Abstract
It is now recognized that the immune system can be a key component of restraint and control during the neoplastic process. Human papillomavirus (HPV)-associated cancers of the anogenital tract and oropharynx represent a significant clinical problem but there is a clear opportunity for immune targeting of the viral oncogene expression that drives cancer development. However, high-risk HPV infection of the target epithelium and the expression of the E6/E7 oncogenes can lead to early compromise of the innate immune system (loss of antigen-presenting cells) facilitating viral persistence and increased risk of cancer. In these circumstances, a succession of interacting and self-reinforcing events mediated through modulation of different immune receptors, chemokine and cytokine responses (CCL20; CCL2; CCR2; IL-6; CCR7; IL-12) further promote the generation of an immune suppressive microenvironment [increased levels of Tregs, Th17, myeloid-derived suppressor cells (MDSCs) and PD-L1]. The overexpression of E6/E7 expression also compromises the ability to repair cellular DNA, leading to genomic instability, with the acquisition of genetic changes providing for the selection of advantaged cancer cells including additional strategies for immune escape. Therapeutic vaccines targeting the HPV oncogenes have shown some encouraging success in some recent early-phase clinical trials tested in patients with HPV-associated high-grade anogenital lesions. A significant hurdle to success in more advanced disease will be the local and systemic immune suppressive factors. Interventions targeting the different immunosuppressive components can provide opportunity to release existing or generate new and effective antitumour immunity. Treatments that alter the protumour inflammatory environment including toll-like receptor stimulation, inhibition of IL-6-related pathways, immune-checkpoint inhibition, direct modulation of MDSCs, Tregs and macrophages could all be useful in combination with therapeutic HPV vaccination. Future progress in delivering successful immunotherapy will depend on the configuration of treatment protocols in an insightful and timely combination.
Keywords: anogenital cancer, cytotoxic T lymphocytes (CTLs), immune-checkpoint inhibitors, myeloid-derived suppressor cells (MDSCs), oropharyngeal cancer, therapeutic HPV vaccines, T regulatory cells (Tregs), tumour microenvironment
Introduction
In spite of the efficacy of ongoing prophylactic vaccination, the full impact on reducing the incidence of cervical and other human papillomavirus (HPV)-associated cancers is still decades away. The key rate-limiting feature in this process is the extent of vaccination coverage which needs to be more than 80% to deliver maximal population protection.1,2 Most vaccination strategies are targeting only adolescent girls, and often the coverage rates are significantly suboptimal. Most importantly, in developing countries, with the highest cervical cancer incidences, the prospect of universal HPV vaccination in the near future is slight. Even in developed counties, there will be a continuing need for treatment of cervical and other HPV-associated cancers. If surgical removal is either not possible or unsuccessful then other approaches are required. Typical cervical cancer survival for the International Federation of Gynecology and Obstetrics (FIGO) stage 1 treated by surgery can be excellent; 5-year survival is 96, 95 and 80–93% in the UK, Germany and the USA, respectively.3–5 However, more advanced, FIGO stage II, III and IV cancers, which are treated with platinum-based chemoradiation, have lower 5-year survival rates, respectively of 54, 75, 58–63; 38, 58, 32–35; 5, 21, 15–16%.3–5 Unfortunately, the treatments that are available currently can cause renal/liver toxicity, fistulas, and with persistent or recurrent disease, there can be painful bone metastases.6 No viable treatment options exist for either metastatic or recurrent disease. Likewise, HPV-associated oropharyngeal squamous cell carcinomas (OPSCCs) that are inoperable are treated by fractionated radiotherapy combined with cisplatin.7 Even though patients with HPV-positive tumours show a better overall 5-year survival than HPV-negative OPSCC, respectively about 65% versus 40%,8 there is a high morbidity and significant mortality in these relatively young populations in developed countries. It is obvious that new and effective treatments are required. Based on a growing body of clinical literature demonstrating proof-of-principle for immune-based therapies for a spectrum of HPV-associated disease9–12 that the immune system can be a key component of restraint and control during the neoplastic process, there is now a new enthusiasm and concrete support (beyond immunologists) by both research funding bodies and industry for developing immunotherapeutic approaches in cancer.
