Abstract
In several retinal degenerative diseases, including age-related macular degeneration, the retinal pigment epithelium, a highly functionalized cell monolayer, becomes dysfunctional. These retinal diseases are marked by early retinal pigment epithelium dysfunction reducing its ability to maintain a healthy retina, hence making the retinal pigment epithelium an attractive target for treatment. Cell therapies, including bolus cell injections, have been investigated with mixed results. Since bolus cell injection does not promote the proper monolayer architecture, scaffolds seeded with retinal pigment epithelium cells and then implanted have been increasingly investigated. Such cell-seeded scaffolds address both the dysfunction of the retinal pigment epithelium cells and age-related retinal changes that inhibit the efficacy of cell-only therapies. Currently, several groups are investigating retinal therapies using seeded cells from a number of cell sources on a variety of scaffolds, such as degradable, non-degradable, natural, and artificial substrates. This review describes the variety of scaffolds that have been developed for the implantation of retinal pigment epithelium cells.
Keywords: Scaffolds, retina, retinal pigment epithelium, age-related macular degeneration
Background
In the pathologies typical of retinal degenerative diseases, retinal pigment epithelial cells become dysfunctional, contributing to the death of neural retinal cells and eventual blindness. For several decades, the transplantation of retinal pigment epithelial cells as a treatment for these diseases has been under investigation. Despite the many advances in this area, diseases such as age-related macular degeneration (AMD), the leading cause of blindness in developed countries, continue to exist without effective treatments.1,2 There have been many promising initial results with free cell therapies, including the preliminary results from an ongoing clinical trial; however, it is recognized that there persist several challenges to such therapies. These challenges include the inability of free cells to attach and form a native architecture, the migration of the transplanted cells, and inflammation.3–6 These challenges have driven research toward tissue engineering solutions, particularly scaffold-based interventions. This review aims to examine tissue engineering scaffolds for AMD, their properties and reported efficacies, and to discuss the hurdles that must be overcome in order to develop a translational therapy using these scaffolds.
Physiology and changes due to AMD
The retina is a complex structure that sits in the posterior of the eye. It consists of the light transducing neural retina, as well as the supportive blood retinal barrier. The blood retinal barrier consists of a cell monolayer, the retinal pigment epithelium (RPE), and its tight junctions, Bruch’s membrane (BM), and the tight junctions of the retinal vascular endothelial cells of the underlying choroid (Figure 1).
Figure 1.
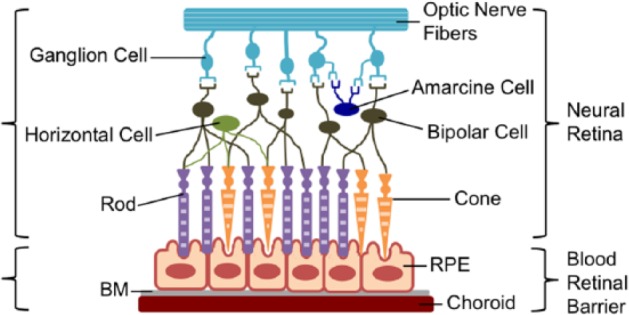
The structural organization of the retina. Diagram illustrating the distribution of retinal cells shows that photoreceptors interact directly with the apical side of the RPE cells. The RPE and other components of the blood retinal barrier maintain a healthy environment for the neural retina.
The RPE cells and blood retinal barrier structure supports the photoreceptors and various cell types in the neural retina in several ways. First, it maintains the retinal microenvironment through highly selective transport into and out of the retina. In addition to this selective transport provided by the blood retinal barrier, the RPE is responsible for the phagocytosis of retinal waste. Second, along with Müller cells and the interphotoreceptor matrix, the RPE provides physical support to the photoreceptors. The apical microvilli of the RPE cells interact with the outer segments of the photoreceptors, providing them structural support. Third, the RPE plays a key role in the visual process by secreting proteins and processing visual cycle by-products. Through these three functions, the RPE and blood retinal barrier support a viable and functional neural retina. Because of its central role to normal retinal function, it is an attractive target for transplantation in the treatment of retinal diseases where the RPE and entire blood retinal barrier become dysfunctional (Figure 2).
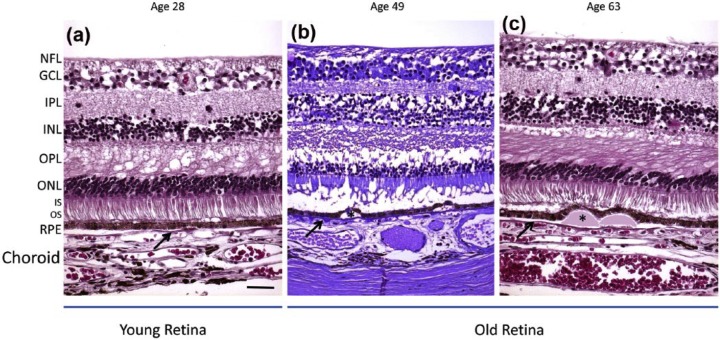
Figure 2.
Histological depiction of young and old retinas. (a) The young retina demonstrates normal retina layers. (b, c) The aged retinas show thinning of the outer nuclear layer (ONL). The aged retinas contain drusen (asterisks) displacing the RPE. The BM is marked with a black arrow. Scale bar: 50 μm. Paraffin sections cut at 4–6 μm.
Source: Reprinted from Ardeljan and Chan.7
NFL: nerve fiber layer; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; IS/OS: inner/outer segments of photoreceptors; RPE: retinal pigment epithelium.
Several changes to the components of the blood retinal barrier occur naturally during aging. These changes, which have been studied and characterized for close to 50 years, include an increase in BM thickness; appearance of coated membrane bodies, phospholipids, and/or drusen; loss of hydraulic conductivity; formation of advanced glycation end-products; or deposits of extracellular material beneath the RPE and within the BM.8–16 In addition to the dysfunction of the RPE and BM, the underlying choriocapillaris, the blood vessels that supply nutrients to the retina, are often damaged during AMD. This is due to the symbiotic relationship between the RPE, BM, and the blood vessels. Although the role of the choroid in AMD has been controversial, there have been observed changes with age. Several studies have observed marked decrease with age and in AMD patients.17–19 These transformations likely play a considerable role in RPE dysfunction in AMD; however, the primary cause of the disease is still unclear.
