9669.2 (1724.1) |
| 2 (6 hr) | 5340 (568.44) | 10557.9 (1158.5) |
| 13 (72 hr) | 5505 (404.17) | 11434.6 (1721.2) |
Table 4.
PK parameters for repeated doses of CN-105 administered as 1 mg/kg i.v. infusions over 30 min
| Subject ID | Dose | AUCTAU1 | AUCTAU2 | Ratio 2/1 | AUCTAU13 | Ratio 13/1 |
|---|---|---|---|---|---|---|
| Mean | 85 | 9669.2 | 10557.9 | 1.1 | 11434.6 | 1.2 |
| Geometric Mean | 84.3 | 9536.1 | 10506.5 | 1.1 | 11328.6 | 1.2 |
| SD | 11.9 | 1724.1 | 1158.5 | 0.2 | 1721.2 | 0.2 |
| CV% | 14 | 17.8 | 11 | 15.1 | ||
| Median | 86 | 9911.7 | 10448.8 | 1.1 | 11600.4 | 1.3 |
| Min | 69.5 | 7425.1 | 8990.5 | 0.9 | 9382.1 | 0.9 |
| Max | 98.8 | 11431.3 | 12581.6 | 1.4 | 14203.2 | 1.5 |
Figure 4.
DISCUSSION
This Phase I, single-center, randomized, double-blind, placebo controlled study is the FIH trial of the apoE-mimetic peptide CN-105. In this trial, we have demonstrated the safety and favorable PK profile of IV CN-105 in healthy adult volunteers. We found that the CL and half-life of CN-105 were relatively consistent across doses within and between subjects, and that the pharmacokinetic data of CN-105 administered as single IV doses between 0.01 and 1.0 mg/kg are consistent with linearity. Furthermore, a dose proportionality analysis of the linear regression of the log area under the plasma drug concentration-time curve versus the log total dose suggests that the doses were proportional to various concentrations, hence CN-105 exposure increases proportionally after a single dose. The median half-life in the terminal elimination phase of CN-105 following a single high dose (0.1, 0.3 and 1.0 mg/kg) and after the last (13th) of a repeated dosing regimen showed that half-life (3.5 hr and 3.6 hr, respectively) did not change with multiple doses.
The observed half-life of 3.6 hours at higher doses in this study was longer than predicted by animal modeling (30 minutes)34. This highlights the difference between human and murine pharmacokinetics and the importance of the study in determining the optimal dosing interval for human subjects. The longer half-life is of practical significance as it permits a repeated dosing regimen rather than a continuous infusion, thereby facilitating emergent administration in the clinical setting. Prior murine studies demonstrated a single weight-based bolus dose achieved adequate CNS concentrations to produce long-term functional improvement, suggesting a pharmacokinetic-pharmacodynamic dissociation that makes continuous exposure unnecessary34. Additionally, the half-life of CN-105 supports the pre-planned dosing paradigm of repeated intravenous administration every 6 hours for 72 hours. This dosing interval was extrapolated from cellular events that lead to peak cerebral edema and secondary neuronal injury. Of note, CN-105 shows minimal accumulation after repeated 6-hour IV doses, and steady state is achieved in the first 24 hrs. Thus, CN-105 has favorable clinical pharmacological properties including a predictable linear PK and dose proportionality and a sufficiently long half-life to permit intermittent dosing. Such intermittent dosing is also more practical within a critical care setting where a patient may be receiving multiple drugs that cannot be infused simultaneously due to diluent or drug-drug interactions.