Immune control in cancer
The importance of immune control in cancer is exemplified by recent studies in colorectal cancer. The TNM Classification of Malignant Tumours (TNM) staging system provides for routine prognostication and treatment of colorectal carcinoma. It summarizes data on tumour burden (T), presence of cancer cells in draining and regional lymph nodes (N) and evidence for distant metastases (M). However, clinical outcome can vary significantly among patients within the same stage. This classification provides limited prognostic information and does not predict response to therapy. Many investigators have published quantitative data on the prognostic value of immune cell infiltrates in solid tumours. One example of such methodology is the ‘immunoscore’, which quantifies the in situ immune infiltrate. In colorectal cancer, the immunoscore of CD8 T cells is a prognostic factor superior to TNM classification.13 Further analysis of tumours with or without metastases identifies large genetic heterogeneity, but no significant differences in chromosomal instability, key cancer-associated mutations or gene expression levels/patterns in metastatic versus nonmetastatic tumours. In metastasis-free cancers, there is increased likelihood of mutations in FBXW7, and the tumours show evidence of increased T-cell proliferation and antigen-presenting cell (APC) function. In metastatic cancers, there is a decreased density of lymphatic vessels plus evidence of reduced immune cytotoxicity. Thus, protection against metastasis, irrespective of genomic instability, is significantly impacted by the cytotoxic immune signature, as well as the density of infiltrates of neovasculature. Cytotoxic T cells are key components of cancer control but they need to both be in the right place, and functional.14
The genetic changes that accumulate throughout the neoplastic process also generate the potential targets for the adaptive immune repertoire of the T cells. This provides for the ability to control even heterogeneous tumours through activation of distinct T cells, recognizing a plethora of tumour-associated antigens (TAAs).15 These are generated by the neoplastic process, which is often driven by the aberrant expression of cellular or viral oncogenes plus any additional mutations resulting from increased genomic instability. With a viral aetiology, the viral oncogenes (e.g. HPV E6 and E7) are potentially targetable for functional and immune intervention strategies. T cells expressing PD-1, an activation marker in the tumour microenvironment (TME), have been reported to be enriched for autologous tumour-reactive T-cells.16 Indeed, recently, immunodominant T-cell reactivity directed at cancer testis antigen 83 (CT83) was identified in a patient who had metastatic cervical cancer that underwent complete regression following infusion with autologous tumour-infiltrating lymphocytes (TILs).17
Early innate immune activation events are critical to the appropriate activation of APCs, such as dendritic cells (DCs) or macrophages, which can react to signals released from damaged cells. Such innate immune recognition of ‘danger’ signals elicited through pathogen-recognition receptors (PPRs) provides for the optimal processing and presentation of the tumour-antigenic milieu and can dictate the flavour of any subsequent adaptive T-cell or antibody response, encoding both specificity and immunological memory.18 Induction of adaptive immunity requires migration of activated APCs to the secondary lymphoid organs along a chemokine gradient. APC migration depends on expression of CCR7, the receptor for lymph node (LN)-homing chemokines CCL19 /CCL21. It is coexpressed on professional APCs with maturation markers CD83 and costimulatory molecules including CD80/CD86.19 Furthermore, expression of the matrix–metalloproteinase (MMP)-9 by APCs supports their migration through the extracellular matrix. In secondary lymphoid organs, the APCs engage with the repertoire of T cells.20 Selection of effective antitumour T cells with specificity for a TAA requires appropriate activation through APCs using a two-signal system whereby TAA-processed antigen in the context of major histocompatibility complex (MHC) class 1 molecules and CD80/CD86 on the APC are required to engage respectively with the specific T-cell receptor (TCR) and CD28 molecules. Without the second costimulation, immune tolerance and T-cell anergy can result.21,22 Subsequently, the balance in provision of cytokines and other intercellular cell-surface molecular interactions controls the particular T-cell differentiation, including cytotoxic T cells. Appropriately activated T cells are equipped to migrate to the tumour site and destroy their tumour target. Necessarily, such cellular immunity must be regulated to protect normal cells from immune attack during infections. Thus, inhibitory signals (immune checkpoints) between T cells and APCs are mediated by CTLA-4/CD28- and PD-1/PD-L1-type interactions, while endogenous T-regulatory cells (Tregs) act to maintain self-tolerance.23–25 Importantly, a variety of cells in the TME, including both specific- and nonspecific-induced Tregs, M2 macrophages, myeloid-derived suppressor cells, tumour cells and associated fibroblasts can also limit and block specific T cells’ function, thereby contributing to persistence of the neoplasia.26
Immune surveillance and natural control of human papillomavirus infection
In the cervix, the clinical course of preinvasive HPV disease is indolent, and, moreover, undergoes regression in a subset of women. This observation suggests that this patient cohort is likely to be informative to gain a better understanding of mechanisms of clearance, as well as persistence. The ability to sample the spectrum of developing neoplasia has provided some unique opportunities to advance our understanding of the key role of immune factors in disease control. These intra-epithelial lesions will also be able to uncover mechanisms of resistance to immune-mediated clearance, thus presenting specific therapeutic targets that may be used in concert with making an effector response to HPV and to encourage the exploitation of immunotherapy in cancer treatment.27