Cell-Based Therapies
Although the altered mechanical properties of the BM are concomitant to AMD rather than causal, any successful treatment should address these properties in the effort to restore a healthy retinal state. The importance of the BM’s mechanical properties is supported by studies that have demonstrated the inability of RPE cells to attach to a damaged BM. Tezel et al.20 cultured passage 1 human RPE cells on BM explants that were modified to mimic a diseased state. The explants were prepared by removing native RPE to expose the basal lamina. Following cell removal, the basal lamina was either left intact or mechanically and enzymatically debrided to expose the inner collagenous layer or the elastin layer. The RPE cell attachment rate, proliferation rate, and mitotic index were all higher on the basal lamina layer when compared to the deeper layers. In a study designed to determine whether attachment to the BM could be improved, the group seeded ARPE19, an established but non-immortalized cell line, and human fetal RPE cells on BM explants that were either unmodified or modified with fibronectin, laminin, and vitronectin.21 This study demonstrated no significant difference between cell types in their attachment to unmodified BMs, indicating that the age of the cell did not influence adherence as much as did the state of the BM.22 Furthermore, studies using modified BM explants showed characteristic RPE morphology and higher viability.21,23 Only cells that were seeded on these protein-enhanced membranes demonstrated substantial proliferation and coverage of RPE cells. The importance of the substrate condition was further confirmed in studies by Gullapalli et al.22,24 in which fetal human RPE cells showed poor cell coverage and morphology on unmodified submacular BMs of donors aged 55 years and older.
For decades, the therapeutic effects of transplanted RPE cells in delaying photoreceptor degeneration have been demonstrated in animal models. In 2001, Lund et al.25 showed significant rescue of visual function using a spontaneously derived cell line (ARPE19) and an extensively characterized genetically engineered human RPE cell line (h1RPE7) as assessed by behavioral or physiological techniques in the Royal College of Surgeons (RCS) rat model that demonstrates retinal degeneration due to the MERTK gene mutation. These positive results were demonstrated through 20 weeks or 140 days post-transplantation. In addition to this study, several other publications have shown similar findings.26–32 Wang et al.33 transplanted ARPE19 cells in the same model and demonstrated the ability of implanted cells to delay inner retinal degeneration. In the same study, ARPE19 cells were also used to preserve cortical visual function in RCS rats. More recently, researchers have turned to embryonic stem cell (ESC)-derived RPE cells. Lu et al.34 implanted these ESC-derived RPE in RCS rats. The cells were able to sustain visual function and photoreceptor integrity in a dose-dependent fashion. This long-term study demonstrated much of the same rescue as the previous studies and additionally, due to its later time points, revealed that the initial rescue began decreasing after post-transplantation day 90 (Figure 3). This reduced efficacy could be due to the injected ESC-derived RPE cells not interacting with the BM and thereby not forming a functional monolayer. The injected cells were observed above and adjacent to the native diseased RPE rather than penetrating and repairing the diseased RPE. Thus, the cells did not address the altered properties of the blood retinal barrier that are associated with retinal degeneration. These diseased-state RPE and BM properties must be addressed in order to promote an efficacious therapy in the long term.
Figure 3.
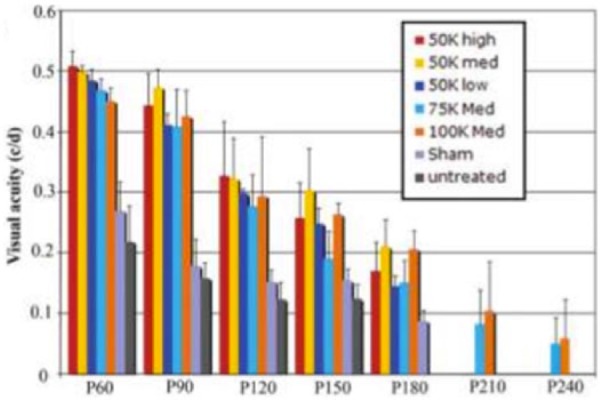
Batch and longevity of the effect of subretinally injected human embryonic stem cell–derived RPE as measured by visual acuity. Rescue of visual function decreases after day 90 and by day 240 only the high-dose groups still have low levels of visual acuity.
Source: Adapted from Lu et al.34
Scaffolds
The two main properties altered during retinal degeneration are the mechanical and transport properties of the BM. In the diseased state, the RPE monolayer is disrupted, which compromises cell–cell junctions and alters cell expression patterns and function on an individual cell basis. In addition, the BM displays a higher level of collagen cross-linking and higher lipid and membrane-coated body content. An optimized scaffold seeded with a mature RPE monolayer can mimic a healthy BM state and address the aforementioned issues associated with retinal degeneration.
Such is the rationale and motivation in exploring scaffolds for RPE cell transplantation. The general consensus is that the ideal scaffold will 35–37
Be biocompatible and not induce inflammation;
Promote and maintain a long-term healthy RPE phenotype;
Mimic healthy BM properties;
Be capable of being fabricated in optimal dimensions (5–90 µm);
Be mechanically robust enough to withstand manipulation during implantation.
In general, scaffolds are composed of materials derived from natural or synthetic sources or are a combination of the two. These are summarized in Table 1.
Table 1.
Summary of scaffolds used to support RPE cells.
