Abstract
Primary cilium is a solitary organelle that emanates from the surface of most postmitotic mammalian cells and serves as a sensory organelle, transmitting the mechanical and chemical cues to the cell. Primary cilia are key coordinators of various signaling pathways during development and maintenance of tissue homeostasis. The emerging evidence implicates primary cilia function in tooth development. Primary cilia are located in the dental epithelium and mesenchyme at early stages of tooth development and later during cell differentiation and production of hard tissues. The cilia are present when interactions between both the epithelium and mesenchyme are required for normal morphogenesis. As the primary cilium coordinates several signaling pathways essential for odontogenesis, ciliary defects can interrupt the latter process. Genetic or experimental alterations of cilia function lead to various developmental defects, including supernumerary or missing teeth, enamel and dentin hypoplasia, or teeth crowding. Moreover, dental phenotypes are observed in ciliopathies, including Bardet-Biedl syndrome, Ellis-van Creveld syndrome, Weyers acrofacial dysostosis, cranioectodermal dysplasia, and oral-facial-digital syndrome, altogether demonstrating that primary cilia play a critical role in regulation of both the early odontogenesis and later differentiation of hard tissue–producing cells. Here, we summarize the current evidence for the localization of primary cilia in dental tissues and the impact of disrupted cilia signaling on tooth development in ciliopathies.
Keywords: craniofacial anomalies, growth/development, mineralized tissue/development, odontoblast(s), oral pathology, signal transduction
Introduction
Primary cilia are nonmotile structures with basal compartments containing a pair of centrioles in the cytoplasm and axoneme. The axoneme, composed of a microtubule skeleton of cilia, is characterized by a 9+0 arrangement of microtubules. Primary cilia serve as a signaling organelles, and they have been observed on a large variety of mammalian cell types (Wheatley et al. 1996). The primary cilium plays a role as a sensory organelle that can respond to mechanical and chemical stimuli from the extracellular environment as a large number of receptors, ion channels, and transporter proteins are localized on the ciliary membrane. Cilia are involved in the signaling of Hedgehog and WNT morphogens and coordinate the switch between cell survival, proliferation, and differentiation (Irigoín and Badano 2011). Ciliary defects often lead to aberrant craniofacial development, which is accompanied by dysmorphologies that include cleft palate, craniosynostosis, or cranioectodermal dysplasia (Brugmann et al. 2010; Schock et al. 2016). Recently, several studies were published emphasizing a key role of primary cilia in odontogenesis (Nakatomi et al. 2013; Liu et al. 2014). Here, we review the localization of primary cilia through all stages of tooth development to provide information about stage and region specificity of their appearance. Next, we focus on the developmental processes and signaling pathways in which primary cilia have been functionally implicated in tooth morphogenesis. We also discuss effects on odontogenesis from defective cilia while emphasizing several principles common to cilia-regulated developmental processes such as 1) shared usage of downstream targets and 2) different proteins functioning in common cellular processes.
Localization of Primary Cilia during Early Odontogenesis
During odontogenesis, the primary cilia have been demonstrated by electron microscopy or immunohistochemical labeling on different dental cells through various stages of fetal and postnatal tooth formation (Sasano 1986; Magloire et al. 2004; Kero et al. 2014; Hisamoto et al. 2016). During the cap stage, the primary cilia were found throughout the whole tooth organ on the cell surfaces of epithelial as well as mesenchymal tooth components (Kero et al. 2014).
At the bell stage, primary cilia were observed in the developing cervical loop and the nearby mesenchyme of dental follicle and dental papilla (Kero et al. 2014). In the E16.5 mouse molar, numerous short cilia were found in the inner and outer enamel epithelium, while fewer cilia were found in the stellate reticulum (Hisamoto et al. 2016). Cilia are also present on the cells of the enamel knot. In the mesenchyme, there are primary cilia widely distributed in areas surrounding the tooth germ as well as in the dental papilla (Hisamoto et al. 2016).
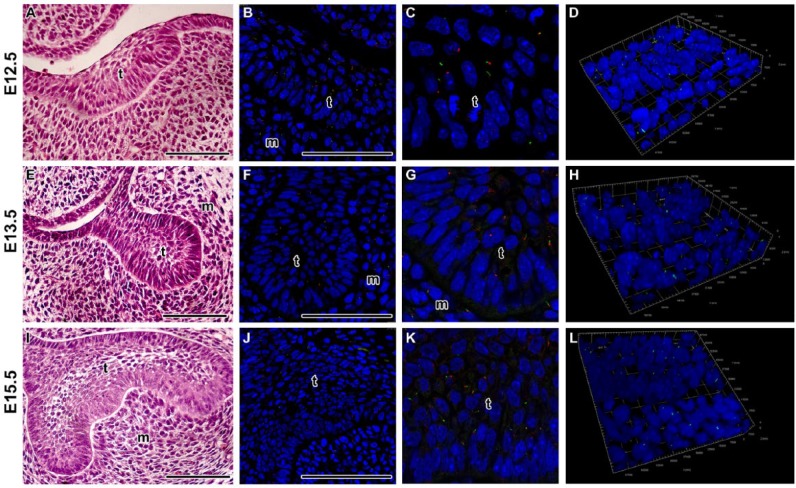
Since there are sparse data on all stages of tooth development, we chose to determine the presence of cilia in the earliest stages of odontogenesis in the epithelial thickening, bud stage and the cap stage. Our analysis revealed mostly short cilia in the epithelial dental tissues as well as mesenchymal cells surrounding the tooth germ (Fig. 1). In the basal epithelial layer of the tooth germ, the primary cilia were located on the apical surface opposed to the basal membrane (Fig. 1), suggesting possible involvement in polarization of cells during early tooth development.
Figure 1.