It is now clear that effective natural cellular immune control accounts for the fact that although many individuals are naturally exposed to an HPV infection, they do not develop the persistent infections associated with the risk for neoplastic progression. There is abundant evidence for the importance of HPV oncogene-specific cytotoxic T lymphocytes (CTLs) in the clearance of cervical intra-epithelial neoplasia (CIN) and significance of the absence of such immune effectors in progressing high-grade lesions and cancers.28 This is supported by the increased susceptibility to HPV-associated lesions in immune-suppressed patients.29 The requirement for objective, quantifiable methods of assessing subject-matched tissue specimens obtained before and after therapeutic intervention is underscored by the history of immune therapies for HPV disease. Clearly much has been learned about the immunobiology of HPV disease initiation and progression, and preclinical models have demonstrated therapeutic efficacy of T cells specific for viral antigens required for disease initiation and persistence. Nevertheless, until relatively recently, clinical trials testing therapeutic vaccination have yielded only limited success.9,10,30–33 In particular, the study by Maldonado et al.9 showed, for the first time, that peripheral vaccination was followed by dramatic changes in the composition, magnitude, and quality of immune responses in the target lesions. Quantitative image analysis-guided molecular profiling of subject-matched tissue specimens obtained before and after vaccination demonstrated that planned therapeutic resection 8 weeks after the third and final vaccination was, in effect, censoring the tissue endpoint. Local factors are pivotal in determining immunologically driven therapeutic outcomes.
Over the past 25 years, it has emerged that high-grade precancers and cancers have a cornucopia of immune escape mechanisms which function at the level of the TME and beyond.34–37 Indeed, there are multiplicities of local immune suppressive factors that can limit antitumour immunity. In particular, tumour hypoxia can attract macrophages and myeloid-derived suppressor cells, both of which can limit T-cell function, including through PD-L1 expression or through IL-10 production modulating APC function and leading to induction of Tregs.34–37 Other myeloid-derived factors inhibiting CTLs include transforming growth factor beta (TGFβ), reactive oxygen species (ROS), reactive nitrogen intermediates, arginase and nitric oxide synthase (NOS) that deplete L-arginine, an important metabolite for CTL function.37
Mature macrophages have functional plasticity dependent upon the TME cytokine profile. Thus, M1 type macrophages, activated by interferon (IFN) from natural killer cells, can act to limit tumour growth in vivo while the M2 phenotype produces immunosuppressive cytokines TGFβ and IL-10.34 This balance of local cytokines acts to functionally polarize CD4-positive T cells, facilitating a T-helper1 (Th1) cell response which can limit tumour progression by enhancing cytotoxic T-cell responses, while protumour Th2 cells skew adaptive responses toward humoral immunity via production of cytokines such as IL-4 and IL-10.21 These and other factors (including the pro-inflammatory cytokine IL-6) can subsequently skew the T-cell response in favour of tumour infiltration of immune-suppressive Th1735 and Treg cells.37 The export of the latter to draining LNs can act to protect subsequently metastasizing tumour cells.
The inherent genetic heterogeneity within an individual patient’s tumour provides the basis for evolution by natural selection in the face of an immune response, for example by loss of MHC class 1 expression, which effectively blindsides otherwise useful tumour-specific T cells.38,39 More recently, it has been shown that cancer cells can exploit the immune-checkpoint mechanisms to escape immune control by expression of inhibitor ligands.22,40 Importantly, blocking tumour-related PD-L1 expression with antibodies is the basis of some therapeutic checkpoint inhibitors that have recently shown remarkable responses in some cancer patients.41,42 To manifest effective immune cancer control or to deliver therapeutic immunotherapy, all negative factors must be overwhelmed by positive antitumour components. Progress for successful immunotherapy in HPV-associated cancer is additionally confounded by their natural history, whereby early events that influence the local microenvironment act as an immunosuppressive subversive influence that promotes the persistent HPV infection and associated neoplasia.
Early subversion of the local immune environment in human papillomavirus infection
In most circumstances the ultimate resolution of a pathogenic infection requires the induction of a local inflammatory response of an appropriate flavour to provide for the recruitment of suitable adaptive immune effectors with specificity to deal with the threat. These early events are part of the nonspecific innate immune response in which either or both local damage and specific pathogen associated molecular patterns engage with pattern recognition receptors on APCs for activation and sampling of the local antigen milieu. These APCs then migrate to local secondary immune sites such as an LN in a CCR7-dependent manner, where they can activate relevant antigen-specific T cells, which then become recruited to the inflammatory site.43 In some apparently normal healthy people, and for, as yet, no clearly identifiable reasons(s), HPV infection can subvert these processes, leading to the potential for a chronic infection and risk of neoplastic progression.45–58 A series of inter-connected events conspire in neoplastic progression to promote immune deviation and the evolution of a metastasizing tumour.