| Scaffold material | Cell types | In vitro characterization/in vivo results | Refs | |
|---|---|---|---|---|
| Native membranes and explants | Anterior lens capsule | Aged human RPE; porcine RPE | In vitro: Over 94% cell viability. Confluent cells expressed F-actin and tight junctions In vivo: 2 weeks post-implantation, lens capsule well tolerated in subretinal space |
38–41 |
| BM explants layers | Fetal RPE | In vitro: Poor cell morphology and low cell density compared to controls In vivo: N/A |
20, 22 | |
| ECM-coated BM | Human fetal RPE; ARPE19 | In vitro: Cleaning explants and ECM protein coating decreased apoptosis, increased proliferation ratios, and formed monolayer after 17 days of culture In vivo: N/A |
21, 24, 42 | |
| Amniotic membrane | Primary rabbit RPE; human RPE | In vitro: RPE showed epithelial cell characteristic gene expression and morphological ultrastructure of including apical microvilli and tight junctions In vivo: N/A |
43–47 | |
| Descemet’s membrane | Porcine and bovine RPE | In vitro: RPE cells formed intact monolayer and characteristic apical microvilli In vivo: N/A |
48 | |
| Natural materials | Collagen | ARPE19, human primary RPE | In vitro: RPE demonstrated hexagonal, cobblestone morphology with F-actin rings, and tight junctions after 9 days of culture; cells could phagocytose In vivo: N/A |
49 |
| Fibrinogen | Human fetal RPE | In vitro: N/A In vivo: cell-seeded fibrinogen matrix in rabbits had photoreceptor loss near transplanted tissue. cell-seeded matrix had minimal choroidal thickening and inflammation |
50 | |
| Gelatin | Porcine RPE sheets | In vitro: N/A In vivo: Subretinal transplants into domestic pigs. Multilayer transplant sheets with outer segment shortening. Cells synthesized basement membrane |
51 | |
| Silk fibroin | ARPE19, human primary RPE | In vitro: cells with characteristic cobblestone morphology after 8 weeks In vivo: N/A |
52–54 | |
| Cryoprecipitate | Human fetal RPE | In vitro: Hexagonal shape, tight junctions, and clear monolayer seen on fiber scaffolds compared to film or glass; phagocytosis ability seen using latex beads In vivo: N/A |
55 | |
| Bacterial cellulose | h-TERT immortalized RPE | In vitro: Acetylated bacterial cellulose demonstrated higher cell adhesion and proliferation compared to unmodified bacterial cellulose In vivo: N/A |
56 | |
| Synthetic polymers | PLGA | Human primary RPE | In vitro: Hexagonal shape, tight junctions, and clear monolayer on fiber scaffolds compared to film or glass; phagocytosis ability seen using latex beads In vivo: N/A |
57, 58 |
| Parylene C | ARPE19; ESC-derived RPE | In vitro: cells on Matrigel-coated scaffolds had hexagonal shape, pigmentation, tight cell–cell junctions, apical microvilli after 4 weeks In vivo: cells transplanted on scaffold had higher survival compared to free cells in athymic nude rats. More macrophages with scaffolds present |
59, 60 | |
| PLLA/PLGA | Mouse RPC | In vitro: Immature RPC markers expression levels decreased after 7 days of culture In vivo: Estimated >50% survival of grafted cells |
58, 61–63 | |
| PCL | Mouse RPC | In vitro: Increased cell attachment, recoverin, rhodopsin, GFAP upregulation; SOX2 downregulation In vivo: N/A |
37 | |
| PEGDMA | Aged human RPE; porcine RPE | In vitro: Over 90% viability; confluent cells expressed F-actin and tight junction In vivo: N/A |
41 | |
| PTMC | Human ESC–RPE | In vitro: PTMC scaffolds supported the maturation of human ESC–RPE promoting a confluent monolayer of cells and RPE-specific gene expression In vivo: N/A |
59 | |
| PDMS | iPSC-derived RPE | In vitro: PDMS enhanced attachment, proliferation, polarization, and maturation of cells In vivo: Subretinal scaffold implantation in porcine eyes showed biocompatibility and ERG revealed preserved macular function up to 2 years after implantation |
64 | |
| Combination scaffolds | Chitosan–PCL/PCL | Mouse RPC | In vitro: Promoted proliferation and differentiation of cells In vivo: N/A |
65 |
| Silk fibroin, PCL, gelatin | Primary adult human RPE | In vitro: Higher cell growth rate and higher expression of characteristic RPE genes compared to PCL and PCL-silk scaffolds In vivo: Subscleral implantation with no inflammation or rejection |
66 |
RPE: retinal pigment epithelium; BM: Bruch’s membrane; ECM: extracellular matrix; h-TERT: human telomerase reverse transcriptase; PLGA: poly(lactic-co-glycolic acid); PLLA: poly(l-lactic acid); RPC: retinal progenitor cell; PCL: poly(caprolactone); GFAP: glial fibrillary acidic protein; SOX2: sex determining region Y-box 2; PEGDMA: polyethylene glycol dimethacrylate; PTMC: poly(trimethylene) carbonate; PDMS: polydimethylsiloxane; iPSC: induced pluripotent stem cell; ERG: electroretinogram.
Natural materials
Bruch’s membrane and other naturally occurring membranes
As with most transplants, one of the first options investigated for RPE transplantation was its native basement membrane, an autologous (e.g. translocated explants) BM. However, the aforementioned age-related changes in the BM present a hurdle for their use.
A unique approach to modifying BM explants involved first seeding corneal endothelial cells on a BM explant, allowing the seeded cells to deposit an extracellular matrix (ECM), removing the corneal cells, and then seeding RPE cells on the deposited ECM.42 This cell-deposited matrix led to significant RPE nuclear density when seeded on aged submacular BM compared to untreated age-matched BM controls. In addition to BM explants, anterior lens capsules as scaffolds for RPE cells have been investigated.38–41 This elastic membrane sits at the back of the lens anterior to the vitreous humor. Similar to the BM, it supports a monolayer of epithelial cells. When compared to synthetic polymer hydrogels, porcine anterior lens capsules supported higher cell density and viability than the hydrogel scaffolds.41 Several other naturally occurring membranes have been investigated for their potential as RPE cell scaffolds, including human amniotic membrane, Descemet’s membrane, and the inner limiting membrane of the retina, and all demonstrated the ability to support characteristic RPE morphology and expression in seeded RPE cells.43–48,67
Because AMD presents with high levels of degeneration in the macula that decrease toward the periphery, translocation of a full thickness choroid–BM–RPE complex has been attempted. This has mainly been performed in patients with the exudative, wet form of AMD but has also been attempted with dry AMD. The translocated grafts show revascularization and delayed degeneration; however, surgical complications remain high and visual improvement has been limited.68–70 In a long-term study by Zeeburg et al., 133 eyes with exudative AMD underwent a graft of the peripheral RPE–choroid complex. Prior to surgery, the average best corrected visual acuity (BCVA) was 20/250. At 4 years post-surgery, 15% of the eyes had BCVA worse than 20/200 and 5% had BCVA worse than or equal to 20/40.71 Although the improvement in some participants’ visual acuity is noteworthy, it is important to look at the bulk of the data which indicates that a vast majority, or 85%, of treated eyes were measured with BCVAs worse than 20/200, which is the cut-off for being characterized as legally blind. Obviously, there is still much progress to be made.
Using natural membranes has its benefits, for instance, they contain the proper native ultrastructure and biochemical cues. However, donor variability and limited material availability motivate the use of non-membranous polymer materials, both natural and synthetic, that can be fabricated into the desired dimensions.
