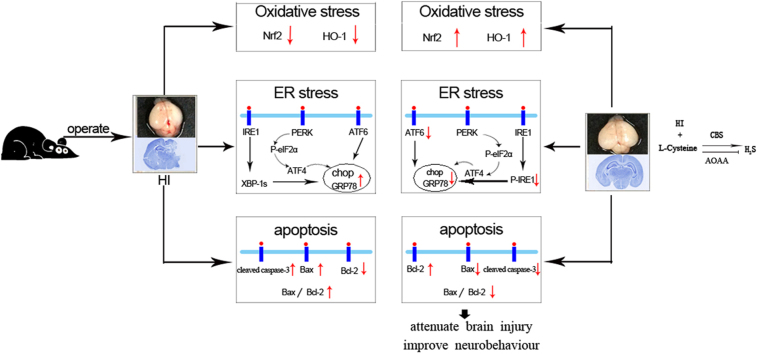
Abstract
Neonatal hypoxic-ischemic (HI) injury is a major cause of neonatal death and neurological dysfunction. H2S has been shown to protect against hypoxia-induced injury and apoptosis of neurons. L-Cysteine is catalyzed by cystathionine-β-synthase (CBS) in the brain and sequentially produces endogenous H2S. The present study was designed to investigate whether L-Cysteine could attenuate the acute brain injury and improve neurobehavioral outcomes following HI brain injury in neonatal mice by releasing endogenous H2S. L-Cysteine treatment significantly attenuated brain edema and decreased infarct volume and neuronal cell death, as shown by a decrease in the Bax/Bcl-2 ratio, suppression of caspase-3 activation, and reduced phosphorylation of Akt and ERK at 72 h after HI. Additionally, L-Cysteine substantially up-regulated NF-E2-related factor 2 and heme oxygenase-1 expression. L-Cysteine also decreased endoplasmic reticulum (ER) stress-associated pro-apoptotic protein expression. Furthermore, L-Cysteine had long-term effects by protecting against the loss of ipsilateral brain tissue and improving neurobehavioral outcomes. Importantly, pre-treatment with a CBS inhibitor significantly attenuated the neuroprotection of L-Cysteine on HI insult. Thus, L-Cysteine exerts neuroprotection against HI-induced injury in neonates via the CBS/H2S pathway, mediated in part by anti-apoptotic effects and reduced oxidative stress and ER stress. Thus, L-Cysteine may be a promising treatment for HI.
Abbreviations: ATF6, activating transcription factor 6; AOAA, aminooxyacetic acid; CBS, cystathionine β synthase; CNS, central nervous system; eIF2α, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; GRP78, glucose-regulated protein 78; HO-1, heme oxygenase-1; HI, hypoxic-ischemic; H2S, hydrogen sulfide; IRE1, inositol-requiring enzyme 1; MAPK, mitogen-activated protein kinase; Nrf2, NF-E2-related factor 2; PERK, pancreatic endoplasmic reticulum kinase; PFA, paraformaldehyde; RT-PCR, reverse transcription–polymerase chain reaction; ROS, reactive oxygen species
Keywords: L-Cysteine, H2S, Hypoxia-ischemia, Oxidative stress, Endoplasmic reticulum stress
Graphical abstract
Highlights
-
•
L-Cysteine administration at 24 h after HI insult has neuroprotective effect.
-
•
L-Cysteine administration attenuated HI-induced oxidative stress and ER stress.
-
•
L-Cysteine administration had long-term effects in improving neurobehavioral function at 14 and 28 days after HI insult.
-
•
Pre-treatment with a CBS inhibitor significantly attenuated the neuroprotection of L-Cysteine on HI in neonatal mice.
1. Introduction
Neonatal hypoxic-ischemic (HI) brain damage is a leading cause of mortality and neurological disabilities in infants and young children [1]. Moreover, even with the best care, there is often little improvement in the overall ability of these children. Therefore, developing effective therapeutic strategies is urgently needed.
Oxidative stress has been shown to play a key role in the development of HI injury. Under normal physiological conditions, a certain level of reactive oxygen species (ROS) is required, and ROS are scavenged by endogenous antioxidant enzymes [2]. Following insults such as HI, ROS production exceeds the capacity of the endogenous antioxidant system, leading to oxidative stress and subsequent neuronal cell death [3]. The toxicity of oxygen-free radicals contributes to lipid peroxidation, DNA damage, protein denaturation, and oxidative injury to tissues. Antioxidants have been proposed as therapeutic candidates for early brain injury after experimental HI or in a clinical setting.
The endoplasmic reticulum (ER) is responsible for protein synthesis, lipid metabolism, calcium handling, metabolic regulation and signaling [4]. In pathological conditions,ER function is impaired, triggering the unfolded protein response (UPR), which is known as “ER-stress”. A growing number of studies have suggested the ER stress plays critical roles in a wide range of diseases, including neurodegeneration, inflammatory disorders, cancer, and diabetes. Moreover, ER stress was shown to trigger neuronal apoptosis and brain damage in ischemic injury [5], [6]. ER stress-associated apoptosis is a potential therapeutic target for improving cerebral ischemia injury.
The functions of hydrogen sulfide (H2S) in the central nervous system (CNS) under various physiological and pathological states have been studied in many reports [7]. In the CNS, L-Cysteine is catalyzed by cystathionine-β-synthase (CBS), which is predominantly expressed in astrocytes, to produce endogenous H2S [8], [9]. L-Cysteine was found to indirectly meditate cellular responses via formation of H2S. For example, treatment with L-Cysteine inhibited electrical stimulation-induced contractions in ileum preparations from fasted mice. L-Cysteine induced vascular relaxation through H2S in vivo and in vitro [10], [11]. We observed that L-Cysteine can regulate the proliferation and neuronal differentiation of neural stem cells via the CBS/H2S system in vitro [12].
The protective effect of H2S application on CNS injury has been shown in many reports using both in vitro and in vivo model systems. For example, H2S reduced oxidative stress-induced injury in primary rat cortical neurons [13]. H2S alleviated microglia activation and pro-inflammatory cytokine production and protected against cognitive dysfunction induced by neuroinflammation [14], [15]. Our previous studies showed that H2S protects neurons from cerebral hypoxia injury [16], [17]. However, the potential therapeutic value of H2S for HI in neonatal animals has not been elucidated. In this study, we investigated whether L-Cysteine treatment could attenuate the acute brain injury and neurobehavioral dysfunction induced by HI in neonatal mice via the CBS/H2S pathway.
2. Materials and methods
2.1. HI model and treatments
The animal experiments were performed in accordance with the International Guiding Principles for Animal Research provided by the Council for International Organizations of Medical Sciences (CIOMS), and procedures were approved by the Animal Ethical and Welfare Committee of Shandong University. Participants who worked with the animal models were trained following Institutional Animal Care and Use Committee Guidebook (IACUC) rules.
The model used in this study was based on the Rice–Vannucci model [18], with minor modifications as described in our previous publication [19]. Briefly, C57 mouse pups (postnatal day 7) were anesthetized under 2.5% isoflurane; then, the right common carotid artery was exposed, and ligation was performed. After a recovery period of 60 min, the pups were placed in a hypoxia chamber (humidified 8% O2 + 92% N2) for 90 min to expose them to hypoxic insult. Then, the animals were removed from the chamber and kept for 60 min before being returned to the dam. Littermate cage-mates that underwent anesthesia and exposure of the carotid artery but no ligation served as sham controls.
L-Cysteine (Sigma, USA) and aminooxyacetic acid (AOAA, Sigma, USA) were administered via intraperitoneal (i.p.) injection. The pups were randomly assigned to five groups: sham + vehicle (saline) group, HI + vehicle (saline) group, HI + L-Cysteine (2.5 mg/kg) group, HI+ L-Cysteine (5.0 mg/kg) group, and HI + L-Cysteine (5.0 mg/kg) + AOAA (5.0 mg/kg) group. The first L-Cysteinetreatment was given 24 h after HI insult and then at 24 h intervals for 3 days. For the fifth groups, animals were pretreated with AOAA (5.0 mg/kg), followed by L-Cysteine (5.0 mg/kg) treatment 30 min later.
In the first experiment, the mice were assessed 72 h after HI for tissue analyses. In the second experiment, the mice were observed for behavioral changes in the open field test 14 days after HI insult. Following the open field test, the same mice then underwent training in the Morris water maze at 28 days after the HI insult(in supplement data Fig. 1).
2.2. Measurement of infarct ratio
In the first experiment, each brain was sliced coronally and processed with 2% TTC staining, and infract volume quantification was carried out as previously described [19].