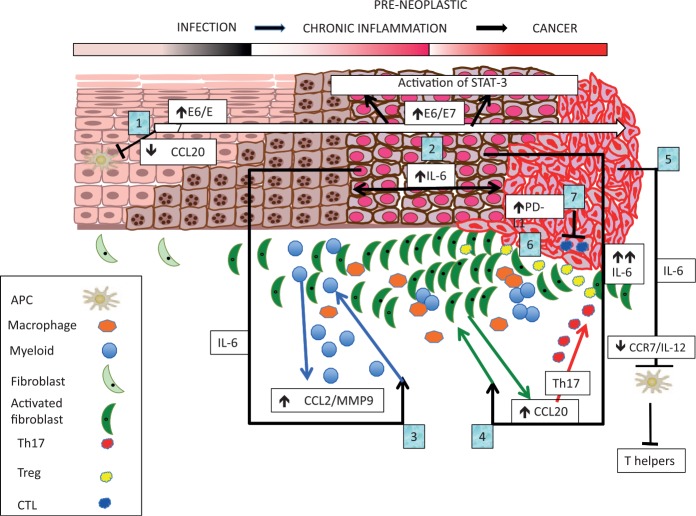
It has been noted that there is often a loss of the APCs and low inflammatory chemokines in HPV-infected tissues.44 Normal keratinocytes produce the CCL20, a chemoattractant for epidermal APCs (Langerhans cells) expressing the receptor CCR6, leading to initiation of immune activation in the skin or mucosa. Notably, mucosal HPV 16 E6 and E7 can suppress the inflammatory NF-κB transcription-factor-dependent CCL20 induction.45,46 It has also been shown that cutaneous HPV 8 E7 oncoprotein affects constitutive, differentiation-dependent CCL20 induction. HPV8 E7 binds to the differentiation-dependent transcription factor C/EBPβ, preventing binding to the promoter of CCL20, thus repressing CCL20 transcription.47 Overall, two major signalling pathways that drive CCL20 can be inhibited by the action of HPV oncogenes.45–47 The consequence is a lack of recruitment of APCs to the HPV-infected epithelium, leading to a failure of optimal innate immune activation and allowing for persistence of virus infection (Figure 1, points 1 and 2).
Figure 1.
The early events which lead to initial immune deviation and further cascades of self-reinforcing events promoting immune escape and lesion progression to cancer: (1) E6/E7 driven loss of CCL20, loss of Langerhans cells; (2) STAT3 activation in human papillomavirus-transformed cells; (3) IL-6 driven CCL2/CCR2 feedback loop promoting myelo-monocytic infiltration; (4) IL-6-driven fibroblast production of CCL20 attracting Th17, sustaining chronic inflammation; (5) IL-6-driven downregulation of antigen-presenting cell migration and IL-12 production blocking; (6) sustained levels of immune-suppressive and tumour-promoting local factors (macrophages and dendritic cells: local MMP-9 production, Tregs and myeloid-derived suppressor cells (MDSCs); (7) immune-factor-driven up-regulation of PD-L1 by tumour or associated immune cells can block antitumour-specific T-cell effectors. The benefits of HPV oncogene vaccination may only be realized if used in combination with standard of care with or without treatments to overcome the local inflammation. These could include toll-like receptor stimulation (dsRNA), STAT3 and possibly IL6 inhibition, immune-checkpoint (PD-1/PD-L1) inhibition, as well as other strategies to directly modulate local tumour MDSCs, Tregs and macrophages.
HPV is necessary but not sufficient for neoplastic progression to cancer. Typically, in people who are immune competent, malignant transformation can take many decades. Although HPV has anti-inflammatory properties, high-grade lesions are infiltrated by myelo-monocytic cells with this process driven by a molecular cascade, whereby HPV-transformed cells produce IL-6 which acts on tumour-associated myeloid and inflammatory cells in a paracrine manner.48 The consequence is rapid activation of STAT3 in monocytes, which translates the signal into CCL2 production, an immune-modulating and tumour-promoting factor.48–50 In addition, CCL2-driven downstream Ca2+ signalling leads to strong MMP-9 expression, which is associated with cancer progression.20,48 The presence of myeloid cell-derived CCL2 is also associated with persistent disease, consistent with its localization with the inflammatory infiltrate in the neoplastic lesions. CCL2 is also a potent monocyte-attracting chemokine and an autocrine loop via CCL2/CCR2 further sustains the inflammatory microenvironment.48 Indeed, low CCL2 in myeloid cells is associated with a better survival in cervical cancer50 (Figure 1, points 3 and 4).