Natural polymer scaffolds
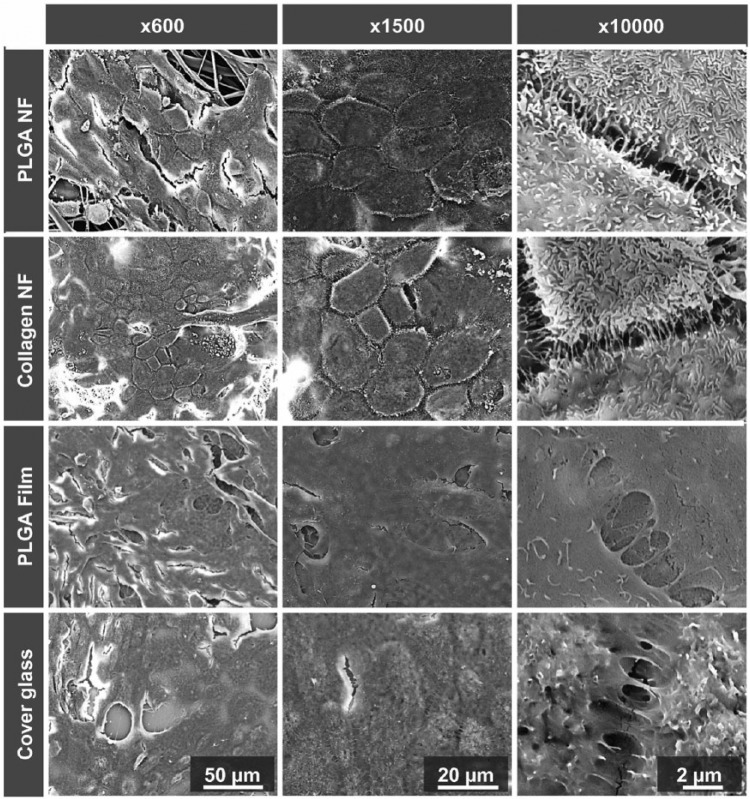
Natural polymers are an attractive option for a number tissue engineering scaffolds. Because the BM consists of various types of collagens, collagen is the most studied natural polymer for BM scaffolds. Collagen shows great promise as a scaffold for many reasons, including its lending itself to a variety of fabrication techniques. As previously mentioned, the ideal scaffold will have similar dimensions to the natural BM, ideally less than 10 µm thick. In 2007, Lu et al. used thin film collagen scaffolds for the culture of RPE cells. These thin films had a thickness of 2.4 ± 0.2 μm and were able to maintain both cell viability and characteristic cell morphology.49 Warnke et al.57 compared thin films to electrospun nanofiber collagen scaffolds and on these nanofiber scaffolds demonstrated better morphology of RPE cells, including more defined apical microvilli, a strong indicator of the health of RPE cells (Figure 4).
Figure 4.
SEM images of RPE cells on PLGA and collagen nanofibrillar membranes (NF), PLGA films and cover glass after 11 days. The RPE cells on NF membranes form an in vivo–like monolayer. Cells on NF membranes also demonstrate long, sheet-like microvilli, while cells on flat surfaces appear less organized.
Source: Reprinted from Warnke et al.55
Interestingly, these collagen nanofiber scaffolds did not show significant differences compared to poly(lactic-co-glycolic acid) (PLGA) nanofiber scaffolds.57 Although collagen is the most highly investigated scaffold material, human cryoprecipitate, gelatin, and crosslinked fibrinogen scaffolds have also been investigated with some promising results.50,51,55,72 Cryoprecipitate offers a unique benefit in that it can be harvested from the patient’s own blood, removing the risk of rejection. Farrokh-Siar et al.55 seeded cryoprecipitate membranes with fetal RPE sheets. The sheets maintained their morphology and proliferated during culture. Both fibrinogen and gelatin were evaluated in vivo in rabbits and pigs, respectively. The crosslinked fibrinogen was prepared into microspheres and seeded with human fetal RPE cells, which survived up to 1 month. However, there was evidence of a mild local inflammatory response. In the Del Priore study that used gelatin, there was a presence of macrophage or macrophage-like cells in the retina, as well as lymphatic cells within the lumen of the choriocapillaris blood vessels underlying the transplant site.51 These indicators of immune response serve as a predictor of the death of the transplanted RPE cells. Reducing the expression of inflammatory cytokines and recruitment of immune cells should be considered during scaffold design.
Silk fibroin is another natural material that has shown promise as a substrate for ocular tissue engineering applications.52–54 Silk fibroin can be obtained from a variety of insect sources such as Bombyx mori and Antheraea pernyi. Shadford et al. evaluated Bombyx mori silk fibroin membranes for culturing ARPE19 cells. These porous scaffolds supported the cells, which maintained their characteristic cobblestone morphology and tight junctions. The fibroin membranes demonstrated similar results with primary RPE cells, though it took longer for the cells to develop the characteristic traits.53 A more recent approach to enhance cell attachment utilized bacterial cellulose coated with urinary bladder matrix, which promoted a cell phenotype comparable to that of native RPE with its characteristic epithelial morphology and characteristic protein expression.56
While these natural polymers have the benefits of biocompatibility and biochemical cues present in the natural extracellular environment, serious drawbacks such as issues with product purity, disease transmission, immune response, and difficulty in functionalization or modification do arise.
Synthetic polymer scaffolds
There have been several synthetic polymers investigated for use as a BM scaffold including poly(l-lactic acid) (PLLA), PLGA, PLLA–PLGA copolymer systems, poly(caprolactone) (PCL), methacrylated hydrogels, polydimethylsiloxane (PDMS), and parylene-C. PLLA and PLGA scaffolds were among the first materials to be investigated for RPE cell delivery and have been investigated by many groups.58,61–64 These scaffolds, mostly fabricated through solvent casting into thin films, have been seeded with D407 RPE cells, human fetal RPE cells, and porcine RPE cells. These scaffolds have repeatedly demonstrated the ability to support viable RPE cells while maintaining their proper morphology and phenotype.36,58,61,62,73 Porous PCL, fabricated using photolithography and ion etching to create a scaffold mold, demonstrated improved markers of maturity and function of seeded fetal human RPE cells compared to non-porous PCL and porous polyester transwells.37 Singh et al.41 compared methacrylate/methacrylamide copolymer hydrogels directly to porcine lens and found each scaffold supported similar cellular densities for both human and porcine RPE cells. The cells also maintained their phenotype and formed monolayers on both materials. More recently, Sorkio et al. used a thin film scaffold of poly(trimethylene) carbonate (PTMC). These PTMC scaffolds were able to support the maturation of human ESC–RPE and promote a confluent monolayer of these cells all while maintaining their RPE-specific gene expression.74
The use of synthetic polymers allows for more control over scaffold parameters such as mechanical and transport properties and degradation characteristics. While degradation may be desirable, the ideal degradation rate has not yet been identified since it depends both on the ability of RPE cells to generate their own matrix and on the state of the BM at the time of cell transplantation. Many synthetic materials have been investigated as scaffolds for RPE cell implantation, though no single material has jumped to the forefront of the field since positive results such as high cell viability, characteristic expression, and cell markers can be obtained on several materials. Besides material selection itself, the scaffold design parameters such as scaffold thickness and transport properties, and the ability to promote cell adhesion, appear to be the most important factors in controlling RPE fate and scaffold success in animal studies.