2.3. Brain histology
The brains were removed and fixed in formalin. The coronal slices of the right hemisphere were sliced into 4 µm sections for Nissl staining, TUNEL staining and immunohistochemical analysis.
The Sections (4 sections/mouse) were assessed with Nissl staining. The slides were stained with 0.5% cresyl violet acetate for 20 min. Then, measurements were performed using Image-Pro Plus 6.0 software. The area of infarction was defined as the loss of the normal cresyl violet staining pattern using the formula (contralateral area − ipsilateral area/contralateral area) × 100 [20].
For immunohistochemical analysis, the sections were dewaxed with a standard procedure as described previously. After the sections were blocked in 10% normal goat serum, they were incubated with anti-CBS antibody (1:200 dilution, Proteintech) at 4 °C overnight. After removal of the primary antibody, the sections were incubated with goat anti-rabbit biotinylated IgG (1:800 dilutions) for 2 h at room temperature. Then, the sections were incubated with avidin-biotin peroxidase enzyme complex followed by diaminobenzidine solution. Six images (20×) were captured randomly for one section per animal (n = 4 each group).
The in situ cell death detection kit (FITC) (Chemicon, Temecula, CA, USA) was used to detect apoptosis in the tissue following the manufacturer's instructionsas described previously [21]. Briefly, the sections were dewaxed and rehydrated by heating the slides at 60 °C. Then, these sections were incubated in a 20 μg/ml proteinase K working solution for 15 min at room temperature. After a wash, the slides were labeled with TUNEL reaction mixture for 1 h at 37 °C. Then, 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich) was used for counterstaining. Ten random fields were captured for one section per animal (n = 4 each group) with a fluorescence microscope (IX71; Olympus, Tokyo, Japan). The proportion of TUNEL-positive cells (with a green fluorescent nucleus) was expressed as the percentage of the total cells counted.
2.4. Tissue preparation
Animals were decapitated after anesthesia, and the brains were dissected. The brain stem and cerebellum were removed from the forebrain. Isolated cortices from the right hemisphere were separated and frozen for further analysis.
The cortices from the ipsilateral hemisphere were homogenized with tissue protein extraction reagent (Pierce Biotechnology, Inc., IL, Rockford, USA) containing protease inhibitors and centrifuged at 12,000g for 10 min. Then, the supernatant was used for subsequent analysis of CBS activity, H2S production and Western blot analysis.
2.5. CBS activity assay and measurement of H2S production
The CBS activity of brain tissue was detected by a CBS assay kit (GENMED SCIENTIFICS INC. China). Data were indirectly reflected via CBS metabolites interacting with NADPH, and the absorbance was measured at 340 nm using a microplate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA). The CBS analysis was performed using standard protocols. and levels were normalized to the protein content. For H2S investigation, a traditional methylene blue method was used. Briefly, the homogenized tissue was incubated with zinc acetate, which generated zinc sulfide to subsequently react with N,N-dimethyl-p-phenylenediamine sulfate (NNDPD). The absorbance value was determined at 670 nm, and H2S was calculated against a calibration curve of NaHS.
2.6. Transmission electron microscopy (TEM)
The cortices from the ipsilateral hemisphere with a volume of 1 mm3 were rapidly dissected on ice and fixed in 2.5% glutaraldehyde for 2 h at 4 °C. Following several washes with PBS, the specimens were fixed in 1% osmium tetroxide for 2 h and then underwent incubation in graded ethanol solutions. They were subsequently infiltrated in 50/50 propylene oxide overnight and embedded. Tissues were prepared for sectioning on an Ultra microtome (EM UC 7, Leica, Germany) (50 nm thickness). After being stained with uranyl acetate, the sections were observed and photographed using Hitachi H-7500 TEM. A total of 3 mice in each group were sacrificed for TEM analysis.
2.7. Western blot analysis
Protein concentration in the supernatant was quantified using a BCA protein assay kit (Pierce Biotechnology, Inc.). Equal amounts of total proteins were loaded onto a 4–20% gradient polyacrylamide gel. The separated proteins were subsequently transferred onto a polyvinylidene difluoride membrane. After they were blocked with 5% milk, the membranes were incubated overnight at 4 °C with the following primary antibodies: anti-phospho-extracellular signal-regulated kinase (ERK)1/2 (1:2000, Cell Signaling Tech.),anti-ERK1/2 (1:2000; Cell Signaling Tech.), anti-glucose-regulated protein 78 (GRP78) (1:1000, Proteintech), anti-C/EBP homologous protein (CHOP) (1:1000, Proteintech), anti-CBS (1:1000, Proteintech), anti-Eukaryotic initiation factor 2α (eIF2α) (1:1000, Proteintech), anti-phospho-eIF2a (1:1000, Cell Signaling Tech.),anti-NF-E2-related factor 2 (Nrf2) (1:1000, Proteintech), anti-heme oxygenase-1 (HO-1) (1:2000, Proteintech.); anti-activating transcription factor (ATF) 4 (1:1000, Cell Signaling Tech.), anti-ATF6 (1:2000, Cell Signaling Tech.), anti-inositol-requiring enzyme1 (IRE1, 1:1000, Cell Signaling Tech.), and anti-phospho-IRE (1:1000, Cell Signaling Tech.),anti-Bcl-2 antibody(1:1000, Santa Cruz Biotechnology), anti-Bax antibody (1:1000, Santa Cruz Biotechnology),anti-caspase-3 (1:500, Cell Signaling Tech.), anti-cleaved Caspase-3 (1:1000, Cell Signaling Tech.). β-actin (1:2000; Sigma-Aldrich) was used as an internal control. After a wash with PBS, the membranes were subsequently incubated with peroxidase-conjugated goat anti-rabbit/mouse IgG (1:5000–1:8000, Sigma-Aldrich). The signals were observed by enhanced chemiluminescence (Pierce, Rockford, IL).
2.8. Reverse transcription–polymerase chain reaction (RT-PCR)
The PCR extraction and PCR reaction protocols were identical to those of a previous report [22]. The primer sequences are listed in Table 1. The intensity of the bands was determined using Image-Pro Plus 6.0 software. The gene expression levels were normalized to β-actin expression.
Table 1.
PCR primers used in this study.
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Bax | GGT TGC CCT CTT CTA CTT TGC | TCT TCC AGA TGG TGA GCG AG |
| Bcl-2 | GGA TGA CTT CTC TCG TCG CTA C | TGA CAT CTC CCT GTT GAC GCT |
| Chop | AGC TGG AAG CCT GGT ATG AG | GAC CAC TCT GTT TCC GTT TC |
| GRP78 | GAA TCC CTC CTG CTC CCC GT | TTG GTC ATT GGT GAT GGT GAT TTT G |
| Nrf2 | ATT TGT AGA TGA CCA TGA GTC GC | ACT GTA ACT CGG GAA TGG AAA |
| HO-1 | GAC AGA AGA GGC TAA GAC CG | TGA ACT AGT GCT GAT CTG GG |
| β -actin | CTA TTG GCA ACG AGC GGT TCC | CAG CAC TGT GTT GGC ATA GAG G |
2.9. Open field test
The open field test was used to measure locomotor activity of the animals. The open-field consists of a 90 cm × 90 cm × 45 cm gray wooden box, divided into 36 equal squares. Each mouse was individually placed in the central square to initiate a 5-min test session. The measurement parameters included the following: crossing (the number of squares crossed); rearing (the frequency of standing on hind limbs); and grooming (number of times the animal was observed grooming the face, licking/cleaning and scratching various parts of the body).
2.10. Morris water maze (MWM) test
The spatial reference memory was assessed using the MWM test as previously described [23]. Briefly, the MWM test was performed in a black cylindrical tank (120 cm in diameter and 60 cm deep), and animal behaviors were recorded with a tracking program(SMART polyvalent video-tracking system, Panlab, Spain). The maze was divided into 4 quadrants of equal area, and a hidden square platform was placed in the center of the II quadrant, submerged 2 cm below the water level. In the training trials (5 consecutive days), all animals were trained to find the escape platform and received one training session per day, each comprising four training trials. The swimming time to find the hidden platform (escape latency) was recorded by the software. In the probe trial, the platform was withdrawn on the sixth day. Animals were allowed to freely swim and monitored for 60 s. The probe trial was expressed as the time to reach the original platform, and the total time spent in the target quadrant was recorded.