Localization of primary cilia during early tooth germs in mouse. (A–D) Primary cilia are dispersed through the epithelial thickening. Ciliated cells are also located in the mesenchymal condensation just below the thickening. (E–H) At the bud stage, cilia are situated mostly on the apical surface of the basal epithelial layer. In the central area, there are only a few ciliated cells with shorter cilia. (I–L) Ciliated cells are dispersed through the enamel organ at the early bell stage. (C, G, K) Detail of interface between the dental epithelium and mesenchyme. D, H, L) Three-dimensional view of detail in the area of interface. Primary cilia are labeled by acetylated alpha tubulin (ALEXA488, green) and pericentrin (ALEXA594, red), and nuclei are stained by DRAQ5 (blue). T, tooth germ; m, mesenchyme. Scale bar = 100 µm.
Localization of Primary Cilia at the Secretory Stage of Odontogenesis
At the late bell stage, primary cilia have been demonstrated in both mouse and human ameloblasts and odontoblasts; these cilia progressively elongate with differentiation status of the cells (Sasano 1986; Magloire et al. 2004; Kero et al. 2014). In odontoblasts, primary cilia are aligned parallel to the dentin walls, and their axonemes are oriented toward the pulp core (Thivichon-Prince et al. 2009; Hisamoto et al. 2016). The regular arrangement of cilia in odontoblasts first appears near the root apex and then extends along the odontoblast layer. Cilia on the ameloblasts project toward the outer enamel epithelium. Thus, the cilia of ameloblasts and odontoblasts are oriented in opposite directions out of the area where the hard tissue is produced (Hisamoto et al. 2016). Ameloblasts seem to have shorter cilia compared with odontoblasts, but a detailed quantitative analysis of cilia length has not been performed yet.
In the incisor, the primary cilia appear during the presecretory phase at the onset of cell differentiation (Sasano 1986), and the data suggest that the differentiation status of cells determines the cilia length. Why the cilia length varies in a developing organ is an open question: the most parsimonious hypothesis would be that differing cilia sizes affect their sensory functions, serving a specific demand of the recipient cell. Ameloblasts and odontoblasts are highly polarized cells producing hard tissues, and the mineral deposition has to be precisely restricted to the secretory region of the cell. Primary cilia were previously shown to mediate Ca2+ response in the kidney epithelium (Raghavan and Weisz 2016); therefore, elongation of cilia in differentiated hard tissue–producing cells can be critical for precise control of mineral deposition. Since both the growth of the ciliary axoneme and trafficking of ions for biomineralization are highly organized and directed processes, we suggest that common cell physiological mechanisms are at play. Mice with defective ciliogenesis due to the lack of Evc proteins (Evc-/- and Evc2-/-) develop hypomineralized incisors. This is further confirmed by defects such as enamel hypoplasia or the presence of undifferentiated odontoblasts. It is unclear whether these defects are manifested early in patterning of the tooth germ or whether the tooth is patterned correctly, but cells are not polarized correctly, resulting in defective hard tissue production and mineralization as both processes could be caused by shorter cilia.
During late bell stages, primary cilia were found also in the dental follicles, which give rise to the periodontium (Hisamoto et al. 2016). Cilia were located in periodontal ligament cells between the dentin/cement and the alveolar bone. In early postnatal and adult mice, cilia were detected on the edges of the periodontal ligament adjacent to both the tooth and alveolar bone; however, there were few ciliated cells in the medial region of the periodontal ligament (Hisamoto et al. 2016). In contrast to the aligned cilia of ameloblasts and odontoblasts, where the odontoblast axoneme points toward the pulp cavity and the ameloblast axoneme points to the outer enamel epithelium, the mesenchymal primary cilia do not show an orderly arrangement in the fetal dental follicle or in the neonatal and adult periodontal ligament (Hisamoto et al. 2016).
In summary, primary cilia are present in all epithelial and mesenchymal compartments of the developing tooth germ, and their length changes significantly during odontogenesis. Cilia are longer in the signaling centers, such as the enamel knot, or in areas undergoing intense cellular differentiation and production of hard tissues. Moreover, cilia are polarized in certain areas already from early stages of tooth development.
Localization of Primary Cilia in the Dental Lamina
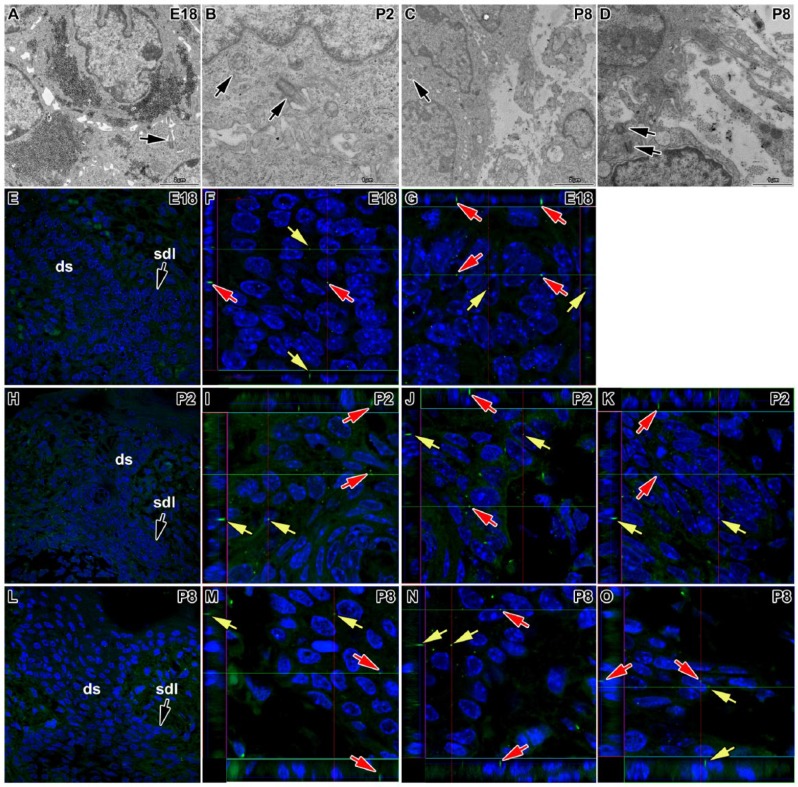
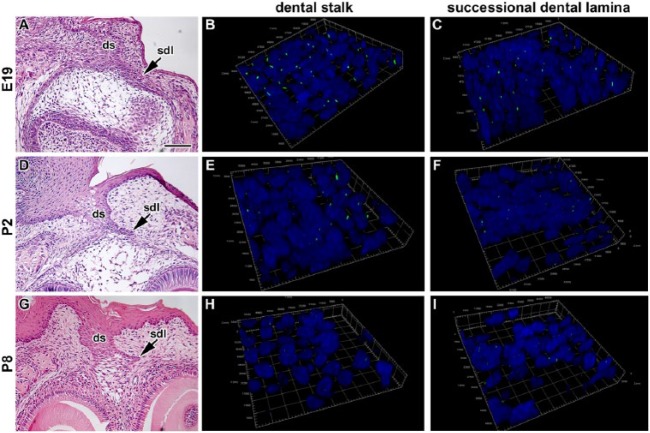