One of the most promising synthetic polymer scaffolds reported is fabricated with soft lithography using parylene-C.59 This sub-micron mesh scaffold, supported by a 6-μm frame, is designed to mimic BM transport properties and is able to support RPE cells in vitro. These scaffolds were seeded with RPE cells, then implanted into the subretinal space of athymic nude rats. When compared to scaffold-free cell suspensions, cells transplanted on parylene-C scaffolds survived in greater numbers. However, an infiltration of macrophages was observed to a higher extent when scaffolds were present.59,60
Combination scaffolds
Many RPE scaffolds fall into the category of natural or synthetic polymers; however, some scaffolds exploit properties of both. Although many synthetic polymer scaffolds are modified with cell adhesion peptides, these are not true combination scaffolds since the bulk material of the scaffold that defines its mechanical and transport properties is the synthetic polymer. The combination approach uses both natural and synthetic materials to compose the bulk of the scaffold, with each material contributing to the scaffold properties. The combination approach provides the advantage of controlling scaffold properties with the synthetic component, while gaining natural cues such as proteins, proteoglycans, and glycosaminoglycans from the natural polymer.
Combination scaffolds have demonstrated success in their ability to grow and maintain retinal progenitor cells and RPE cells in vitro and in vivo. Using electrospinning, Chen et al.65 fabricated chitosan-graft-PCL/PCL hybrid scaffolds. These scaffolds promoted the proliferation and differentiation of mouse retinal progenitor cells. Another novel approach taken by Xiang et al. resulted in an ultrathin porous nanofibrous membrane of Antheraea pernyi silk fibroin, PCL, and gelatin.66 This 3- to 5-μm scaffold induced a higher cell growth rate and a higher expression of characteristic RPE genes in primary human RPE compared to when these cells were seeded on PCL alone or PCL–silk fibroin scaffolds. Subscleral implantation of these combination scaffolds demonstrated biocompatibility with no evidence of inflammation or rejection.
The combination approach using natural and synthetic polymers to optimize cell growth and expression while also controlling the mechanical, transport, and degradation properties has been explored in other areas that use cell scaffolds, but in RPE tissue engineering, this approach is a novel and promising one. The hurdle of reproducibility when using natural polymers still exists in a combination approach. Future work should address this challenge while also characterizing cells to ensure optimal clinical results.75
Scaffold surface design and modification
In addition to scaffold dimension and transport property design, scaffold surface design and modification, specifically topographical cues and plasma treatment, have been investigated. The most common topography for fabricated RPE scaffolds is that of electrospun nanofibers.75 Depending on the parameters used during fabrication, nanofiber diameter can be controlled. Lui et al. determined that the optimal nanofiber diameter was 200 nm. In addition, Steedman et al.76 demonstrated that retinal progenitor cells had significantly higher adherence to PCL scaffolds with a surface microtopography compared to PCL with a smooth surface or tissue culture polystyrene.
In addition to topography, surface modification to enhance hydrophilicity is a common approach to encourage cell attachment and spreading. Oxygen, air, and ammonia gas plasma treatments to increase scaffold hydrophilicity have all induced a variety of positive effects in cells cultured on these scaffolds. For instance, oxygen plasma–treated poly(hydroxybutyrate-co-hydroxyvalerate) thin film scaffolds investigated by Tezcaner et al.77 demonstrated that hydrophilicity increased with increasing oxygen treatment, while surface roughness decreased. The oxygen treatment increased attachment and spreading of D407 RPE cells. However, this improvement was modest and not statistically significant. Williams et al.78 investigated commercially available polyurethanes treated with air plasma to increase the substrate wettability. Prior to treatment, only a few ARPE19 cells attached and those that did remained aggregated. However, after treatment cells grew into a monolayer with the characteristic RPE cobblestone morphology.78 ARPE19 cells were also used by Krishna et al.79 on ammonia plasma–treated expanded polytetrafluoroethylene scaffolds. The ammonia treatment resulted in enhanced growth and monolayer formation with phagocytic ability. Recently, Shahmoradi et al. investigated the effects of both the morphology and the hydrophilicity of a PCL scaffold. This study determined that a lower fiber diameter of 185.8 nm, close to the 200-nm optimal diameter determined by Lui et al., and an increased hydrophilicity were best for the culture of human RPE cells.80
Future considerations and conclusion
Despite current clinical trials where free cell suspensions are transplanted into AMD-damaged RPE layers, there is an ongoing need to rebuild a healthy blood retinal barrier in order for cell therapy to be a successful long-term treatment for AMD. A major hurdle that must be overcome is inflammation. While the retina possesses qualities of an immune privileged site, this is only true when a healthy RPE and BM are present. This is not the case during AMD, and therefore in many in vivo studies there is considerable macrophage infiltration. Many of the scaffolds discussed have not yet been evaluated in vivo. Of the scaffolds that have reported results in animal models, the animals are often athymic and therefore inflammation is not studied. Once further studies are performed, it will be interesting to see how scaffold material plays a role in inflammation. Previous reports show inflammation following RPE grafts despite immunosuppression. Crafoord et al.81 reported no significant difference between cyclosporine-treated rabbits and cyclosporine-untreated rabbits 6 months after RPE graft transplantation. Both treatment groups showed macrophage invasion and photoreceptor damage. Zhang and Bok82 investigated the immune response in the subretinal space following RPE transplantation. The investigators used two RCS rat strains, BD IX and LEJ, that have incompatible major histocompatibility complexes (MHC). This study demonstrated that chronic rejection of RPE grafts in rats is mediated by the MHC. Mechanistic studies suggest that interferon gamma (IFN-γ) activation of the RPE leads to increased MHC class II expression. When IFN-γ was used to stimulate cells prior to implantation, those cells were acutely rejected.83 Therefore, future scaffolds should downregulate the expression of this cytokine, as well as other inflammatory markers that have previously been characterized in RPE cells.84–88 These cytokines include tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), and monocyte chemoattractant protein 1 (MCP-1). Liu et al.89 demonstrated a significant correlation between the expression of these cytokine genes and the activation of microglial cells in the retina following retinal detachment in rats.