In addition, High Risk (HR) HPV gene actions during early infection influence immune evasion by several complementary strategies. The viral HPV E5 interferes with trafficking of MHC class I molecules through a physical interaction,51 while E7 reduces the density of surface MHC molecules and antigen presentation through its effects on STAT1 signalling and the suppression of IRF-1.52 The E6 protein associates with Tyk2, thereby impairing Jak-STAT activation by IFNα.53 All these effects compromise the MHC class processing and presentation of viral peptides at the infected cell surface and possibly desensitize the target susceptibility to IFN. This reduces the visibility/susceptibility of infected cells to innate and adaptive immune–effector mechanisms favouring viral persistence.
Further compromise of immune activation and communication
That transformed epithelial cells are able to activate STAT3 in bystanding myeloid cells is a pivotal event in carcinogenesis. IL-6, overexpressed in malignant HPV-transformed cells in vitro, becomes highly upregulated during cervical carcinogenesis in vivo54 and is associated with poor prognosis in cancer.55 Once established, even weak paracrine signals can drive powerful amplification machinery promoting protumourigenic and immunosuppressive responses. It turns out that IL-6 produced during cervical carcinogenesis interferes with expression of the migration receptor CCR7 by mature APCs (DCs). In fact, IL-6 dissociates CCR7 expression from other maturation markers (CD83) and costimulatory molecules (CD80/CD86) resulting in mature but functionally impaired DCs.56,57 This inhibition specifically impairs the migration of activated APCs to the chemokines directing them to secondary lymphoid tissues.56 In addition, the suppression of DC IL-12 production has significant influences on the Th1-type responses (Smola et al. unpublished). Notably, the second factor in DC migration, MMP-9, is not suppressed but upregulated by IL-6 in myeloid DCs. Thus, DCs and other myeloid cells accumulate in the tumour stroma and their MMP-9 expression in the TME may promote tumour growth and angiogenesis. Activation of the IL-6/STAT3 pathway in tissue-localized immune-cell infiltrates induces the recruitment of myelo-monocytic cells via CCL2.47 The tumour cells themselves can also produce IL-6, which is followed by induction of, and subsequent consequent attraction of CD4/IL-17/CCR6-positive T cells.58 Infiltration with these protumourigenic T cells is clearly associated with advanced FIGO stages.58 CCL20 transcription is also regulated by paracrine production of IL-6, which activates the C/EBPβ pathway in the tumour-associated fibroblasts, again underscoring the importance of IL-6 in carcinogenesis.58 Thus in early cervical carcinogenesis, CCL20 is suppressed by HPV oncoproteins in the neoplastic epithelial cells, while in advanced stages, CCL20 becomes preferentially expressed in the stroma and amplifies the chronic inflammatory response.58 The combination of local immune factors also upregulates the expression of the checkpoint-inhibitor ligand, PD-L1, by tumour or associated immune cells and this acts to block any antitumour-specific T-cell effectors22 (Figure 1, points 5–7). The increased knowledge and understanding of the complex interactions that may limit either endogenous or induced immunologically driven resolution of HPV-associated neoplasia has provided opportunities for new or improved strategies for therapeutic intervention.
Human papillomavirus and signalling pathway interaction
As in many human solid tumours, STAT3 activation is a primary event in cervical neoplasia.59 Indeed, inhibition of STAT3 can affect tumour growth and enhance the response of some types of tumour to chemotherapy or indirectly through immune mechanisms. While STAT3 activation levels increase with grade of cervical neoplasia, there is an overall decline of STAT3 activation during progression to malignancy. However, STAT3 activation is heterogeneous with higher levels found at the invasive margins of tumours (Smola et al. unpublished). The overall pattern of activated STAT3 expression in cervical cancer may be influenced by paracrine effects from the diverse microenvironment. Major activators of the STAT3 pathway include the IL-6 and oncostatin M (OSM) cytokines, which bind to receptors IL-6R (IL-6α, gp80) and OSMR-β, respectively. Downstream, the ubiquitously expressed common chain gp130 recruits Janus kinases, leading to phosphorylation at tyrosine 705 of STAT3. IL-6 signalling is limited by the availability of the gp80 chain (transmembrane or soluble forms). While cultured cervical cancer cells do not express significant gp80, the soluble form can induce trans-signalling and leading to STAT3 activation in vitro.48,54 Expression patterns of gp80 in vivo and the potential source for soluble gp80 that might drive this signalling pathway are still to be identified.