As there is no available information about primary cilia in the dental lamina and the dental stalk, we analyzed the distribution of primary cilia in these areas in mouse using an antibody specific for acetylated alpha tubulin to label the axoneme of the primary cilia. We observed only very short cilia in the central area of the dental stalk as well as in the rudimentary successional dental lamina (Figs. 2 and 3). Primary cilia protruded as small projections beyond the cell surface into tight intercellular spaces between epithelial cells (Fig. 2) and were oriented in a rostro-caudal direction (Fig. 3), while no specific orientation of cilia was observed in the mesenchyme. We found only small differences between the buccal and lingual side of the dental stalk around birth (E18 and P2). At P8, however, more primary cilia were observed on the lingual side of the dental stalk (Fig. 2). While numerous cilia were observed in the dental stalk at the late embryonic stage (E18), the number of cilia decreased with age. Just before tooth eruption at P14, almost no cilia were detected as keratinization progressed (Dosedělová et al. 2015). The disappearance of primary cilia during keratinization was previously described in the skin and hairs (Lehman et al. 2009). The decrease of primary cilia number with age was observed also in the rudimental successional dental lamina (Fig. 2). While in odontoblasts and ameloblasts, the cilia increased their length during differentiation, the dental stalk cells decreased the cilia length and became sparse during keratinization. This suggests that the differentiation state of the cells can affect both the ciliary length and the number or ciliated cells.
Figure 2.
Primary cilia in the dental stalk and rudimentary successional dental lamina of mouse. Primary cilium (arrow) is located between epithelial cells in the central area of the dental stalk (ds) at E18 (A) and in the epithelial cells of the successional dental lamina (sdl) at P2 (B). (C) Cross-section of the primary cilia in the epithelial cells of the basal layer on the lingual side of the dental stalk at P8. (D) Mesenchymal cell (mes) with primary cilia facing to the epithelium of the dental stalk at P8. (E) Lower-power view of the transversal section of the dental stalk and the rudimental successional lamina at E18. (F, G) A high number of primary cilia is observed at E18. Cilia are scattered through the dental stalk (ds) and in the successional dental lamina (sdl). (H) Lower-power view of the transversal section of the dental stalk and the rudimental successional lamina at P2. (I–K) Numerous primary cilia are located in the dental stalk as well as successional dental lamina at P2. (L) Lower-power view of the transversal section of the dental stalk and the rudimental successional lamina at P8. (M–O) The number of primary cilia decreases at P8. A higher number of primary cilia is located on the lingual side of the dental stalk (K) while only few cilia in the rudimental successional lamina (L). Numerous cilia in the epithelium are very short or oriented in the rostro-caudal direction. Primary cilia are labeled by acetylated alpha tubulin (FITC, green), and nuclei are stained by DAPI (blue). Arrows point to the primary cilia. Details of sections are shown also as a profile view on the side of details to visualize cilia oriented in the rostro-caudal direction. Red and green lines indicate the section where the profile is visualized. Arrows in the same color show identical cilia on the transversal section as well in profile view.
Figure 3.
Three-dimensional analysis of primary cilia directionality in the dental stalk and rudimentary successional dental lamina of mouse. (A, D, G) Lower-power view of the dental stalk and rudimentary successional dental lamina stained by hematoxylin and eosin. (B, E, H) Three-dimensional view of the area of interface between the dental stalk epithelium and mesenchyme. (C, F, I) Three-dimensional view of the area of interface between the successional dental lamina epithelium and mesenchyme. Cilia are short in both areas during late embryonic and postnatal stages and oriented mostly in the rostro-caudal direction in epithelial tissues. The number of cilia decreased with the age of animals. Primary cilia are labeled by acetylated alpha tubulin (ALEXA488, green), and nuclei are stained by DRAQ5 (blue). Ds, dental stalk; sdl, successional dental lamina. Scale bar = 100 µm.
Signaling Pathways Depending on Primary Cilia
Primary cilia maintains a subset of cytoplasmic and membrane molecules that contribute to the modulation of Hedgehog (HH), Wingless (WNT), platelet-derived growth factor (PDGF), and TGF-β signaling cascades (Lee and Gleeson 2011; Christensen et al. 2016).
The function of the primary cilia depends on the intraflagellar transport (IFT), which transports cargo through the cilium in either the anterograde (to the cilia tip) or retrograde direction (to the cilia base). In the basal body region, the cargo molecules are typically concentrated in large protein complexes, which are then transported along the axoneme in the anterograde direction by the action of the kinesin-II complex (Kif3A, Kif3B, and Kap) and later in retrograde transport provided by dyneins (Cole et al. 1998). The amount and speed of the entering or exiting proteins are regulated in the transition zone, where the gatekeeper proteins function. The transition zone resembles a nuclear pore in its composition and regulates a selective protein entry into the cilia.