Another challenge to the development of a long-term treatment of AMD concerns the damaged underlying blood vessels of the choriocapillaris. With the thinning of these blood vessels and an insufficient nutrient supply to any implanted scaffolds and seeded RPE cells, it is likely that a dysfunctional or diseased phenotype will eventually present in implanted cells. Scaffold design that promotes healthy vessel formation, slows thinning of the aged-choroid, and potentially delivers therapeutics must be investigated further in order to establish the symbiotic relationship that maintains healthy RPE and choroid epithelial cells.
Much progress has been made in the development of a cell therapy for the treatment of AMD in the past several decades. Using transplanted cells, there has been partial visual rescue or delayed degeneration in several animal models and even in human clinical trials. However, most of the previous results motivate the development of scaffolds to better support cell transplantation and re-establish a healthy blood retinal barrier. Design parameters that include material type, scaffold mechanical properties, and surface modification will play an important role in overcoming the hurdles that the field is currently facing.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.E.W. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 GM8339 from the NIGMS.
References
- 1. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96(5): 614–618. [DOI] [PubMed] [Google Scholar]
- 2. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82(11): 844–851. [PMC free article] [PubMed] [Google Scholar]
- 3. Binder S, Stanzel BV, Krebs I, et al. Transplantation of the RPE in AMD. Prog Retin Eye Res 2007; 26(5): 516–554. [DOI] [PubMed] [Google Scholar]
- 4. Gouras P, Flood MT, Kjeldbye H. Transplantation of cultured human retinal cells to monkey retina. An Acad Bras Cienc 1984; 56(4): 431–443. [PubMed] [Google Scholar]
- 5. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 2012; 379(9817): 713–720. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015; 385(9967): 509–516. [DOI] [PubMed] [Google Scholar]
- 7. Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 2013; 37: 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hogan MJ. Bruch’s membrane and disease of the macula. Role of elastic tissue and collagen. Trans Ophthalmol Soc U K 1967; 87: 113–161. [PubMed] [Google Scholar]
- 9. Killingsworth MC. Age-related components of Bruch’s membrane in the human eye. Graefes Arch Clin Exp Ophthalmol 1987; 225(6): 406–412. [DOI] [PubMed] [Google Scholar]
- 10. Pauleikhoff D, Barondes MJ, Minassian D, et al. Drusen as risk factors in age-related macular disease. Am J Ophthalmol 1990; 109(1): 38–43. [DOI] [PubMed] [Google Scholar]
- 11. Pauleikhoff D, Harper CA, Marshall J, et al. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology 1990; 97(2): 171–178. [PubMed] [Google Scholar]
- 12. Piguet B, Wells JA, Palmvang IB, et al. Age-related Bruch’s membrane change: a clinical study of the relative role of heredity and environment. Br J Ophthalmol 1993; 77(7): 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okubo A, Rosa RH, Jr, Bunce CV, et al. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Invest Ophthalmol Vis Sci 1999; 40(2): 443–449. [PubMed] [Google Scholar]
- 14. Sarks SH, Arnold JJ, Killingsworth MC, et al. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol 1999; 83(3): 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curcio CA, Millican CL, Bailey T, et al. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci 2001; 42(1): 265–274. [PubMed] [Google Scholar]
- 16. Ruberti JW, Curcio CA, Millican CL, et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch’s membrane. Invest Ophthalmol Vis Sci 2003; 44(4): 1753–1759. [DOI] [PubMed] [Google Scholar]
- 17. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 2009; 147(5): 811–815. [DOI] [PubMed] [Google Scholar]
- 18. Ding X, Li J, Zeng J, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci 2011; 52(13): 9555–9560. [DOI] [PubMed] [Google Scholar]
- 19. Spencer C, Abend S, McHugh KJ, et al. Identification of a synergistic interaction between endothelial cells and retinal pigment epithelium. J Cell Mol Med. Epub ahead of print 12 April 2017. DOI: 10.1111/jcmm.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tezel TH, Kaplan HJ, Del Priore LV. Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Invest Ophthalmol Vis Sci 1999; 40(2): 467–476. [PubMed] [Google Scholar]
- 21. Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Invest Ophthalmol Vis Sci 2004; 45(9): 3337–3348. [DOI] [PubMed] [Google Scholar]
- 22. Gullapalli VK, Sugino IK, Van Patten Y, et al. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res 2005; 80(2): 235–248. [DOI] [PubMed] [Google Scholar]
- 23. Del Priore LV, Tezel TH. Reattachment rate of human retinal pigment epithelium to layers of human Bruch’s membrane. Arch Ophthalmol 1998; 116(3): 335–341. [DOI] [PubMed] [Google Scholar]
- 24. Gullapalli VK, Sugino IK, Van Patten Y, et al. Retinal pigment epithelium resurfacing of aged submacular human Bruch’s membrane. Trans Am Ophthalmol Soc 2004; 102: 123–137; discussion 137–138. [PMC free article] [PubMed] [Google Scholar]
- 25. Lund RD, Adamson P, Sauvé Y, et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc Natl Acad Sci U S A 2001; 98(17): 9942–9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saigo Y, Abe T, Hojo M, et al. Transplantation of transduced retinal pigment epithelium in rats. Invest Ophthalmol Vis Sci 2004; 45(6): 1996–2004. [DOI] [PubMed] [Google Scholar]
- 27. Abe T, Tomita H, Kano T, et al. Autologous iris pigment epithelial cell transplantation in monkey subretinal region. Curr Eye Res 2000; 20(4): 268–275. [PubMed] [Google Scholar]