A recent study has shown that IL-6/STAT3 signalling is able to sensitize cervical cancer cells in vitro to chemotherapeutic drugs.48 This observation was completely unexpected, since the IL-6/STAT3 pathway was previously characterized as a cancer survival pathway in other tumours. IL-6/STAT3-induced sensitization is mediated by the IFN regulatory factor IRF-1, which has been identified as the STAT3-inducible pro-apoptotic factor. Obviously, IL-6/STAT3-signalling can overcome the HPV oncogene mediated inhibition of IRF-1 activity.60,61 Significantly, IRF-1 expression by tumours in vivo correlates with response to radio/chemotherapy.48 Thus, IRF-1 expression in pretherapeutic biopsies might serve a novel predictive marker for therapy response in cervical cancer patients.
Many commercial IL-6 and STAT3 inhibitors are available for deployment in treating cervical neoplasia and cancer.62 Blocking IL-6/STAT3 signalling could well revert paracrine protumourigenic effects in the microenvironment as well as local immune deviation. However, pivotal data have indicated that such a treatment will also lower the sensitivity to radio/chemotherapy.48 Likewise, while STAT3 inhibitors may be useful in overriding immune suppression and tumour progression, in cervical cancer they should not be deployed directly before or simultaneously with chemotherapy because they may reduce the latter’s efficacy.
Vaccination strategies
In the early 1990s, developing therapeutic vaccines aimed at generating specific effector T cells targeting the constitutive and functionally obligate expression of E6 and E7 oncogenes seemed relatively straightforward. The reality has been that progress has been, until recently, very disappointing. Successions of vaccine designs based on HPV 16 E6 and E7 oncogenes delivered by viral or bacterial vectors or naked nucleic acid, or as peptides or proteins, or variously targeted to or by dendritic cells have been tested.28 In most cases, while these approaches proved immunogenic and efficacious in preclinical animal models, they failed to deliver sufficient evidence of effect in early-phase clinical trials to sustain development. A contributing factor is the difficulty in designing a clinical trial in patients with, for example, cervical cancer that is sufficiently powered to measure a clinical impact. Moreover, relevant immune responses are much more likely to be identified in the TME, than in the peripheral blood. Early-stage cervical cancer patients treated with surgery have a high long-term survival rate, which makes the number of patients and the follow-up times required impossible to configure. Later-stage patients are treated by chemoradiation, which together with the vast array of immunological escape mechanisms acquired during its natural history can compromise the ability to measure immunogenicity and possibly clinical impact. Another difficulty is that the ability to assess the capacity of a vaccine to induce a relevant cytotoxic T-cell response is mostly only measurable by sampling from the peripheral blood. It is obvious that a key factor is the requirement to deliver the therapeutic effectors to the tumour with all its immune-avoidance properties. As documented here and elsewhere,28 the development of high-grade cervical intraepithelial neoplastic lesions is associated with immune deviation favouring persistence. In addition, CIN3 lesions can also show evidence of Human Leukocyte Antigen (HLA) class I downregulation undermining the effectiveness of otherwise useful anti-HPV CTL.63 Recent work has highlighted how CIN3 regression is dependent on entry of α4β7 CD8 T cells into the lesion epithelial areas via expression of the ligand for α4β7, MAdCAM-1 on the endothelium of lesion-associated neovasculature.64 Thus, assessing vaccine impact on cervical intraepithelial neoplastic lesions is also very demanding on trial design to measure relevant immune factors in tissue specimens obtained both before and after intervention.
In spite of the difficulties in testing therapeutic vaccines in patients with cervical intraepithelial neoplasia, a recent study of a DNA plasmid targeting HPV16/18 E6/E7 (VGX-3100), administered by electroporation was able to meet its primary clinical endpoint of histologic regression of CIN2/3.10 Moreover, in vaccinated subjects who experienced histologic regression, HPV became undetectable. In contrast, subjects who received placebo and experienced histologic regression were unlikely to clear virus. A key to future utility is to identify patients most likely to respond to such immune therapy. Unfortunately, therapeutic vaccination that induced an immune response to vaccine antigens detectable in the peripheral blood without ex vivo manipulation did not reliably identify subjects whose lesions were likely to regress. Quantitative image analyses of the intensity and colocalization of immune-related antigens of interest suggested a phenotype that was likely to respond to vaccination. These observations are driving the development of methods allowing molecular profiling of specific cell subsets of interest in the TME.27 Another HPV E6/E7 DNA therapeutic vaccine (GX-188) has the HPV 16 and 18 E6 and E7 sequences fused to the extracellular domain of Fms-like tyrosine kinase-3 ligand and the signal sequence of tissue plasminogen activator with the aim of promoting antigen presentation and trafficking of the fused protein to the secretory pathway.65 Electroporation-enhanced immunization promotes an E6/E7-specific T-helper-1-polarized response and induces polyfunctional HPV16-specific CD8 T-cells in CIN3 patients. Importantly, seven out of nine patients showed complete lesion regression and viral clearance within 9 months of follow up.65 Further clinical trials are required to confirm that this vaccine is able to induce sufficient high-quality T cells that can traffic to the lesion and deliver a curative payload. A cautionary thought is that given that the surgical cure rate for diagnosed high-grade lesions is virtually 100%,66 it may be asking a lot to deliver immune therapy better than standard of care. Nevertheless, the avoidance of surgical intervention by therapeutic vaccination may prove to be an attractive alternative for many patients.