2.11. Statistical analysis
Data were analyzed by the software SPSS. All values are expressed as the mean ± standard. The data from the training trials in the MWM were averaged for each mouse (total data/total number of trials per day) and analyzed using repeated measures ANOVA. When significant day×treatment interactions were observed, one-way ANOVA using a post-hoc Tukey's test was used to investigate the treatment effect for each day. Other data were analyzed statistically by the one-way ANOVA using the post-hoc Tukey's test for multiple comparisons of means. A p value <0.05was considered statistically significant.
3. Results
3.1. L-Cysteine prevented HI-induced brain injury
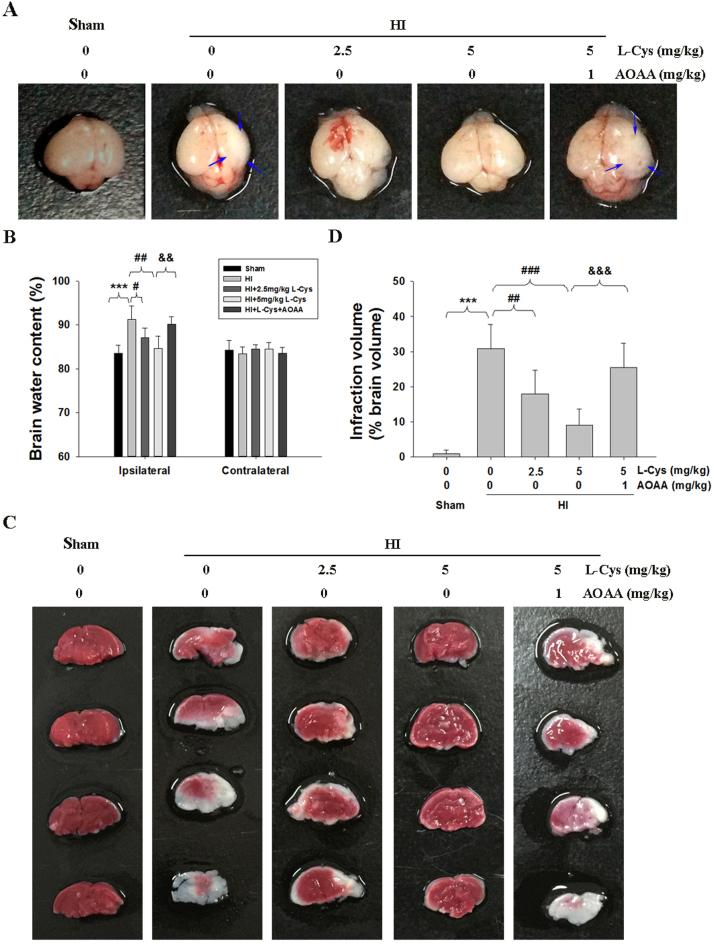
As shown in Fig. 1A, the ipsilateral side of the brain was visibly edematous 3 days after HI insult compared to that of the Sham controls. Post-insult treatment with L-Cysteine (2.5 and 5 mg/kg) alleviated edema and morphological damage in the HI group. Moreover, HI insult significantly increased the water content of the ipsilateral hemispheres compared to that of the Sham group (Fig. 1B, p < 0.001). L-Cysteine treatment at doses of 2.5 mg/kg and 5 mg/kg alleviated the brain water content of ipsilateral hemispheres in the HI group (p < 0.01 and p < 0.001, respectively).
Fig. 1.
L-Cysteine administration alleviated HI-induced brain injury. (A) Representative brain photos of mice were taken 3days after HI insult. The black arrows indicate the significant change of morphology in the ipsilateral hemisphere. (B) Brain water content was measured at 3 days after HI insult, n = 6 in each group. (C) Representative TTC-stained brain sections from each group. (D) Analysis of infarct volume from result C, n = 6 in each group. Values represent the mean ± SD, ***p < 0.001 HI VS Sham; #p < 0.05, ##p < 0.01, ###p < 0.001 HI + L-Cysteine (L-Cys) VS HI; &&p < 0.01, &&&p < 0.001 HI + L-Cys+AOAA VS HI + L-Cys.
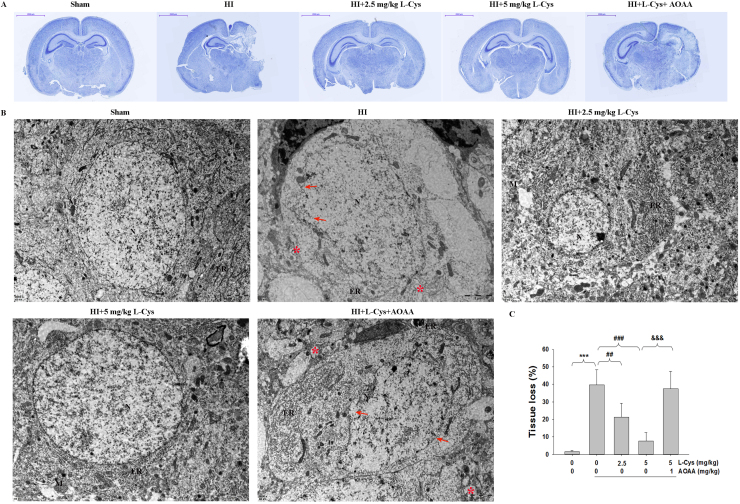
TTC staining showed that HI insult substantially increased brain infarction area, which was reversed by L-Cysteine treatment at two doses (Fig. 1C-D). In addition, ipsilateral volume loss was identified by Nissl staining. The results showed that there was a large area of tissue loss in the ipsilateral hemisphere of the HI group (Fig. 2A-C, p < 0.001), which was decreased in the L-Cysteine-treated group(p < 0.01, p < 0.001 respectively).
Fig. 2.
Effects of L-Cysteine administration on neuronal cell loss and ultrastructural changes in neurons of the ipsilateral cortex. (A) Representative Nissl staining of brain sections performed 3 days after HI insult. Scale bar = 2000 µm. (B) RepresentativeTEM results from each group showing the nucleus (N), rough endoplasmic reticulum (ER), mitochondria (M), and ribosomes, n=3 in each group. Arrow indicates the necrotic cells, Asterisk (*) indicates swelling and vacuolized mitochondria. Scale bar = 2 µm. (C) The quantification of tissue loss was expressed as (contralateral area − ipsilateral area/contralateral area) × 100. Values representthe mean ± SD. n = 6 in each group. Values represent the mean ± SD, ***p < 0.001 HI VS Sham; ##p < 0.01, ###p < 0.001 HI + L-Cys VS HI; &&&p < 0.001 HI + L-Cys+AOAA VS HI + L-Cys.
TEM demonstrated that the neurons of ipsilateral hemispheres in the Sham groups had abundant cytoplasm and complete cell membranes. However, the neurons of the ipsilateral hemispheres in the HI group were severely damaged; the organelles were significantly decreased, the mitochondria and ER were swollen with some vacuoles, and the nuclear membranes were unclear or had partially disappeared. Treatment with L-Cysteine alleviated this morphological damage. In these animals, the ultra-microstructure was similar to those of neurons in the Sham group (Fig. 2B).
Furthermore, pre-treatment with AOAA significantly prevented the L-Cysteine-mediated inhibition of HI insult in mice (Fig. 1, Fig. 2). At the same time, the brain injury in the animals pretreated with AOAA alone was not more severe than that in the HI group (in Supplement data Fig. 2).
3.2. Effects of L-Cysteine on CBS expression and H2S production after HI
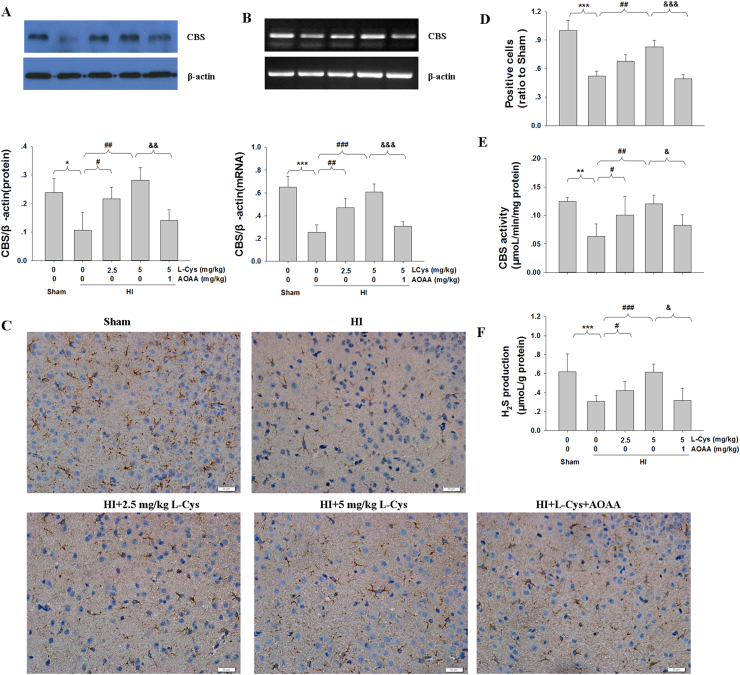
Western blot and RT-PCR determination revealed that HI insult significantly decreased the levels of CBS mRNA and protein in the lesioned cortex compared with those in the Sham group. A significant increase in CBS expression in the lesioned cortex was observed after L-Cysteine treatment in the HI group (Fig. 3A-B). Moreover, immunohistochemical staining also revealed that HI insult significantly reduced CBS expression compared with that of the Sham group. However, post-insult treatment with L-Cysteine dramatically upregulated CBS expression in the HI group 3days after HI insult (Fig. 3C-D).