Although apoE-mimetic peptides have demonstrated robust benefits in preclinical models of acute (traumatic) brain injury 22–33, spontaneous (nontraumatic) ICH may be the optimal initial therapeutic target for a number of strategic reasons. Epidemiologically, ICH is a deadly condition that lacks effective treatment options and carries a high rate of morbidity and mortality1 that has not improved in the last 20 years 5. To date, multiple surgical clinical trials in ICH have failed to show any significant benefit 35,36, and despite further ongoing surgical trials, a paradigm shift towards alternative therapies targeting other mechanisms of neuronal injury may be necessary. ICH is a clinically devastating condition with an urgent need for an alternative therapeutic entity; this makes it an attractive initial clinical outlet for pharmacological interventions designed to reduce neuroinflammatory responses. It is logistically feasible because patients present acutely and are cared for in the controlled environment of a neurointensive care or step-down unit 6. Unlike TBI, patients are more likely to be accompanied by a legally authorized representative, facilitating recruitment. As opposed to ischemic stroke, in which a core area of brain tissue may undergo early irreversible injury, secondary neuronal injury, such as progressive cerebral edema and mass effect, following ICH tends to occur over a more protracted period raising the possibility of a prolonged therapeutic window 10. Furthermore, clinically relevant radiographic surrogates (brain computed tomography) are readily available and standardized to accurately localize and quantify hematoma size and to characterize the evolution of perihematomal cytotoxic edema and the resultant mass effect 37. These radiographic measures of mass effect and serum biochemical markers of glial activation, neuronal injury and neuroinflammation serve as important objective measures to capture target engagement in the initial trials of CN-105 in patients with ICH. Phase 2 trial design for ICH is also facilitated by the availability of clinically validated prognostic scoring systems based on hemorrhage size 38, location, neurological exam, and patient characteristics 39. These considerations are particularly important given the absence of any previous successful studies in this area.
CONCLUSION
CN-105 is the leading candidate in the class of apoE-mimetic peptides derived from the receptor-binding region with the potential to improve outcomes in ICH patients when compared to the current standard of care. This Phase 1 FIH study demonstrated a linear and predictable pharmacokinetic profile. Likewise, the safety profile was reassuring, demonstrating only mild, transient adverse effects. Taken together, this favorable PK and safety profile, the wealth of preclinical data demonstrating a histologically and functionally therapeutic benefit across different models of acute brain injury, and the current clinical need for better therapeutic options for ICH patients support further development of CN-105 for treatment of this population.
Supplementary Material
Acknowledgments
The authors wish to recognize the contributions of the Data Monitoring Committee members who volunteered their time in support of this study: David Huang (chair and medical monitor; The University of North Carolina – Chapel Hill), Nada El Husseini (Wake Forest Baptist Health), Gerald A Grant (Stanford University), Scott Kasner (University of Pennsylvania), and John Lynch (Medical College of Wisconsin). This study was supported by Aegis-CN and an institutional grant to Duke University from the North Carolina Center for Biotechnology. DTL is an officer of Aegis-CN (study sponsor). JTG is supported by K23NS085049.
Footnotes
Disclosures: This study was supported by Aegis-CN and an institutional grant to Duke University from the North Carolina Center for Biotechnology. DTL is an officer of Aegis-CN (study sponsor). JTG is supported by K23NS085049.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Stocchetti N, Taccone FS, Citerio G, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care. 2015;19:186. doi: 10.1186/s13054-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner DS, James ML, Laskowitz DT, Wijdicks EF. Translational research in acute central nervous system injury: lessons learned and the future. JAMA Neurol. 2014;71(10):1311–1318. doi: 10.1001/jamaneurol.2014.1238. [DOI] [PubMed] [Google Scholar]
- 5.Rincon F, Mayer SA. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19(1):95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurthy K, Laskowitz DT. Cellular and Molecular Mechanisms of Secondary Neuronal Injury following Traumatic Brain Injury. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): 2016. [PubMed] [Google Scholar]
- 8.Hanrahan F, Campbell M. Neuroinflammation. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): 2016. [PubMed] [Google Scholar]