Defects in cilia and their components modify HH signaling by altered mobilization of proteins toward the tip and base of cilia along the microtubule-based axoneme. This transport was shown to play a crucial and vertebrate-specific role in HH signal transduction (Huangfu et al. 2003). In the absence of the HH ligand, Ptch1 represses the transmembrane protein Smo and prevents Smo from entering the cilia (Rohatgi et al. 2007). When the HH ligand binds to Ptch1, Ptch1 exits from the cilium and Smo translocates into the cilium. The balance of Ptch1 and Smo regulates the balance of Gli transcription factors between the activator and repressor form, which can then be translocated to the nucleus and modulate gene expression (Huangfu et al. 2003; Haycraft et al. 2005; Goetz and Anderson 2010).
Deficiencies in Odontogenesis Caused by Defective Primary Cilia Signaling
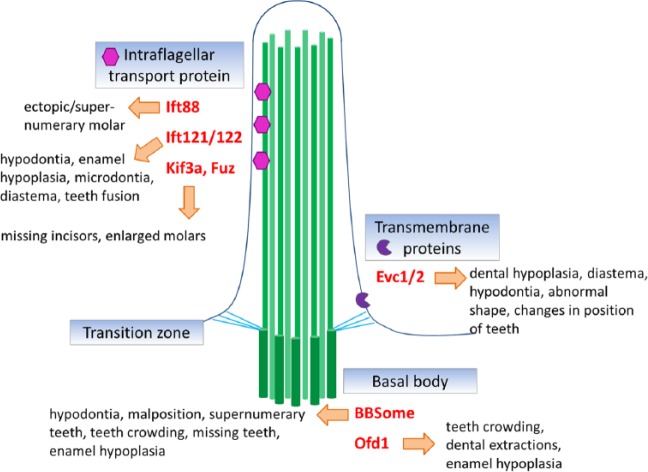
Disruption of ciliary protein transport affects HH signaling and results in several developmental disorders with diverse tooth phenotypes (Figure 4). Mutations in ciliary transport have pleiotropic effects on tooth development because Sonic hedgehog (SHH) is required for tooth germ initiation, growth, morphogenesis, and cellular differentiation as well as for the establishment of cellular polarity (Dassule et al. 2000).
Figure 4.
Primary cilia components and dental disorders. Schema of main compartments of the primary cilium and gene product localization with association to dental phenotype.
IFT88 (also known as Polaris or TG737) is a component of the anterograde IFT machinery; mice with mutant alleles of IFT88 develop a strong dental phenotype. A hypomorphic allele of IFT88 (Tg737orpk) has increased HH signaling and formation of supernumerary molars in the diastema of both the lower and upper jaws (Ohazama et al. 2009; Zhang et al. 2003). Interestingly, IFT88 seems to have different functions in the mesenchyme and the epithelium. Mice with mesenchymal deletion of IFT88 develop supernumerary teeth and die at birth, while animals with epithelial deletion of IFT88 in the dental epithelial cells survive and show no supernumerary teeth formation (Ohazama et al. 2009). Therefore, for tooth initiation, HH signaling in the primary cilia is required in the dental mesenchyme cells but not the epithelial cells.
While SHH regulation within the primary cilia is well studied, the role of the cilium in WNT/β-catenin signaling is less explored. In fact, suppression of primary cilia resulted in up-regulation of the WNT pathway (Lancaster et al. 2011). Similarly, tooth buds from Wnt1-cre Kif3af/f mice exhibit a loss of HH signaling and a concomitant gain of WNT/β-catenin signaling in the dental mesenchyme. The gain of Wnt signaling in the mesenchyme has a secondary effect on the dental epithelium, where it increases proliferation and results in multiple invaginations instead of single secondary enamel knot (Liu et al. 2014). These data suggest that the primary cilium integrates WNT and SHH signaling between epithelium and mesenchyme during odontogenesis (Liu et al. 2014). The Wnt1cre::Kif3a deletion also resulted in developmental arrest of incisors, sometime after their initiation (Brugmann et al. 2010; Liu et al. 2014). Focal domains of WNT and SHH signaling are known to define the sites where teeth will develop (Sarkar et al. 2000); therefore, these expression changes could cause defects in incisor initiation. Surprisingly, molar germ was enlarged and exhibited only failure of mesenchymal condensation and the misshapen enamel organ, leading to disrupted enamel production in Wnt1Cre+Kif3afl/fl animals.
A similar region-specific response was observed in Fuz-/- mice, which exhibit missing incisors in the upper and lower jaw; however, all molar germs develop normally (Zhang et al. 2011). FUZ is a planar cell polarity effector protein, which is necessary for trafficking of cargo to basal bodies and through the cilia (Gray et al. 2009). The phenotype similarity between PCP and isolated ciliary mutants such as Kif3a suggests cell-specific cilia functions in tooth development. What determines the different response to cilia loss in the molar and incisor area is not known and should be addressed by future research.
Ciliopathy Syndromes with Dental Phenotype
Ciliopathies are human disorders caused by defective function of cilia. The group of ciliopathies is rapidly expanding as the diagnostic methods of genetic disorders has improved in the past decades (Lee and Gleeson 2011). Ciliopathies are often accompanied with numerous facial defects (Brugmann et al. 2010) and usually also exhibit disrupted odontogenesis. Here, we summarize ciliopathies displaying defective odontogenesis (Table) with the aim to uncover common features connected with disrupted primary cilia and shared usage of downstream targets through SHH signaling in primary cilia.
Table.