Fig. 3.
Effects of L-Cysteine administration on CBS expression and H2S production after HI. The CBS levels of the ipsilateral cortex were measured 3 days after HI by Western blot (A), RT-PCR (B) and immunohistochemical staining (C). Bar graphs show quantification of CBS levels from Western blot and RT-PCR. n = 4 in each group. Scale bar = 50 µm. (D) Quantitative analysis of the number of CBS-positive cells. Values are expressed relative to those of the Sham group. n = 4 in each group. (E) The CBS activity of the ipsilateral cortex was measured by a commercial kit, n = 6 in each group. (F) The content of H2S in the ipsilateral cortex was measured by the NNDPD method, n = 6 in each group. Values represent the mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001 HI VS Sham; #p < 0.05, ##p < 0.01, ###p < 0.001 HI + L-Cys VS HI; &p < 0.05, &&p < 0.01, &&&p < 0.001 HI + L-Cys+AOAA VS HI + L-Cys.
Next, we tested the CBS activity following L-Cysteine treatment. The results showed that HI insult significantly decreased the CBS activity in the lesioned cortex compared to that of the Sham group (Fig. 3E, p < 0.01). In contrast, L-Cysteine treatment at 2.5 mg/kg and 5 mg/kg significantly reversed HI-inhibited CBS activity in mice (Fig. 3E). At the same time, administration of L-Cysteine increased the H2S level of the lesioned cortex in the HI group (Fig. 3F).
After AOAA injection, the CBS activity was decreased again. Importantly, the L-Cysteine-treated group displayed the highest level of CBS activity compared to that in the HI groups, and this effect was inhibited by AOAA. These results further suggested that CBS played an important role in the generation of H2S after L-Cysteine injection in the lesioned cortex.
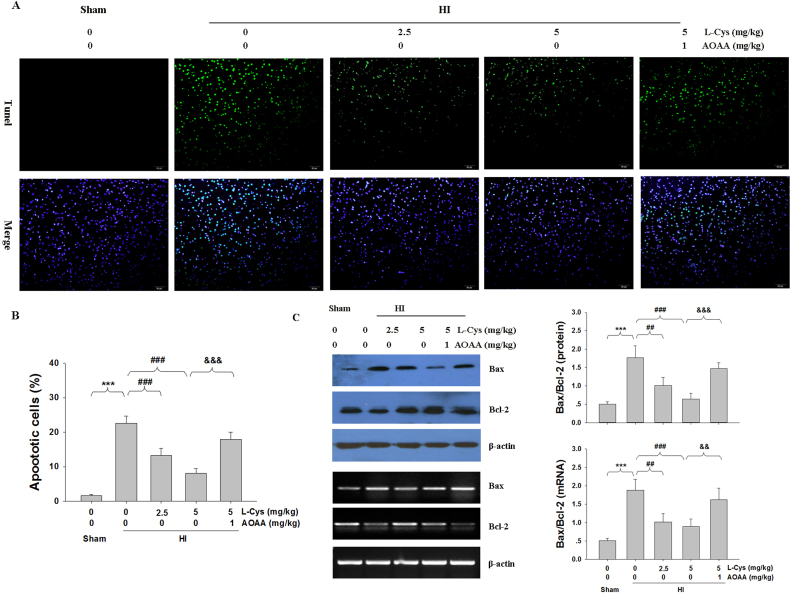
3.3. L-Cysteine decreased TUNEL-positive cells and apoptotic markers
Apoptosis was evaluated using the TUNEL assay. The result showed that HI insult generated an increase in TUNEL-positive cells in the ipsilateral hemispheres compared to those of the Sham group. In contrast, treatment with L-Cysteine significantly decreased HI-induced apoptosis in the lesioned cortex (Fig. 4A-B).
Fig. 4.
L-Cysteine alleviates HI-induced apoptosis. (A) TUNEL staining was performed 3 days after HI injury. (B) Analysis of TUNEL-positive cells from result A. Scale bar = 50 µm. Images are representative of triplicate sets. Values represent the mean ± SD. n = 4 in each group. (C) The levels of Bax and Bcl-2 of the ipsilateral cortex were measured 3 days after HI by Western blot and RT-PCR. Quantification of the Bax and Bcl-2levels from Western blot and RT-PCR. Values represent the mean ± SD, ***p < 0.001 HI VS Sham; ##p < 0.01, ###p < 0.001 HI + L-Cys VS HI; &&p < 0.01, &&&p < 0.001 HI + L-Cys + AOAA VS HI + L-Cys.
HI insult significantly increased the Bax/Bcl-2 ratio at the protein and mRNA levels, while L-Cysteine treatment prevented these effects (Fig. 4C). Furthermore, pre-treatment with AOAA significantly decreased the L-Cysteine-mediated inhibition of HI-induced apoptosis in mice.
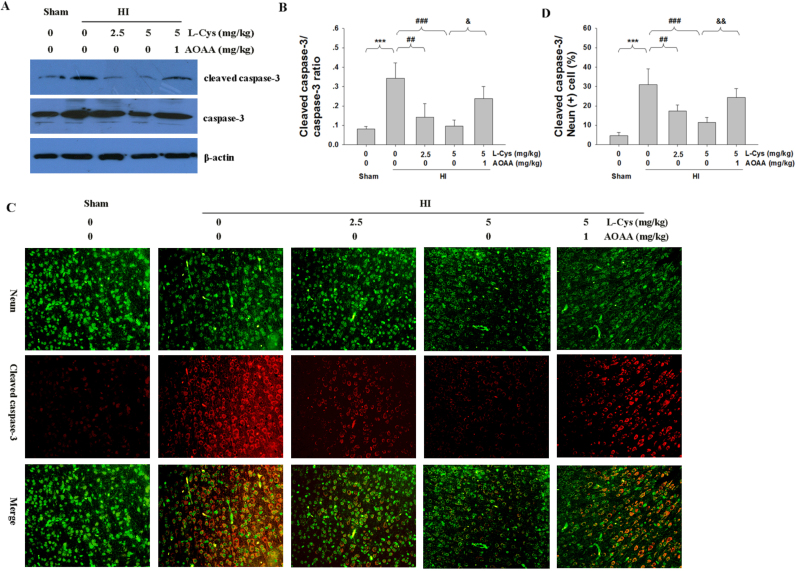
3.4. L-Cysteine attenuated HI–induced caspase-3 activation
Activated caspase-3 in the lesioned cortex was examined to quantify the apoptotic response using Western blotting. As shown in Fig. 5A-B, HI insult increased the level of cleaved caspase-3 compared to that of the Sham group (p < 0.001), while active caspase-3 was dramatically decreased following L-Cysteine treatment (p < 0.01, p < 0.001 respectively).
Fig. 5.
The effect of L-Cysteine on caspase-3 activation after HI injury. (A) Representative Western blot of cleaved caspase-3 and caspase-3 in the ipsilateral cortex of each group. (B) Quantification of cleaved caspase-3 and caspase-3 levels from Western blot. n = 4 in each group. (C) Representative immunofluorescence staining of the ipsilateral cortex performed 3 days after HI injury. Scale bar = 50 µm. (D)Quantification of active caspase-3/NeuN-positive cells from result C. n = 4 in each group. Values represent the mean ± SD, ***p < 0.001 HI VS Sham; ##p < 0.01, ###p < 0.001 HI + L-Cys VS HI; &p < 0.05, &&p < 0.01 HI + L-Cys+AOAA VS HI + L-Cys.