- 28. Li LX, Turner JE. Transplantation of retinal pigment epithelial cells to immature and adult rat hosts: short- and long-term survival characteristics. Exp Eye Res 1988; 47(5): 771–785. [DOI] [PubMed] [Google Scholar]
- 29. Arnhold S, Heiduschka P, Klein H, et al. Adenovirally transduced bone marrow stromal cells differentiate into pigment epithelial cells and induce rescue effects in RCS rats. Invest Ophthalmol Vis Sci 2006; 47(9): 4121–4129. [DOI] [PubMed] [Google Scholar]
- 30. Lopez R, Gouras P, Kjeldbye H, et al. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci 1989; 30(3): 586–588. [PubMed] [Google Scholar]
- 31. Lavail MM, Li L, Turner JE, et al. Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Exp Eye Res 1992; 55(4): 555–562. [DOI] [PubMed] [Google Scholar]
- 32. Sauve Y, Klassen H, Whiteley SJ, et al. Visual field loss in RCS rats and the effect of RPE cell transplantation. Exp Neurol 1998; 152(2): 243–250. [DOI] [PubMed] [Google Scholar]
- 33. Wang S, Lu B, Wood P, et al. Grafting of ARPE-19 and Schwann cells to the subretinal space in RCS rats. Invest Ophthalmol Vis Sci 2005; 46(7): 2552–2560. [DOI] [PubMed] [Google Scholar]
- 34. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009; 27(9): 2126–2135. [DOI] [PubMed] [Google Scholar]
- 35. Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol 2010; 248(6): 763–778. [DOI] [PubMed] [Google Scholar]
- 36. Lu L, Yaszemski MJ, Mikos AG. Retinal pigment epithelium engineering using synthetic biodegradable polymers. Biomaterials 2001; 22(24): 3345–3355. [DOI] [PubMed] [Google Scholar]
- 37. McHugh KJ, Tao SL, Saint-Geniez M. Porous poly(ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Invest Ophthalmol Vis Sci 2014; 55(3): 1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartmann U, Sistani F, Steinhorst UH. Human and porcine anterior lens capsule as support for growing and grafting retinal pigment epithelium and iris pigment epithelium. Graefes Arch Clin Exp Ophthalmol 1999; 237(11): 940–945. [DOI] [PubMed] [Google Scholar]
- 39. Lee CJ, Vroom JA, Fishman HA, et al. Determination of human lens capsule permeability and its feasibility as a replacement for Bruch’s membrane. Biomaterials 2006; 27(8): 1670–1678. [DOI] [PubMed] [Google Scholar]
- 40. Nicolini J, Kiilgaard JF, Wiencke AK, et al. The anterior lens capsule used as support material in RPE cell-transplantation. Acta Ophthalmol Scand 2000; 78(5): 527–531. [DOI] [PubMed] [Google Scholar]
- 41. Singh S, Woerly S, McLaughlin BJ. Natural and artificial substrates for retinal pigment epithelial monolayer transplantation. Biomaterials 2001; 22(24): 3337–3343. [DOI] [PubMed] [Google Scholar]
- 42. Sugino IK, Rapista A, Sun Q, et al. A method to enhance cell survival on Bruch’s membrane in eyes affected by age and age-related macular degeneration. Invest Ophthalmol Vis Sci 2011; 52(13): 9598–9609. [DOI] [PubMed] [Google Scholar]
- 43. Capeans C, Piñeiro A, Pardo M, et al. Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand 2003; 81(3): 271–277. [DOI] [PubMed] [Google Scholar]
- 44. Ohno-Matsui K, Ichinose S, Nakahama K, et al. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Mol Vis 2005; 11: 1–10. [PubMed] [Google Scholar]
- 45. Ohno-Matsui K, Mori K, Ichinose S, et al. In vitro and in vivo characterization of iris pigment epithelial cells cultured on amniotic membranes. Mol Vis 2006; 12: 1022–1032. [PubMed] [Google Scholar]
- 46. Singhal S, Vemuganti GK. Primary adult human retinal pigment epithelial cell cultures on human amniotic membranes. Indian J Ophthalmol 2005; 53(2): 109–113. [DOI] [PubMed] [Google Scholar]
- 47. Stanzel BV, Espana EM, Grueterich M, et al. Amniotic membrane maintains the phenotype of rabbit retinal pigment epithelial cells in culture. Exp Eye Res 2005; 80(1): 103–112. [DOI] [PubMed] [Google Scholar]
- 48. Thumann G, Schraermeyer U, Bartz-Schmidt KU, et al. Descemet’s membrane as membranous support in RPE/IPE transplantation. Curr Eye Res 1997; 16(12): 1236–1238. [DOI] [PubMed] [Google Scholar]
- 49. Lu JT, Lee CJ, Bent SF, et al. Thin collagen film scaffolds for retinal epithelial cell culture. Biomaterials 2007; 28(8): 1486–1494. [DOI] [PubMed] [Google Scholar]
- 50. Oganesian A, Gabrielian K, Ernest JT, et al. A new model of retinal pigment epithelium transplantation with microspheres. Arch Ophthalmol 1999; 117(9): 1192–1200. [DOI] [PubMed] [Google Scholar]
- 51. Del Priore LV, Tezel TH, Kaplan HJ. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest Ophthalmol Vis Sci 2004; 45(3): 985–992. [DOI] [PubMed] [Google Scholar]
- 52. Chirila T, Barnard Z, Zainuddin, et al. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng Part A 2008; 14(7): 1203–1211. [DOI] [PubMed] [Google Scholar]
- 53. Shadforth AM, George KA, Kwan AS, et al. The cultivation of human retinal pigment epithelial cells on Bombyx mori silk fibroin. Biomaterials 2012; 33(16): 4110–4117. [DOI] [PubMed] [Google Scholar]
- 54. Harkin DG, George KA, Madden PW, et al. Silk fibroin in ocular tissue reconstruction. Biomaterials 2011; 32(10): 2445–2458. [DOI] [PubMed] [Google Scholar]
- 55. Farrokh-Siar L, Rezai KA, Patel SC, et al. Cryoprecipitate: An autologous substrate for human fetal retinal pigment epithelium. Curr Eye Res 1999; 19(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 56. Goncalves S, Rodrigues IP, Padrão J, et al. Acetylated bacterial cellulose coated with urinary bladder matrix as a substrate for retinal pigment epithelium. Colloids Surf B Biointerfaces 2016; 139: 1–9. [DOI] [PubMed] [Google Scholar]
- 57. Warnke PH, Alamein M, Skabo S, et al. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater 2013; 12: 9414–9422. [DOI] [PubMed] [Google Scholar]
- 58. Lu L, Garcia CA, Mikos AG. Retinal pigment epithelium cell culture on thin biodegradable poly(DL-lactic-co-glycolic acid) films. J Biomater Sci Polym Ed 1998; 9(11): 1187–1205. [DOI] [PubMed] [Google Scholar]
- 59. Lu B, Zhu D, Hinton D, et al. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices 2012; 14(4): 659–667. [DOI] [PubMed] [Google Scholar]