Summary of Dental Phenotypes and Gene Mutation Caused by Disruption of Morphology or Function in the Primary Cilia.
| Tooth Phenotype | Gene | Disease | Defects in Cilia | Reference | |
|---|---|---|---|---|---|
| Tooth Number | Reduced tooth number/missing teeth | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia | Baujat and Le Merrer 2007 Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 |
| Evc2 | Weyers | Loss of Smoothened/EVC protein complex from transition zone in primary cilia | Curry and Hall 1979 Roubicek and Spranger 1984 Ye et al. 2006 Dorn et al. 2012 | ||
| Ift121/Ift122 | CED | Swollen shape, defective retrograde intraflagellar transport | Levin et al. 1977 Walczak-Sztulpa et al. 2010 Qin et al. 2011 | ||
| Ofd1 | OFD | Absent cilia |
Dodge and Kernohan 1967
Ferrante et al. 2006 Singla et al. 2010 Jerman et al. 2014 |
||
| Fuz | Fewer, shorter cilia | Zhang et al. 2011 | |||
| Kif3a | Fewer cilia | Liu et al. 2014 | |||
| Supernumerary teeth | Ofd1 | OFD | Cilia absent |
Thivichon-Prince et al. 2009
Ferrante et al. 2006 Jerman et al. 2014 Singla et al. 2010 |
|
| Ift88 | Cilia absent | Ohazama et al. 2009 | |||
| Tooth Size | Enlarged molar tooth germ | Kif3a | Fewer cilia |
Liu et al. 2014
Brugmann et al. 2010 |
|
| Microdontia | Ift121/Ift122 | CED | Swollen shape, defective retrograde intraflagellar transport | Sensenbrenner et al. 1975 Walczak-Sztulpa et al. 2010 Qin et al. 2011 | |
| Tooth Shape | Abnormal shape | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia | Baujat and Le Merrer 2007 Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 |
| Evc2 | Weyers | Curry and Hall 1979 Roubicek and Spranger 1984 Ye et al. 2006 Dorn et al. 2012 | |||
| Serrated incisal margins | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia |
Baujat and Le Merrer 2007
Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 |
|
| Disorganized tooth germ | Ofd1 | OFD | Absent primary cilia |
Thivichon-Prince et al. 2009
Ferrante et al. 2006 Singla et al. 2010 Jerman et al. 2014 |
|
| Tooth position/spacing | Dental transposition | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia | Baujat and Le Merrer 2007 Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 |
| Evc2 | Weyers | Curry and Hall 1979 Roubicek and Spranger 1984 Ye et al. 2006 Dorn et al. 2012 | |||
| Dental malposition | Ofd1 | OFD | Absent primary cilia | Dodge and Kernohan 1967 Ferrante et al. 2006 Singla et al. 2010 Jerman et al. 2014 | |
| Diastema/widely spaced teeth | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia | Baujat and Le Merrer 2007 Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 | |
| Ift121/Ift122 | CED | Swollen shape, defective retrograde intraflagellar transport | Young 1989 Qin et al. 2011 | ||
| Tooth crowding | Bbs | BBS | Disrupted intraflagellar transport; mislocalization of ciliary proteins |
Blacque and Leroux 2006
Beales et al. 1999
Waters and Beales 2011 |
|
| Disrupted differentiation of cells | Enamel hypoplasia | Evc1/2 | EvC | Loss of Smoothened/EVC protein complex from transition zone in primary cilia |
Baujat and Le Merrer 2007
Nakatomi et al. 2013 Ruiz-Perez et al. 2000 Ruiz-Perez et al. 2003 Dorn et al. 2012 |
| Ofd1 | OFD | Absent primary cilia |
Dodge and Kernohan 1967,Ferrante et al. 2006; Singla et al. 2010,Jerman et al. 2014 |
||
| Ift121/Ift122 | CED | Swollen shape, defective retrograde intraflagellar transport |
Walczak-Sztulpa et al. 2010
Qin et al. 2011
Young 1989 |
||
| Bbs | BBS | Disrupted intraflagellar transport; mislocalization of ciliary proteins |
Blacque and Leroux 2006
Beales and others 1999 |
||
| Dentin hypoplasia | Ofd1 | OFD | Absent primary cilia |
Dodge and Kernohan 1967
Ferrante and others 2006 Jerman et al. 2014 Singla et al. 2010 |
BBS, Bardet-Biedl syndrome; CED, cranioectodermal dysplasia; EVC, Ellis-van Creveld syndrome; OFD, oral-facial-digital syndrome.
Bardet-Biedl syndrome (BBS) is an autosomal recessive condition with a wide spectrum of clinical features. There are 16 known genes associated with BBS (BBS1–BBS16; Waters and Beales 2011), and a subset of BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9) are located at the basal bodies or along the ciliary axonemes (Forsythe and Beales 2013). These proteins form the BBSome complex, which mediates protein trafficking to the cilia and contributes to loading IFT cargo proteins at the base of the cilia (Blacque and others 2004). Mutations of Bbs genes disrupt IFT, causing mislocalization of ciliary proteins (Blacque and Leroux 2006). Moreover, BBS proteins are involved in Hedgehog signaling through their interaction with Smo, and they facilitate trafficking of Smo to the cilia (Seo et al. 2011).
Among other craniofacial defects, dental anomalies are common in BBS (Borgström et al. 1996). The affected individuals suffer from crowding of the teeth, dental extractions, enamel hypoplasia, and micrognathia (Beales et al. 1999). Furthermore, hypodontia, small teeth, and short roots (Borgström et al. 1996) or taurodontism (Andersson et al. 2013) were observed in human patients. Recently, a single central upper incisor was described in Bbs3-/- mouse (Kawasaki et al. 2016). Other mouse BBS models have not been evaluated for dental phenotype yet (Blacque and Leroux 2006). Therefore, it will be necessary to correlate specific dental phenotypes with individual BBS mutations and/or disrupted IFT in the future.
Ellis-van Creveld syndrome (EvC) is caused by mutation in Evc and Evc2 genes (Ruiz-Perez et al. 2000, 2003). EVC and EVC2 are transmembrane proteins that function as SHH signaling regulators (Blair et al. 2011). The formation of the Smo-Evc2 complex at the EvC zone is necessary for proper Hh signal transmission (Dorn et al. 2012). Primary cilia in cells of patients with EvC syndromes are ultrastructurally normal. The pathophysiology of the syndrome is caused by failure of EVC2 to localize to cilia, thus preventing SHH pathway activity (Dorn et al. 2012).