- 9.Shichita T, Ito M, Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Frontiers in cellular neuroscience. 2014;8:319. doi: 10.3389/fncel.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Frontiers in cellular neuroscience. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisgraber KH. Apolipoprotein E: structure-function relationships. Advances in protein chemistry. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- 12.Woo D, Kaushal R, Chakraborty R, et al. Association of apolipoprotein E4 and haplotypes of the apolipoprotein E gene with lobar intracerebral hemorrhage. Stroke. 2005;36(9):1874–1879. doi: 10.1161/01.STR.0000177891.15082.b9. [DOI] [PubMed] [Google Scholar]
- 13.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18(5):465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Duan RS, Chen Z, Dou YC, et al. Apolipoprotein E deficiency increased microglial activation/CCR3 expression and hippocampal damage in kainic acid exposed mice. Exp Neurol. 2006;202(2):373–380. doi: 10.1016/j.expneurol.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS. Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke. 1999;30(5):1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- 16.Aono M, Bennett ER, Kim KS, et al. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116(2):437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- 17.Christensen DJ, Ohkubo N, Oddo J, et al. Apolipoprotein E and peptide mimetics modulate inflammation by binding the SET protein and activating protein phosphatase 2A. J Immunol. 2011;186(4):2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- 18.Hoe HS, Pocivavsek A, Chakraborty G, et al. Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J Biol Chem. 2006;281(6):3425–3431. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]
- 19.Sheng Z, Prorok M, Brown BE, Castellino FJ. N-methyl-D-aspartate receptor inhibition by an apolipoprotein E-derived peptide relies on low-density lipoprotein receptor-associated protein. Neuropharmacology. 2008;55(2):204–214. doi: 10.1016/j.neuropharm.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskowitz DT, Thekdi AD, Thekdi SD, et al. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167(1):74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 21.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76(1–2):70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 22.James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40(2):632–639. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laskowitz DT, Lei B, Dawson HN, et al. The apoE-mimetic peptide, COG1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care. 2012;16(2):316–326. doi: 10.1007/s12028-011-9641-5. [DOI] [PubMed] [Google Scholar]
- 24.Lynch JR, Wang H, Mace B, et al. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp Neurol. 2005;192(1):109–116. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Hoane MR, Pierce JL, Holland MA, et al. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J Neurotrauma. 2007;24(7):1108–1118. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- 26.Laskowitz DT, McKenna SE, Song P, et al. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24(7):1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- 27.Hoane MR, Kaufman N, Vitek MP, McKenna SE. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J Neurotrauma. 2009;26(1):121–129. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Anderson LG, Lascola CD, et al. ApolipoproteinE mimetic peptides improve outcome after focal ischemia. Exp Neurol. 2013;241:67–74. doi: 10.1016/j.expneurol.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Tukhovskaya EA, Yukin AY, Khokhlova ON, Murashev AN, Vitek MP. COG1410, a novel apolipoprotein-E mimetic, improves functional and morphological recovery in a rat model of focal brain ischemia. J Neurosci Res. 2009;87(3):677–682. doi: 10.1002/jnr.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Pang J, Peng J, et al. An apoE-derived mimic peptide, COG1410, alleviates early brain injury via reducing apoptosis and neuroinflammation in a mouse model of subarachnoid hemorrhage. Neurosci Lett. 2016;627:92–99. doi: 10.1016/j.neulet.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Wang H, Sheng H, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care. 2006;4(1):25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Hong J, Lu M, et al. ApoE mimetic ameliorates motor deficit and tissue damage in rat spinal cord injury. J Neurosci Res. 2014;92(7):884–892. doi: 10.1002/jnr.23371. [DOI] [PubMed] [Google Scholar]
- 33.Gu Z, Li F, Zhang YP, et al. Apolipoprotein E Mimetic Promotes Functional and Histological Recovery in Lysolecithin-Induced Spinal Cord Demyelination in Mice. Journal of neurology & neurophysiology. 2013;2014(Suppl 12):10. doi: 10.4172/2155-9562.S12-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei B, James ML, Liu J, et al. Neuroprotective pentapeptide CN-105 improves functional and histological outcomes in a murine model of intracerebral hemorrhage. Sci Rep. 2016;6:34834. doi: 10.1038/srep34834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF. Surgical trials in intracerebral hemorrhage. Stroke. 2013;44(6 Suppl 1):S79–82. doi: 10.1161/STROKEAHA.113.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81(2):107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb AJ, Ullman NL, Morgan TC, et al. Accuracy of the ABC/2 Score for Intracerebral Hemorrhage: Systematic Review and Analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke. 2015;46(9):2470–2476. doi: 10.1161/STROKEAHA.114.007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