To further verify the anti-apoptotic effects of L-Cysteine in neurons, we used active caspase-3/NeuN double-staining immunofluorescence to examine the apoptosis in the lesioned cortex. Fig. 5C-D shows that the number of active caspase-3/NeuN double positive-stained neurons were significantly higher in the lesioned cortex of HI group than that in the Sham group (p < 0.001). Post-insult treatment with L-Cysteine substantially reduced the number of positive-stained neurons compared with those in vehicle-treatment HI mice (p < 0.01, p < 0.001 respectively, Fig. 5C-D). Moreover, pre-treatment with AOAA significantly blocked the effect of L-Cysteine in decreasing the HI-induced caspase-3 activation in mice.
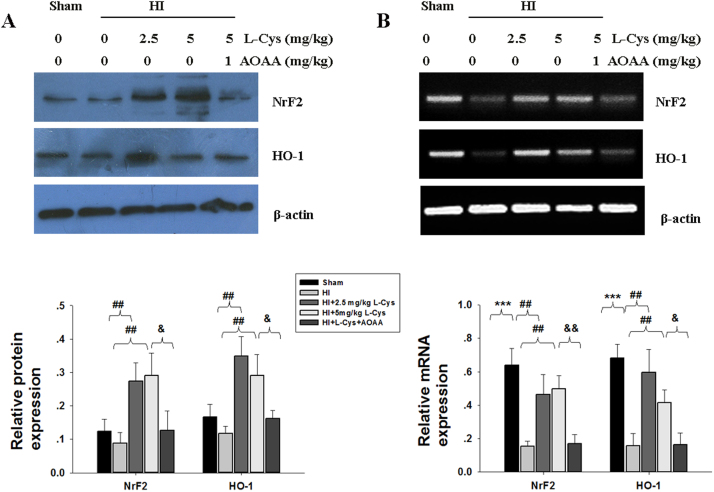
3.5. Effects of L-Cysteine on Nrf2 and HO-1 levels after HI insult
Exposure to HI slightly decreased the levels of Nrf2 and HO-1 protein in the ipsilateral cortex, but the differences between the HI and Sham groups were not statistically significant (Fig. 6A, p > 0.05, p > 0.05 respectively). However, L-Cysteine administration significantly increased the levels of Nrf2 and HO-1 in the HI group.
Fig. 6.
Effect of L-Cysteine on Nrf2 and HO-1 expression. The levels of Nrf2 and HO-1 of the ipsilateral cortex were examined 3 days after HI by Western blot (A) and RT-PCR (B). Bar graphs show quantification of the protein and mRNA levels from Western blot and RT-PCR, n = 3 in each group. ***p < 0.001 HI VS Sham; ##p < 0.01 HI+ L-Cys VS HI; & p < 0.05, && p < 0.01 HI + L-Cys + AOAA VS HI + L-Cys.
Down-regulation of Nrf2 and HO-1 mRNA in the lesioned hemisphere was found in the HI group (Fig. 6B, p < 0.001, p < 0.001, respectively), and these changes were significantly blocked by L-Cysteine treatment at both doses. Pre-treatment with AOAA decreased Nrf2 and HO-1 levels compared to those in the HI + L-Cys group.
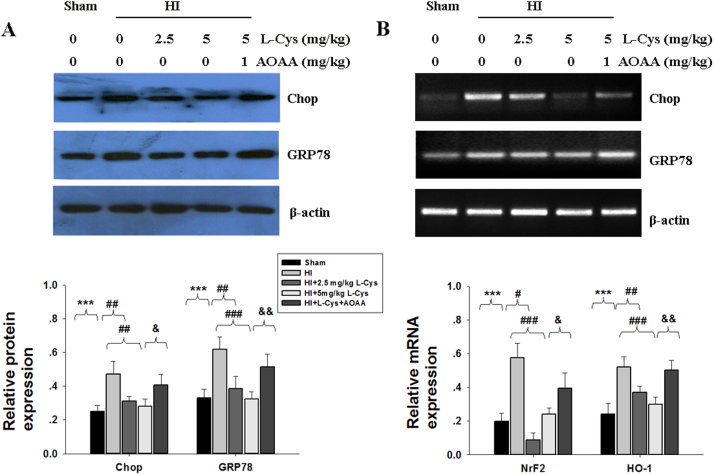
3.6. The effect of L-Cysteine on ER stress
GRP78 and Chop, molecular markers of ER stress, were measured by Western blot and RT-PCR. Up-regulation of GRP78 and Chop mRNA and protein in the lesioned hemisphere was found in the HI group, but these increases were significantly blocked by L-Cysteine treatment (Fig. 7A-B).
Fig. 7.
Effect of L-Cysteine on GRP78 and Chop expression. The levels of GRP78 and Chop of the ipsilateral cortex were examined 3 days after HI by Western blot (A) and RT-PCR (B). Bar graphs show quantification of protein and mRNA levels from Western blot and RT-PCR. Values represent the mean ± SD, n = 4 in each group. ***p < 0.001 HI VS Sham; #p < 0.05, ##p < 0.01, ###p < 0.001 HI+ L-Cys VS HI; & p < 0.05, && p < 0.01 HI + L-Cys + AOAA VS HI + L-Cys.
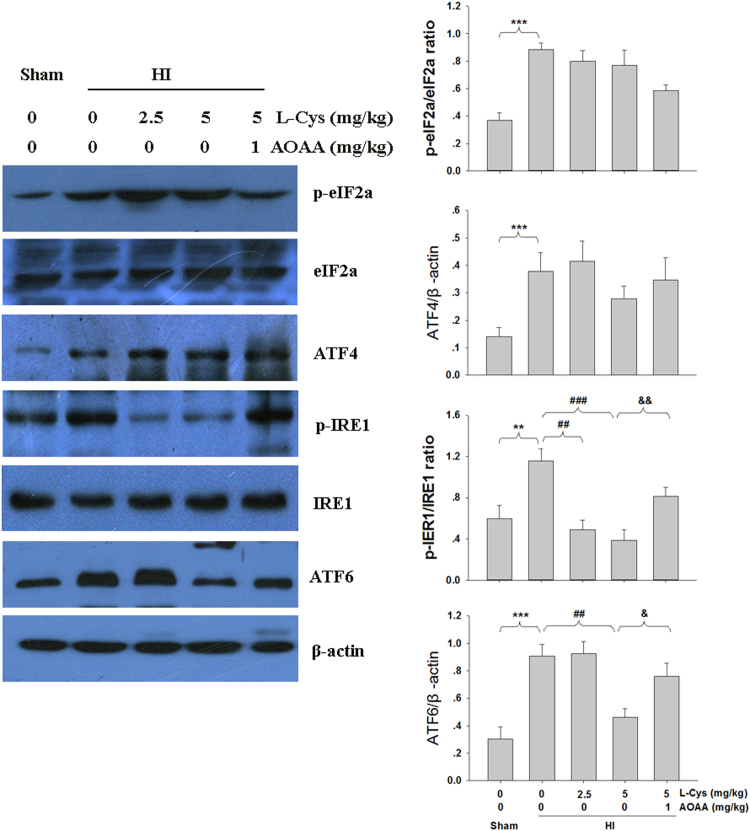
Next, we investigated the effect of L-Cysteine on the ER pathway. As shown in Fig. 8, HI stimulated expression of phospho-IRE1, phospho-eIF2α, ATF4 and ATF6. Treatment with L-Cysteine decreased levels of phospho-IRE1α and ATF6. L-Cysteine had no effect on the HI-induced phospho-eIF2α and ATF4. Furthermore, pre-treatment with AOAA significantly decreased the effect of L-Cysteine in inhibiting the HI-induced ER stress.
Fig. 8.
Effect of L-Cysteine on phospho-eIF2α, ATF4, phospho-IRE1 and ATF6 expression. The levels of phospho-eIF2α, ATF4, phospho-IRE1 and ATF6 of the ipsilateral hemisphere were examined 3 days after HI by Western blot. Bar graphs show quantification of the protein levels. Values represent the mean ± SD, n = 3 in each group, ***p < 0.001 HI VS Sham; ##p < 0.01, ###p < 0.001 HI + L-Cys VS HI; & p < 0.05, && p < 0.01 HI + L-Cys +AOAA VS HI + L-Cys.