EvC syndrome is characterized by numerous defects during all stages of odontogenesis, including formation of serrated incisal margins, dental transposition, diastema, conical teeth, enamel hypoplasia, hypodontia, premature eruption or exfoliation of teeth, and natal teeth (Baujat and Le Merrer 2007). Loss of Evc in mice results in incisor hypoplasia and the fusion of first and second molars, disruption of molar symmetry, molar microdontia, abnormal root development with their fusions, and delayed cell differentiation (Nakatomi et al. 2013). Interestingly, in the Evc mutant, the response to SHH signaling decreased from buccal to lingual axis (Nakatomi et al. 2013). Our analysis of cilia distribution did not reveal, with the exception of the dental stalk, any significant spatial differences in cilia number during embryonic stages. Therefore, the control of signal trafficking through the cilia seems to be also region specific even within a single tooth germ. As Shh mutants exhibit growth retardation on their lingual aspect (Dassule et al. 2000), it was proposed that EVC restrains the signaling by antagonizing SHH in buccal region (Nakatomi et al. 2013). Another possibility is that there are different requirements of SHH signaling in labial and lingual sides of the tooth germs. In polyphyodont species, Shh expression was observed in the dental-oral interface specifically in cells on the lingual side (Buchtova et al. 2008). This means that remnants of this ancestral expression or responsiveness to SHH pathway can be present in mouse even when it cannot be visualized with in situ hybridization.
Moreover, while the first molars are robustly disrupted in Evc-deficient animals, only root development is affected in the second molar, and the third molar is unaffected. The sensitivity to Evc loss may be explained by differences in developmental timing of the mouse molars. The first and second molars are initiated during embryonic development in close proximity to oral epithelium, while the third molar is initiated perinatally by overgrowth of the second molar (Chlastakova et al. 2011). Thus, the first and second molars develop closely together in time and location within the oral-dental interface and are more sensitive to Evc loss, while the cells that contribute to the third molar have different embryonic timing and do not rely on Evc for morphogenesis.
EvC syndrome is modeled in the mouse by introducing a premature stop codon into exon 12 (named Evc2ex12/ex12) to model the common human mutation. These mice exist as either a global knockout or a conditional floxed allele. Evc2ex12/ex12 animals develop significant tooth defects. Both the upper and lower incisors are short and hypomineralized; in addition, the upper incisors do not curve toward the lower jaw but extend distally from the jaw (Zhang et al. 2017). Intriguingly, deleting Evc2 using the neural-crest specific, P0-cre, causes similar, but less severe phenotypes in the incisors and molar teeth (Zhang et al. 2015). These data suggest that the primary cilia residing in the neural crest–derived mesenchyme are necessary for normal mineralization of the tooth. It is unknown if Evc2 is required for tissue-specific early patterning and morphogenesis of the tooth germ.
Weyers acrofacial dysostosis (Curry Hall syndrome) is phenotypically considered to be less serious form of EvC syndrome with milder phenotypes. This disorder is also a result of loss-of-function mutations in Evc or Evc2 genes (Howard et al. 1997; Ruiz-Perez et al. 2000; Ye et al. 2006). Among other craniofacial defects, people with this syndrome have abnormal shape, number, and position of the teeth (Curry and Hall 1979; Roubicek and Spranger 1984).
Cranioectodermal dysplasia (CED), also known as Sensenbrenner syndrome, is a ciliopathy caused by mutation in either Ift122 (Walczak-Sztulpa et al. 2010) or Ift121 genes (Gilissen et al. 2010). IFT121 and IFT122 proteins are necessary for proper IFT and localization of signaling molecules in the primary cilia. When the Ift122 gene is disrupted in the mouse, primary cilia exhibit swollen shape and have defective retrograde IFT (Qin et al. 2011). IFT122 was shown to negatively regulate SHH signaling downstream of Smoothened but upstream of transcription factor Gli2 (Qin et al. 2011).
In human patients, CED is characterized by dental deficiencies including hypodontia and microdontia (Sensenbrenner et al. 1975; Levin et al. 1977). Teeth can be widely spaced or fused with enamel hypoplasia (Young 1989). This is accompanied by reduced frequency and length of the primary cilia (Alazami et al. 2014).
Oral-facial-digital syndrome (OFD), also called Papillon-League or Psaume syndrome, is caused by mutation in the Ofd1 gene encoding protein expressed in centrosome and basal bodies of primary cilia (Romio et al. 2004). OFD1 is required for the recruitment of IFT88 protein to the centrosome. The functional connection between OFD1 and IFT88 is apparent from the identical phenotypes of their mutants. OFD1 forms a signaling complex together with polycystins, flotillins, and epidermal growth factor receptor (Jerman et al. 2014). When one of these proteins is defective in the primary cilia of dental pulp–derived odontoblasts, it decreases the assembly and/or stability of the entire complex (Jerman et al. 2014). Cells with mutations in Ofd1 do not form primary cilia, and inactivation of Ofd1 results in defective SHH signaling (Ferrante et al. 2006; Singla et al. 2010).
OFD syndrome is characterized by developmental defects including frequent dental abnormalities such as malposition, supernumerary teeth, missing teeth, and enamel hypoplasia (Dodge and Kernohan 1967). Analysis of Ofd1-deficient mice revealed that abnormalities are caused by the lack of axoneme, which results in disorganization of tooth germ structure including cusp formation and the disruption of odontoblast differentiation and polarization (Thivichon-Prince et al. 2009).