Of course, an approaching 100% effective therapeutic HPV vaccine against major oncogenic types would also act as a prophylactic vaccine. Given the change in cervical cancer screening approaches from PAP smear to HPV testing, the market for treating HPV infection by therapeutic vaccine may be a viable one. In addition, the clinical need in HPV-associated head and neck cancer has provided opportunity for clinical trials of VGX-3100 [ClinicalTrials.gov identifier: NCT02163057] and a Listeria based HPV E7 vaccine (ADXS 11-001) [ClinicalTrials.gov identifiers: NCT02291055 and NCT02002182]. ADXS11-001 is a live, bioengineered Listeria monocytogenes (Lm) vector containing a plasmid insert that facilitates secretion of HPV-16 E7 oncoprotein fused to an attenuated Lm virulence factor. ADXS11-001 induces immune responses in HPV-related cancers through multiple mechanisms.67,68 Unfortunately, a case of systemic listeriosis was recently reported during a phase I trial of ADXS11-001 [ClinicalTrials.gov identifier: NCT01598792] in HPV-positive oropharyngeal cancer.69
HPV-associated vulvar intraepithelial neoplasia (VIN) has offered some options for useful vaccine trial design because in many cases, the high-grade lesion surgery is not an option and the other treatments are not curative. For example, Kenter and colleagues used an adjuvanted HPV 16 E6/E7 long-peptide vaccine that showed good T-cell immunogenicity and significant clinical lesion responses.30 Other studies have shown the clinical impact of local treatment with imiquimod in VIN.70 Imiquimod is a topically applied innate immune response toll-like receptor 7/8 agonist and acts to change local immunosuppressive factors.71 This could alter the potential of additional immunotherapeutic approaches such as photodynamic therapy (PDT) or vaccination. PDT-related responses work directly by lesion ablation but also influence local immune factors and recruitment of CD8 T cells. A combination of imiquimod followed by PDT induced long-term response in high-grade VIN patients where local T-cell levels correlated with clinical outcome.72 A vaccine based on a fusion of HPV16 L2E6E7 administered after local lesion treatment with imiquimod elicited clinical responses in immune-competent subjects who had VIN. Responders had a significant increased T-cell proliferative response to vaccine, whereas nonresponders did not.31 While these results are encouraging there is still a long way to go until the licensing of a therapeutic vaccine for treatment of HPV-associated cancer. It is likely that combination with other immune modulatory treatments may be a viable and necessary development.
Immune-checkpoint inhibitors
In the clinical setting of normal tissue, immune checkpoints have a vital homeostatic function. Tumours can hijack these homeostatic pathways to evade the immune system and allow uncontrolled growth. Checkpoint inhibitors block these regulatory pathways and can thereby enhance immune surveillance and tumour clearance. Compelling clinical trial outcome data are establishing the efficacy of checkpoint inhibitors across a range of previously treatment-refractory cancers with the licensing of anti-CTLA-4 and anti-PD-1 antibodies for treatment of metastatic melanoma and some other cancers.73 Several clinical trials are currently exploring anti-CTLA-4 or anti-PD-1 therapy in cervical cancer [ClinicalTrials.gov identifiers: NCT01711515, NCT02054806 and NCT02488759] and OPSCC [ClinicalTrials.gov identifier: NCT01848834].