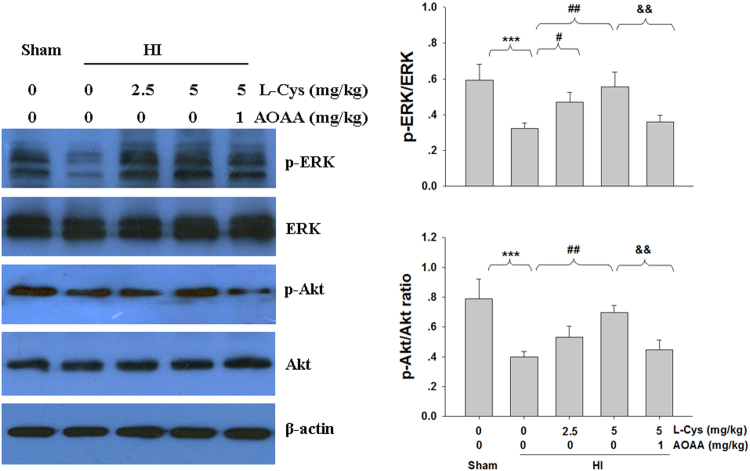
3.7. L-Cysteine prevented the acute dephosphorylation of Akt and ERK1/2 after HI insult
Activation of Akt and ERK is important for neuronal survival in the ischemic brain. Thus, we investigated whether Akt and ERK1/2 activation was associated with the neuroprotective effect of L-Cysteine in mice. The results showed that phosphorylation of Akt and ERK1/2 in the lesioned cortex was decreased in the HI group compared to that in the Sham group (Fig. 9). Again, this dephosphorylation of Akt and ERK1/2in the lesioned cortex was partially reversed by L-Cysteine treatment (Fig. 9).
Fig. 9.
Effect of L-Cysteine on Akt and ERK phosphorylation. The levels of phospho-Akt (p-Akt) and Akt (A); phospho-ERK (p-ERK) and ERK (B) were examined 3 days after HI by Western blot. Bar graphs show quantification of the protein levels, n = 4 in each group. Values representthe mean ± SD. ***p < 0.001 HI VS Sham; #p < 0.05, ##p < 0.01 HI + L-Cys VS HI; && p < 0.01 HI+ L-Cys + AOAA VS HI + L-Cys.
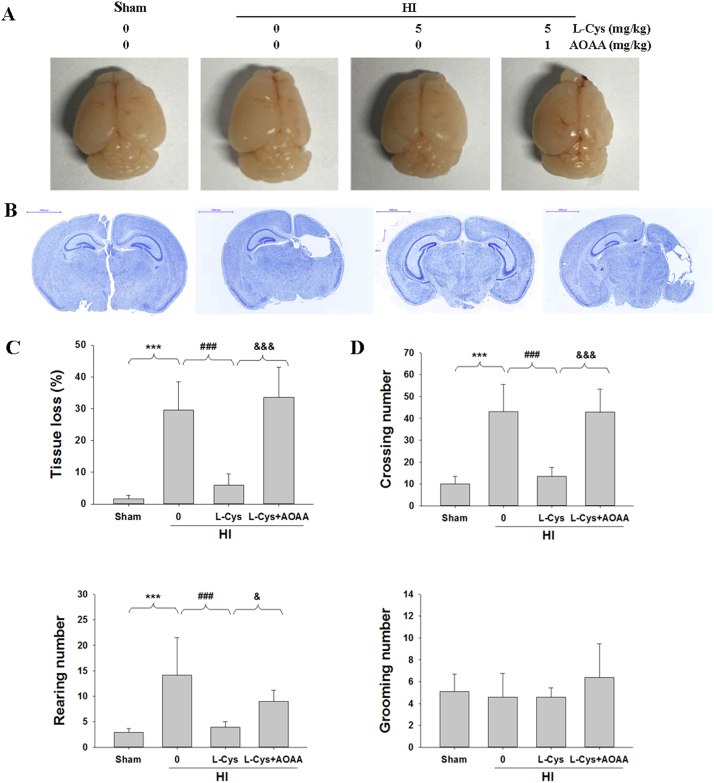
3.8. L-Cysteine reduced brain atrophy and locomotor activity 2 weeks after neonatal HI
Two weeks after HI, mice in the HI group showed a significant loss of ipsilateral brain tissue (atrophy) and brain asymmetry (Fig. 10A). Treatment with L-Cysteine after HI alleviated the atrophy; however, a small degree of asymmetry was still visible (Fig. 10A). Meanwhile, Nissl staining revealed that HI insult led to a large area of tissue loss in the ipsilateral cortex, which was attenuated in the L-Cysteine-treated group (p < 0.001, Fig. 10B-C).
Fig. 10.
Effects of L-Cysteine on brain atrophy and behavior changes in the open field test at 14 days following HI insult. (A) Representative brain photos of mice were taken14 days after HI insult. Note the brain asymmetry and atrophy in the ipsilateral hemisphere after HI. Treatment with L-Cysteine after HI (HI + L-Cys) prevented the atrophy; however, a small degree of asymmetry was still visible (lower left image). (B) Nissl staining of the brain was performed 14 days after HI insult. (C) The quantification of tissue loss was expressed as (contralateral area−ipsilateral area/contralateral area) × 100. Values representthe mean ± SD, n = 6 in each group. (D) The locomotion was measured using an open field test, n=10 in each group. Values represent the mean ± SD, **p < 0.01, ***p < 0.001 HI VS Sham; #p < 0.05, ##p < 0.01 HI + L-Cys VS HI; && p < 0.01, &&& p < 0.001 HI + L-Cys + AOAA VS HI + L-Cys.
The results of the open field test showed significant differences between the groups in crossings [F(4, 49) = 44.623, p < 0.001] and rearing [F(4, 49) = 17.480, p < 0.001] but not in grooming [F(4,49) = 1.645, p > 0.05]. HI insult resulted in significantly increased crossing (p < 0.001) and rearing (p < 0.001) compared to that in the Sham group. Treatment with L-Cysteine attenuated the HI-induced increase in crossing (p < 0.001) and rearing (p < 0.001). There was no significant difference in grooming between the groups. Again, the effect of L-Cysteine on brain atrophy and behavioral deficits at juvenile stages was partially reversed by AOAA treatment (Fig. 10D).
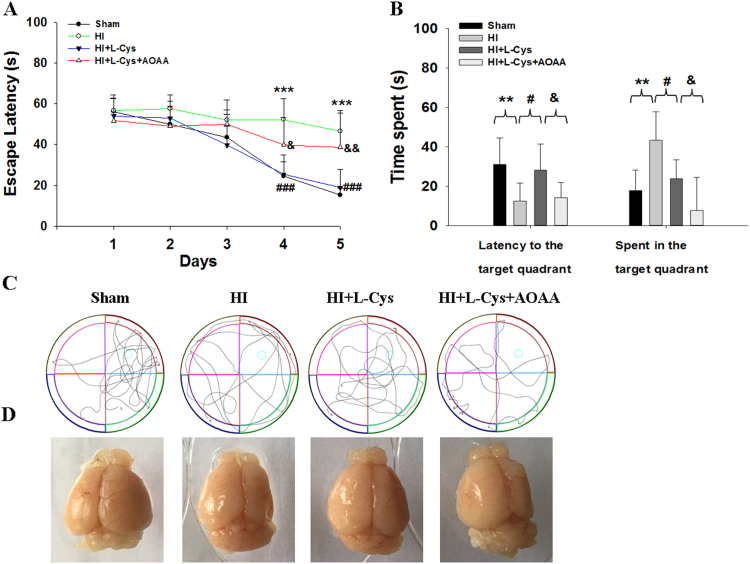
3.9. Effects of L-Cysteine on the MWM test after HI
The spatial reference memory was assessed using the MWM test on post-insult28day in each group to observe whether post-insult treatment with L-Cysteine could attenuate the behavioral deficits generated by the hypoxic injury.
During acquisition training, the escape latencies of each group were determined and are presented in Fig. 11A. All groups showed a progressive reduction in escape latencies across 5 training days [F (3,36) = 15.41, p < 0.001], which indicates all mice learned the location of the platform. In additional, the day×treatment interaction was not significant [F (3,36 = 2.27, p > 0.05, repeated measures ANCOVA], which suggested that animals in each group behaved similarly regardless of the previous treatment. Importantly, there were longer latencies to find the platform on day 4 (p < 0.001) and day 5 (p < 0.001) in the HI group than that of the Sham group, suggesting that HI insult led to cognitive impairment. Furthermore, L-Cysteine administration showed a progressive reduction in escape latencies on day 4 (p < 0.001) and day 5 (p < 0.001).
Fig. 11.