Meckel syndrome (MKS) is a ciliopathy caused by a mutation in MKS proteins. Five MKS proteins are located in the centrosomes or ciliary membranes. Mutation in MKS1 leads to defective cilia formation and reduced Hedgehog signaling, where MKS1 has been found to act downstream of Ptch1 and Smo (Weatherbee et al. 2009). In humans, the phenotype includes cystic kidneys, encephalocele, and polydactyly, as well as natal teeth. Based on our data here, showing the primary cilia in the dental stalk, and combined with the accelerated eruption of teeth in Meckel’s patients, we hypothesize that the primary cilia need to be lost on the dental stalk cells for tooth eruption to occur.
Conclusions
Recent findings suggest that the primary cilia play a critical role during odontogenesis. Moreover, cilia represent an essential structure-adjusting region-specific response of cells to extracellular as well as intracellular signals. During odontogenesis, cilia have been observed in all analyzed cell types. Changes in the cilia distribution and length were detected during development, correlating mainly with the differentiation status of the cells producing hard tissues.
Primary cilia are important signaling centers; therefore, any defect of their structure or function results in altered odontogenesis. The specific consequences are changes in the tooth number, size, shape, position, and odontoblast/ameloblast differentiation. Unique dental phenotypes are not correlated with a single gene or with increased or decreased signaling through a particular pathway. A tissue-specific response including complex signaling regulation is responsible for the final phenotype of affected individuals, but it is still necessary to answer the question as to why the same mutations display such variable tooth phenotypes.
While most studies on ciliopathies focus on anomalies that are critical for patient survival such as craniofacial, kidney, or limb anomalies, little attention is paid to the teeth. We propose that morphology and function of the tooth should be included in these assessments, to fully understand the role of primary cilia during development. Further investigation will be necessary to focus on individual ciliary protein function during odontogenesis to reveal causes of complex symptoms in human patients.
Author Contributions
M. Hampl, H. Dosedelova, contributed to data acquisition, analysis, and interpretation, drafted the manuscript; P. Cela, H.L. Szabo-Rogers, contributed to data interpretation, drafted the manuscript; M. Kunova Bosakova, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; P. Krejci, contributed to conception and design, critically revised the manuscript; M. Buchtova, contributed to conception and design, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Jana Dumková for transmission electron microscopy imaging.
Footnotes
This work was supported by the project “EXCELLENCE in Molecular Aspects of the Early Development of Vertebrates” from the Operational Programme Research, Development and Education (CZ.02.1.01/0.0/0.0/15_003/0000460), by the Czech Science Foundation (17-14886S), and by institutional support (RVO:67985904). The authors are also grateful for the support from the National Institutes of Health DE020740 (HLSR) and start-up funds from the University of Pittsburgh School of Dental Medicine (HLSR).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alazami AM, Seidahmed MZ, Alzahrani F, Mohammed AO, Alkuraya FS. 2014. Novel IFT122 mutation associated with impaired ciliogenesis and cranioectodermal dysplasia. Mol Genet Genomic Med. 2(2):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson EM, Axelsson S, Gjølstad LF, Storhaug K. 2013. Taurodontism: a minor diagnostic criterion in Laurence-Moon/Bardet-Biedl syndromes. Acta Odontol Scand. 71(6):1671–1674. [DOI] [PubMed] [Google Scholar]
- Baujat G, Le Merrer M. 2007. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. 1999. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Leroux MR. 2006. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 63(18):2145–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, et al. 2004. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 18(13):1630–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HJ, Tompson S, Liu YN, Campbell J, MacArthur K, Ponting CP, Ruiz-Perez VL, Goodship JA. 2011. Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC Biol. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgström MK, Riise R, Tornqvist K, Granath L. 1996. Anomalies in the permanent dentition and other oral findings in 29 individuals with Laurence-Moon-Bardet-Biedl syndrome. J Oral Pathol Med. 25(2):86–89. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA. 2010. A primary cilia-dependent etiology for midline facial disorders. Hum Mol Genet. 19(8):1577–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtova M, Handrigan GR, Tucker AS, Lozanoff S, Town L, Fu K, Diewert VM, Wicking C, Richman JM. 2008. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Dev Biol. 319(1):132–145. [DOI] [PubMed] [Google Scholar]
- Chlastakova I, Lungova V, Wells K, Tucker AS, Radlanski RJ, Misek I, Matalova E. 2011. Morphogenesis and bone integration of the mouse mandibular third molar. Eur J Oral Sci. 119(4):265–274. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB. 2016. Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor beta (TGF-beta) signaling. Cold Spring Harb Perspect Biol [epub ahead of print 16 September 2016]. doi: 10.1101/cshperspect.a028167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. 1998. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 141(4):993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry CJ, Hall BD. 1979. Polydactyly, conical teeth, nail dysplasia, and short limbs: a new autosomal dominant malformation syndrome. Birth Defects Orig Artic Ser. 15(5B):253–263. [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. 2000. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 127(22):4775–4785. [DOI] [PubMed] [Google Scholar]
- Dodge JA, Kernohan DC. 1967. Oral-facial-digital syndrome. Arch Dis Child. 42(222):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn KV, Hughes CE, Rohatgi R. 2012. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell. 23(4):823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosedělová H, Dumková J, Lesot H, Glocová K, Kunová M, Tucker AS, Veselá I, Krejčí P, Tichý F, Hampl A, et al. 2015. Fate of the molar dental lamina in the monophyodont mouse. PLoS One. 10(5):e0127543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. 2006. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 38(1):112–117. [DOI] [PubMed] [Google Scholar]
- Forsythe E, Beales PL. 2013. Bardet-Biedl syndrome. Eur J Hum Genet. 21(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, et al. 2010. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet. 87(3):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. 2010. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 11(5):331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. 2009. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 11(10):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. 2005. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1(4):e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto M, Goto M, Muto M, Nio-Kobayashi J, Iwanaga T, Yokoyama A. 2016. Developmental changes in primary cilia in the mouse tooth germ and oral cavity. Biomed Res. 37(3):207–214. [DOI] [PubMed] [Google Scholar]