- 60. Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 2013; 54(7): 5087–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Giordano GG, Thomson RC, Ishaug SL, et al. Retinal pigment epithelium cells cultured on synthetic biodegradable polymers. J Biomed Mater Res 1997; 34(1): 87–93. [DOI] [PubMed] [Google Scholar]
- 62. Hadlock T, Singh S, Vacanti JP, et al. Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng 1999; 5(3): 187–196. [DOI] [PubMed] [Google Scholar]
- 63. Lu L, Nyalakonda K, Kam L, et al. Retinal pigment epithelial cell adhesion on novel micropatterned surfaces fabricated from synthetic biodegradable polymers. Biomaterials 2001; 22(3): 291–297. [DOI] [PubMed] [Google Scholar]
- 64. Peng CH, Chuang JH, Wang ML, et al. Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget 2016; 40: 64631–64648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen H, Fan X, Xia J, et al. Electrospun chitosan-graft-poly (varepsilon-caprolactone)/poly (varepsilon-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int J Nanomedicine 2011; 6: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xiang P, Wu KC, Zhu Y, et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014; 35(37): 9777–9788. [DOI] [PubMed] [Google Scholar]
- 67. Beutel J, Greulich L, Lüke M, et al. Inner limiting membrane as membranous support in RPE sheet-transplantation. Graefes Arch Clin Exp Ophthalmol 2007; 245(10): 1469–1473. [DOI] [PubMed] [Google Scholar]
- 68. Joussen AM, Heussen FM, Joeres S, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol 2006; 142(1): 17–30. [DOI] [PubMed] [Google Scholar]
- 69. Van Meurs JC, ter Averst E, van Hagen PM, et al. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol 2004; 88(1): 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. MacLaren RE, Uppal GS, Balaggan KS, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology 2007; 114(3): 561–570. [DOI] [PubMed] [Google Scholar]
- 71. van Zeeburg EJ, Maaijwee KJ, Missotten TO, et al. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am J Ophthalmol 2012; 153(1): 120–127.e2. [DOI] [PubMed] [Google Scholar]
- 72. Tezel TH, Del Priore LV. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 1997; 235(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 73. Thomson RC, Giordano GG, Collier JH, et al. Manufacture and characterization of poly(alpha-hydroxy ester) thin films as temporary substrates for retinal pigment epithelium cells. Biomaterials 1996; 17(3): 321–327. [DOI] [PubMed] [Google Scholar]
- 74. Sorkio A, Haimi S, Verdoold V, et al. Poly(trimethylene carbonate) as an elastic biodegradable film for human embryonic stem cell-derived retinal pigment epithelial cells. J Tissue Eng Regen Med. Epub ahead of print 4 January 2017. DOI: 10.1002/term.2221. [DOI] [PubMed] [Google Scholar]
- 75. Hotaling NA, Khristov V, Wan Q, et al. Nanofiber Scaffold-based tissue-engineered retinal pigment epithelium to treat degenerative eye diseases. J Ocul Pharmacol Ther 2016; 32(5): p. 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Steedman MR, Tao SL, Klassen H, et al. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices 2010; 12(3): 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tezcaner A, Bugra K, Hasirci V. Retinal pigment epithelium cell culture on surface modified poly(hydroxybutyrate-co-hydroxyvalerate) thin films. Biomaterials 2003; 24(25): 4573–4583. [DOI] [PubMed] [Google Scholar]
- 78. Williams RL, Krishna Y, Dixon S, et al. Polyurethanes as potential substrates for sub-retinal retinal pigment epithelial cell transplantation. J Mater Sci Mater Med 2005; 16(12): 1087–1092. [DOI] [PubMed] [Google Scholar]
- 79. Krishna Y, Sheridan C, Kent D, et al. Expanded polytetrafluoroethylene as a substrate for retinal pigment epithelial cell growth and transplantation in age-related macular degeneration. Br J Ophthalmol 2011; 95(4): 569–573. [DOI] [PubMed] [Google Scholar]
- 80. Shahmoradi S, Yazdian F, Tabandeh F, et al. Controlled surface morphology and hydrophilicity of polycaprolactone toward human retinal pigment epithelium cells. Mater Sci Eng C Mater Biol Appl 2017; 73: 300–309. [DOI] [PubMed] [Google Scholar]
- 81. Crafoord S, Algvere PV, Kopp ED, et al. Cyclosporine treatment of RPE allografts in the rabbit subretinal space. Acta Ophthalmol Scand 2000; 78(2): 122–129. [DOI] [PubMed] [Google Scholar]
- 82. Zhang X, Bok D. Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci 1998; 39(6): 1021–1027. [PubMed] [Google Scholar]
- 83. Liversidge J, Forrester JV. Antigen processing and presentation in the eye: a review. Curr Eye Res 1992; 11: 49–58. [DOI] [PubMed] [Google Scholar]
- 84. Jaffe GJ, Peters WP, Roberts W, et al. Modulation of macrophage colony stimulating factor in cultured human retinal pigment epithelial cells. Exp Eye Res 1992; 54(4): 595–603. [DOI] [PubMed] [Google Scholar]
- 85. Jaffe GJ, Van Le L, Valea F, et al. Expression of interleukin-1 alpha, interleukin-1 beta, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp Eye Res 1992; 55(2): 325–335. [DOI] [PubMed] [Google Scholar]
- 86. Elner SG, Delmonte D, Bian ZM, et al. Differential expression of retinal pigment epithelium (RPE) IP-10 and interleukin-8. Exp Eye Res 2006; 83(2): 374–379. [DOI] [PubMed] [Google Scholar]
- 87. Elner VM, Scales W, Elner SG, et al. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res 1992; 54(3): 361–368. [DOI] [PubMed] [Google Scholar]
- 88. Cao S, Walker GB, Wang X, et al. Altered cytokine profiles of human retinal pigment epithelium: oxidant injury and replicative senescence. Mol Vis 2013; 19: 718–728. [PMC free article] [PubMed] [Google Scholar]
- 89. Liu Y, Yang X, Utheim TP, et al. Correlation of cytokine levels and microglial cell infiltration during retinal degeneration in RCS rats. PLoS ONE 2013; 8(12): e82061. [DOI] [PMC free article] [PubMed] [Google Scholar]