Effects of L-Cysteine administration on learning and memory deficits 28 days after HI. (A) Animal performance during the acquisition trial consisting of 4 trials per day for 5 days. Values indicate the average escape latency (in seconds). (B) Performance during the probe trial where the platform was removed. Values indicate the escape latency and percent time spent in the target quadrant. (C) Display of tracks of all groups during the probe trial. (D) Representative image of the brain taken after the MWM test. Values represent the mean ± SD, n = 10 in each group, **p < 0.01, ***p < 0.001 HI VS Sham; #p < 0.05, ### p < 0.001 HI + L-Cys VS HI; & p < 0.05, && p < 0.01 HI + L-Cys +AOAA VS HI + L-Cys.
In the probe trial, the HI group spent a longer time searching for the original platform than that of the Sham group (p < 0.01; Fig. 11B-C). However, the L-Cysteine administration group showed decreased escape latency compared to that of the HI group (p < 0.05; Fig. 11B-C). Relative to the Sham group, post-hoc testing demonstrated that the HI group significantly reduced the time spent in the target quadrant, which was reversed by L-Cysteine treatment (p < 0.05; Fig. 11B-C). In addition, post-HI treatment with L-Cysteine significantly improved HI-induced brain atrophy (Fig. 11D). Pre-treatment with AOAA significantly blocked the L-Cysteine effect on the MWM test. All these findings suggest that L-Cysteine could attenuate the impairment of learning and memory caused by HI insult in mice.
4. Discussion
Post-ischemic neuroprotective interventions may be of more therapeutic value than pre-ischemic treatments, given the difficulty of prospectively identifying which fetuses are at risk for antenatal or intrapartum HI. In this study, L-Cysteine intraperitoneally injected 24 h post-HI could reduce brain edema, ameliorate infarct size, and attenuate cell death in the ipsilateral hemisphere. Furthermore, L-Cysteine significantly attenuated HI-induced oxidative stress and ER stress. L-Cysteine therapy also had long-term effects in improving neurobehavioral function, as evidenced by ameliorating HI-induced brain volume loss. Importantly, pre-treatment with a CBS inhibitor significantly attenuated the neuroprotection of L-Cysteine on HI in neonatal mice. Taken together, L-Cysteine exerts neuroprotection against HI-evoked injury in neonates via the CBS/H2S pathway.
H2S has a neuroprotective effect in cerebral ischemia in animal models and is associated with attenuated brain atrophy and improved long-term cognitive functional deficits [24]. Consistent with these previous studies, L-Cysteine in this study is the preferred substrate for H2S generation, providing neuronal protection at 3 days after HI. Moreover, administration of L-Cysteine reduced the tissue loss and brain atrophy 2 weeks after HI. Importantly, L-Cysteine improved the learning and memory performance of HI animals 4 weeks after HI.
Increasing evidence has shown that Nrf2 up-regulation is involved in antioxidant protection in various CNS diseases, including cerebral ischemic injury [25], traumatic brain injury [26], and neurodegenerative disorders [27]. Following oxidative insults, Nrf2 rapidly migrates to and accumulates in the nucleus, where it actives a battery of antioxidant genes, including HO-1, NAD(P)H: quinone oxidoreductase 1, glutamate-cysteine ligase, and glutathione peroxidase [28]. In the CNS, HO-1 was reported to protect cells against oxidative damages by catalyzing heme to biologically active products, including carbon monoxide, biliverdin, and ferrous iron [29]. Therefore, pharmacological activation of the Nrf2/HO-1 system may provide novel opportunities for the protection of cells against oxidative damages related to brain injury [30], [31]. In the study, L-Cysteine administration ameliorated HI injury, which was linked to the up-regulation of Nrf2and HO-1 expression in the lesioned cortex. These data suggested that Nrf2 nuclear translocation occurred in the brain upon HI stimulation, leading to the transcription and expression of HO-1. L-Cysteine exhibited potent protective effects against HI insult, which were associated with its antioxidant effects and were dependent on activation of the Nrf2/HO-1 system.
ER stress can also activate apoptotic signals, resulting in cell apoptosis and deterioration of neurological symptoms. Under ER stress conditions, misfolded proteins accumulate in the ER, and GRP78 is released by three ER stress sensors, protein kinase RNA-like ER kinase (PERK), ATF6 and IRE1. Then, the stress sensors are activated and up-regulate GRP78 and CHOP expression [32]. CHOP is believed to be play a critical role in ER stress-mediated apoptosis [33]. Our experiments showed that HI clearly up-regulated GRP78 and Chop in the lesioned hemisphere, consistent with previous studies [5], [34]. Following ER stress, GRP78 is displaced from the stress sensors IRE1, PERK and ATF6 in the ER lumen, activating three signaling pathways: the IRE1 pathway, the PERK/eif2α/ATF4 pathway and the ATF6α pathway [35]. All three stress sensing pathways increase the transcription factor CHOP and are capable of altering levels of Bcl-2 family members to elicit apoptosis [36]. We examined these pathways in response to L-Cysteine treatment. The PERK pathway was monitored by the levels of eiF2α phosphorylation and ATF4 expression. The activation of PERK results in phosphorylation of eIF2α and then up-regulates the expression of ATF4 and several downstream genes. This enhances the activation of CHOP, which induces apoptosis in cells. Our study showed that HI insult up-regulated expression levels of GRP78, Chop, phospho-eIF2α and ATF4, and these results are similar to those in the previous studies [34], [37]. L-Cysteine could relieve the expression of GRP78 and Chop. However, no changes in phospho-eIF2α and ATF4 were observed after L-Cysteine treatment. In the second pathway, the activation of IRE1 by phosphorylation induces splicing of X box protein-1 (XBP-1) mRNA via cleavage of its intron. Then, spliced XBP-1 finally induces cell apoptosis. Next, we monitored the levels of IRE1 phosphorylation. HI insult induced a large increase in phospho-IRE1 that was completely inhibited by L-Cysteine administration. For the last pathway, activated ATF6 translocates from the ER membrane to the Golgi apparatus, where it is cleaved. Cleaved ATF6translocates to the nucleus and then up-regulates several ER chaperones and UPR target genes [33]. We found that the HI-induced AFT6 expression was decreased by L-Cysteine administration. These data suggested that the neuroprotective effect of L-Cysteine against HI-induced injury is closely connected with ER stress in neonatal mice.
Crosstalk between oxidative stress and ER stress is linked to many diseases, such as diabetes, neurodegeneration, atherosclerosis and HI injury [38], [39]. HI insult induces overwhelming ROS production, which impairs the redox state in the ER lumen and leads to excess ER stress and neuronal cell death [40], [41]. However, excessive ER stress promotes ROS accumulation and thus exacerbates the oxidative stress [38]. Thus, combination therapies that block both oxidative stress and ER stress provide an effective way to protect the brain against HI injury. Our data demonstrated that post-insult treatment with L-Cysteine can counteract oxidative stress and ER stress processes.
Accumulating evidence has shown that H2S is decreased in Alzheimer's disease (AD) [42], aging [43], and hyperglycemia [44]. In vitro and in vivo models demonstrated that restoration of H2S levels promoted cell growth and improved mitochondrial function as well as protecting against oxidative stress factors, such as amyloid beta peptides and malondialdehyde [45]. In the present study, HI insult reduced CBS expression, which was associated with the decreased H2S levels in the lesioned cortex. The exogenously administered L-Cysteine could produce more H2S, consistent with its role as an H2S precursor. Using the CBS inhibitor AOAA could reverse L-Cysteine-induced H2S level in the lesioned cortex, which is also consistent with this interpretation. Moreover, AOAA markedly block the neuroprotection of L-Cysteine against HI-evoked injury in neonates. This supports the hypothesis that L-Cysteine could generate H2S by CBS to alter the apoptosis, oxidative stress and ER stress in HI-evoked injury in neonates. Taken together, the results suggest that oxidative stress and/or ER stress may be the major cause of the impaired CBS/H2S system in HI insult, leading to diminished neuronal apoptosis and dysfunction. Antioxidative and/or ER stress therapies targeting up-regulation of the endogenous CBS/H2S system may be an effective strategy to improve neurological function.
There are several limitations to our study. First, we used AOAA to block CBS activity and L-Cysteine function, but the CNS has other enzymes to produce H2S, such as 3-mercaptopyruvate sulfur transferase, which remain to be investigated in future studies. Second, the mechanism of activation of the Nrf2/HO-1 system by L-Cysteine has not been completely elucidated and should be investigated in further studies. Third, the crosstalk between oxidative stress and ER stress in the effect on L-Cysteine against HI insult was not investigated.