- Howard TD, Guttmacher AE, McKinnon W, Sharma M, McKusick VA, Jabs EW. 1997. Autosomal dominant postaxial polydactyly, nail dystrophy, and dental abnormalities map to chromosome 4p16, in the region containing the Ellis-van Creveld syndrome locus. Am J Hum Genet. 61(6):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426(6962):83–87. [DOI] [PubMed] [Google Scholar]
- Irigoín F, Badano JL. 2011. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics. 12(4):285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman S, Ward HH, Lee R, Lopes CA, Fry AM, MacDougall M, Wandinger-Ness A. 2014. OFD1 and flotillins are integral components of a ciliary signaling protein complex organized by polycystins in renal epithelia and odontoblasts. PLoS One. 9(9):e106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Izu Y, Hayata T, Ideno H, Nifuji A, Sheffield VC, Ezura Y, Noda M. 2016. Bardet-Biedl syndrome 3 regulates development of cranial base midline structures. Bone. 101;179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kero D, Novakovic J, Vukojevic K, Petricevic J, Kalibovic Govorko D, Biocina-Lukenda D, Saraga-Babic M. 2014. Expression of Ki-67, Oct-4, g-tubulin and a-tubulin in human tooth development. Arch Oral Biol. 59(11):1119–1129. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG. 2011. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol. 13(6):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. 2011. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 3(9):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Laag E, Michaud EJ, Yoder BK. 2009. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol. 129(2):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LS, Perrin JC, Ose L, Dorst JP, Miller JD, McKusick VA. 1977. A heritable syndrome of craniosynostosis, short thin hair, dental abnormalities, and short limbs: cranioectodermal dysplasia. J Pediatr. 90(1):55–61. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen S, Cheng D, Jing W, Helms JA. 2014. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J Dent Res. 93(5):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire H, Couble ML, Romeas A, Bleicher F. 2004. Odontoblast primary cilia: facts and hypotheses. Cell Biol Int. 28(2):93–99. [DOI] [PubMed] [Google Scholar]
- Nakatomi M, Hovorakova M, Gritli-Linde A, Blair HJ, MacArthur K, Peterka M, Lesot H, Peterkova R, Ruiz-Perez VL, Goodship JA, et al. 2013. Evc regulates a symmetrical response to Shh signaling in molar development. J Dent Res. 92(3):222–228. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, et al. 2009. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 136(6):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. 2011. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Natl Acad Sci U S A. 108(4):1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V, Weisz OA. 2016. Discerning the role of mechanosensors in regulating proximal tubule function. Am J Physiol Renal Physiol. 310(1):F1–F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science. 317(5836):372–376. [DOI] [PubMed] [Google Scholar]
- Romio L, Fry AM, Winyard PJ, Malcolm S, Woolf AS, Feather SA. 2004. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol. 15(10):2556–2568. [DOI] [PubMed] [Google Scholar]
- Roubicek M, Spranger J. 1984. Weyers acrodental dysostosis in a family. Clin Genet. 26(6):587–590. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, et al. 2000. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 24(3):283–286. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Tompson SW, Blair HJ, Espinoza-Valdez C, Lapunzina P, Silva EO, Hamel B, Gibbs JL, Young ID, Wright MJ, et al. 2003. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 72(3):728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. 2000. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A. 97(9):4520–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano Y. 1986. Dynamic behavior of ciliated centrioles in rat incisor ameloblasts during cell differentiation. Arch Histol Jpn. 49(4):437–448. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner JA, Dorst JP, Owens RP. 1975. New syndrome of skeletal, dental and hair anomalies. Birth Defects Orig Artic Ser. 11(2):372–379. [PubMed] [Google Scholar]
- Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. 2011. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and smoothened. PLoS Genet. 7(11):e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EN, Struve JN, Chang CF, Williams TJ, Snedeker J, Attia AC, Stottmann RW, Brugmann SA. 2016. A tissue-specific role for intraflagellar transport genes during craniofacial development. PLoS One. 12(3): e0174206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. 2010. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 18(3):410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivichon-Prince B, Couble ML, Giamarchi A, Delmas P, Franco B, Romio L, Struys T, Lambrichts I, Ressnikoff D, Magloire H, et al. 2009. Primary cilia of odontoblasts: possible role in molar morphogenesis. J Dent Res. 88(10):910–915. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. 2010. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 86(6):949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. 2011. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 26(7):1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee SD, Niswander LA, Anderson KV. 2009. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 18(23):4565–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN, Wang AM, Strugnell GE. 1996. Expression of primary cilia in mammalian cells. Cell Biol Int. 20(1):73–81. [DOI] [PubMed] [Google Scholar]
- Ye X, Song G, Fan M, Shi L, Jabs EW, Huang S, Guo R, Bian Z. 2006. A novel heterozygous deletion in the EVC2 gene causes Weyers acrofacial dysostosis. Hum Genet. 119(1-2):199–205. [DOI] [PubMed] [Google Scholar]
- Young ID. 1989. Cranioectodermal dysplasia (Sensenbrenner’s syndrome). J Med Genet. 26(6):393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Takeda H, Tsuji T, Kamiya N, Kunieda T, Mochida Y, Mishina Y. 2017. Loss of function of Evc2 in dental mesenchyme leads to hypomorphic enamel. J Dent Res. 96(4):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Takeda H, Tsuji T, Kamiya N, Rajderkar S, Louie K, Collier C, Scott G, Ray M, Mochida Y, et al. 2015. Generation of Evc2/Limbin global and conditional KO mice and its roles during mineralized tissue formation. Genesis. 53(9):612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. 2003. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 227(1):78–90. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wlodarczyk BJ, Niederreither K, Venugopalan S, Florez S, Finnell RH, Amendt BA. 2011. Fuz regulates craniofacial development through tissue specific responses to signaling factors. PLoS One. 6(9):e24608. [DOI] [PMC free article] [PubMed] [Google Scholar]