In summary, L-Cysteine reduces brain injury and restores long-term neurobehavioral function after HI insult in neonatal mice via the CBS/H2S system to counteract various processes underlying HI pathology (apoptosis, oxidative stress, and ER stress). The findings suggest the clinical potential of H2S-releasing molecules in the regulation of HI pathophysiology.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (No. 81671213), the Fundamental Research Funds of Shandong University (2015JC008) and the Natural Science Foundation of Shandong Province (No. ZR2016HM33).
Acknowledgments
Authors contribution
ZW: study design, data interpretation and writing of the manuscript
SL: performed the majority of the laboratory work;
DQX: animal model and Western blot analysis;
LXW, TTZ, XMB, TL, YKX, and SSB: the animal model;
HX, manuscript revising;
DXL: manuscript design, proof reading, and editing;
the authors have no conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.06.007.
Appendix A. Supplementary material
Supplementary material
References
- 1.Lorenz J.M., Wooliever D.E., Jetton J.R., Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch. Pediatr. Adolesc. Med. 1998;152:425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 2.Moro M.A., Almeida A., Bolanos J.P., Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic. Biol. Med. 2005;39:1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q., Chen W., Sinha B., Tu Y., Manning S., Thomas N., Zhou S., Jiang H., Ma H., Kroessler D.A. Neuroprotective agents for neonatal hypoxic-ischemic brain injury. Drug Discov. Today. 2015;20:1372–1381. doi: 10.1016/j.drudis.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Tajiri S., Oyadomari S., Yano S., Morioka M., Gotoh T., Hamada J.I., Ushio Y., Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 5.Carloni S., Albertini M.C., Galluzzi L., Buonocore G., Proietti F., Balduini W. Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia-ischemia. J. Pineal Res. 2014;57:192–199. doi: 10.1111/jpi.12156. [DOI] [PubMed] [Google Scholar]
- 6.Paschen W., Doutheil J. Disturbances of the functioning of endoplasmic reticulum: a key mechanism underlying neuronal cell injury? J. Cereb. Blood Flow Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yetik-Anacak G., Sevin G., Ozzayim O., Dereli M.V., Ahmed A. Hydrogen sulfide: a novel mechanism for the vascular protection by resveratrol under oxidative stress in mouse aorta. Vasc. Pharmacol. 2016;87:76–82. doi: 10.1016/j.vph.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Al-Magableh M.R., Hart J.L. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn Schmiede. Arch. Pharmacol. 2011;383:403–413. doi: 10.1007/s00210-011-0608-z. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Liu D.X., Wang F.W., Zhang Q., Du Z.X., Zhan J.M., Yuan Q.H., Ling E.A., Hao A.J. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H(2)S pathway. Neuroscience. 2013;237:106–117. doi: 10.1016/j.neuroscience.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 14.Xuan A., Long D., Li J., Ji W., Zhang M., Hong L., Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer's disease. J. Neuroinflamm. 2012;9:202. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu L.F., Wong P.T., Moore P.K., Bian J.S. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z., Zhan J., Wang X., Gu J., Xie K., Zhang Q., Liu D. Sodium hydrosulfide prevents hypoxia-induced behavioral impairment in neonatal mice. Brain Res. 2013;1538:126–134. doi: 10.1016/j.brainres.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Yuan L., Liu D., Wang J., Wang S., Zhang Q., Gong Y., Liu H., Hao A., Wang Z. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol. Res. 2014;84:32–44. doi: 10.1016/j.phrs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Vannucci R.C., Vannucci S.J. A model of perinatal hypoxic-ischemic brain damage. Ann. N. Y. Acad. Sci. 1997;835:234–249. doi: 10.1111/j.1749-6632.1997.tb48634.x. [DOI] [PubMed] [Google Scholar]
- 19.Bai X., Liu S., Yuan L., Xie Y., Li T., Wang L., Wang X., Zhang T., Qin S., Song G. Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 2016;1646:410–417. doi: 10.1016/j.brainres.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Arteaga O., Revuelta M., Uriguen L., Martinez-Millan L., Hilario E., Alvarez A. Docosahexaenoic acid reduces cerebral damage and ameliorates long-term cognitive impairments caused by neonatal hypoxia-ischemia in rats. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0221-8. [DOI] [PubMed] [Google Scholar]
- 21.Hu Q., Li T., Wang L., Xie Y., Liu S., Bai X., Zhang T., Bo S., Xin D., Xue H. Neuroprotective effects of a smoothened receptor agonist against early brain injury after experimental subarachnoid hemorrhage in rats. Front. Cell Neurosci. 2016;10:306. doi: 10.3389/fncel.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q., Liu S., Li T., Yuan L., Liu H., Wang X., Wang F., Wang S., Hao A., Liu D., Wang Z. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget. 2016 doi: 10.18632/oncotarget.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T., Liu H., Xue H., Zhang J., Han X., Yan S., Bo S., Liu S., Yuan L., Deng L. Neuroprotective effects of hydrogen sulfide against early brain injury and secondary cognitive deficits following subarachnoid hemorrhage. Brain Pathol. 2016 doi: 10.1111/bpa.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Wang Y., Xie Y., Yang Z., Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem. Res. 2011;36:1840–1849. doi: 10.1007/s11064-011-0502-6. [DOI] [PubMed] [Google Scholar]
- 25.Son T.G., Camandola S., Arumugam T.V., Cutler R.G., Telljohann R.S., Mughal M.R., Moore T.A., Luo W., Yu Q.S., Johnson D.A. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin W., Wang H., Ji Y., Hu Q., Yan W., Chen G., Yin H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 2008;44:135–140. doi: 10.1016/j.cyto.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 27.van Muiswinkel F.L., Kuiperij H.B. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr. Drug Targets CNS Neurol. Disord. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 28.Magesh S., Chen Y., Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syapin P.J. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br. J. Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu L., Wang C., Wang J., Zhang Y., Zhang X., Yang Y., Zhuo J., Liu J. The neuroprotection of hypoxic preconditioning on rat brain against traumatic brain injury by up-regulated transcription factor Nrf2 and HO-1 expression. Neurosci. Lett. 2016;611:74–80. doi: 10.1016/j.neulet.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H., Mitchell S., Ciechanowicz S., Savage S., Wang T., Ji X., Ma D. Argon protects against hypoxic-ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid-derived 2)-like 2. Oncotarget. 2016;7:25640–25651. doi: 10.18632/oncotarget.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozutsumi Y., Segal M., Normington K., Gething M.J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 33.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Tu L., Li Y., Di C., Wang S. Notoginsenoside R1 protects against neonatal cerebral hypoxic-ischemic injury through estrogen receptor-dependent activation of endoplasmic reticulum stress pathways. J. Pharmacol. Exp. Ther. 2016 doi: 10.1124/jpet.115.230359. [DOI] [PubMed] [Google Scholar]
- 35.Hammadi M., Oulidi A., Gackiere F., Katsogiannou M., Slomianny C., Roudbaraki M., Dewailly E., Delcourt P., Lepage G., Lotteau S. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J. 2013;27:1600–1609. doi: 10.1096/fj.12-218875. [DOI] [PubMed] [Google Scholar]
- 36.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 37.Chavez-Valdez R., Flock D.L., Martin L.J., Northington F.J. Endoplasmic reticulum pathology and stress response in neurons precede programmed necrosis after neonatal hypoxia-ischemia. Int. J. Dev. Neurosci. 2016;48:58–70. doi: 10.1016/j.ijdevneu.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 39.Nakka V.P., Prakash-babu P., Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol. Neurobiol. 2016;53:532–544. doi: 10.1007/s12035-014-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi T., Saito A., Okuno S., Ferrand-Drake M., Dodd R.L., Nishi T., Maier C.M., Kinouchi H., Chan P.H. Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J. Cereb. Blood Flow Metab. 2003;23:1117–1128. doi: 10.1097/01.WCB.0000089600.87125.AD. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi T., Saito A., Okuno S., Ferrand-Drake M., Dodd R.L., Chan P.H. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J. Cereb. Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- 42.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer's disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 43.Jin S., Pu S.X., Hou C.L., Ma F.F., Li N., Li X.H., Tan B., Tao B.B., Wang M.J., Zhu Y.C. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid. Med. Cell Longev. 2015;2015:758358. doi: 10.1155/2015/758358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coletta C., Modis K., Szczesny B., Brunyanszki A., Olah G., Rios E.C., Yanagi K., Ahmad A., Papapetropoulos A., Szabo C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol. Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y.Y., Bian J.S. Hydrogen sulfide protects amyloid-beta induced cell toxicity in microglia. J. Alzheimers Dis. 2010;22:1189–1200. doi: 10.3233/JAD-2010-101002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material