A down side to these encouraging responses and improved cancer survival rates to checkpoint-inhibitor treatments are the significant toxicities seen in some patients. Across the board, only a proportion of patients respond to blockade of immune checkpoints.74 Ongoing efforts to identify a predictive biomarker of response to PD-1 blockade is complicated by the fact that PD-L1 expression is dynamic over time and displays intratumoural heterogeneity. Pembrolizumab (anti-PD-1) recently gained approval in non-small lung cancer with a companion diagnostic assay to guide its use.75,76 Identifying the patients who are most likely to respond is a key area of interest for all solid tumours and this has been investigated in cervical and OPSCC cancers in particular, by looking at the expression of PD-1/PD-L1 axis. A cross-sectional immunohistochemical study of PD-L1 expression in squamous and adenocarcinomas of the cervix, and a subset of subject-matched primary and metastatic tumours, was unable to show any correlation with PD-L1 expression and survival.77 Marginal PD-L1 expression at tumour/stromal interface was associated with a more favourable prognosis, which may be related to T-cell activation and release of gamma IFN. In adenocarcinomas, expression of PD-L1 by macrophages appears to be associated with poorer survival. This demonstrates the importance of the different components that may influence the potential clinical response in time and space. It has been shown that IL-6 and PGE2 drives monocyte differentiation to PD-L1+ CD163+ and possibly CD14+ macrophages (M2) that induce Tregs, influencing lower IL-12 and increased IL-10 with consequent negative influence on T-cell activation.78 A recent pivotal observation is that an abundance of suppressive PD-L1+/CD14+ M2 macrophages, myeloid-derived suppressor cells (MDSCs), PD-1 or CTLA-4 positive T cells and Tregs is found in the tumour positive but not negative lymph nodes.79 In situ the Tregs and PD-L1 positive immune cells form an immune-suppressive cordon around the metastatic tumour cells. Importantly, it seems that delineated fields of Tregs are associated with immunosuppression and anatomically colocalized in tumour positive LNs enabling metastatic spread.80 This once again emphases the importance of the early events that set up the local immune deviation in an HPV associated intraepithelial lesion.
Interestingly in HPV positive (compared to negative) oral pharyngeal squamous cell carcinoma (OPSCC) patients, the improved clinical outcome is in part a consequence of increased radiosensitivity (residual p53 activity compared to HPV negative tumours where p53 is most often mutated).81 HPV positive OPSCC has also been associated with different patterns of immune cell infiltration in the tumours. In one study, CD8 T cell density in the stroma was significantly linked to improved outcome.82 In another increased PD-1 positive T cells were associated with good clinical outcome.83 Lyford-Pike et al presented evidence of potential interaction between PD-L1 on tumour and infiltrating macrophages with the high density of PD-1 positive T lymphocyte fronts in HPV positive OPSCC.84 A recent study examined this further by multiplex scoring of CD8, PD-L1 and CD68 markers concluded that the best prognosis patients with HPV positive OPSCC had high-density CD8 T cells in the stroma with low tumour levels of PD-L1. Interestingly, improved prognosis in HPV negative tumour patients was associated with high CD68 PD-L1 positive macrophages infiltration.85 It appears that there are different immune populations that can influence outcome in HPV positive or negative OPSCC. At this point there is no simple way to use the PD-L1 biomarker as a means to decide how to allocate checkpoint inhibitor treatment in OPSCC. It is likely that any useful prognostic will have to utilize multiple marker and spatial information. Ongoing treatment trials will hopefully illuminate the potential of checkpoint inhibitor treatment in OPSCC.
Overcoming the limitations of the local micro-environment: changing the balance
The overall lessons from investigation of the natural history of HPV associated cancers is the complex early interplay between the viral infection and changes in the local microenvironment leading to immune deviation. In persistent infection this is characterized by an inflammatory infiltration that undermines and blunts the innate immune response by interfering with APC recruitment and migration and promoting infiltration of protumourigenic myeloid cells.
Since IL-6 orchestrates many of these responses via the STAT3 or C/EBPβ signalling pathways in myeloid cells47 or tumour-associated fibroblasts58 leading to Th17 attraction, the IL-6 signalling pathway is an attractive target to revert immunosuppressive and protumourigenic responses in the microenvironment. In cervical cancer, however, IL-6/STAT3-inhibitors should not be deployed directly before or simultaneously with chemotherapy, since they can lower the sensitivity to radio/chemotherapy.
The potential efficacy of therapeutic vaccination strategies targeting HPV oncogenes is intertwined with the immune deviation of the local microenvironment. It is going to be necessary to combine different approaches to deal with the many aspects of these barriers to optimize the potential of immunization to recover or induce effective cellular immunity that can functionally clear HPV associated lesions. This might include combination of treatments that influence APCs, (MDSCs), macrophages function to optimize the impact of therapeutic vaccination locally.86 This type of approach is being investigated and some are illustrated with the following few examples.
The realization that some chemotherapeutic agents actually target immunosuppressive immune populations in patients has begun to be exploited. The combination of HPV16-SLP vaccination with standard carboplatin and paclitaxel chemotherapy was investigated in mouse tumour models and in patients with advanced cervical cancer.87 In both, a progressing tumour was associated with abnormal frequencies of circulating myeloid cells. Treatment of tumour-bearing mice with chemotherapy and vaccination significantly improved survival and was directly correlated with the chemotherapy altering the myeloid cell population in the blood and tumour; chemotherapy had no effect on tumour-specific T-cell responses.87 In advanced cervical cancer patients, carboplatin–paclitaxel also normalized the abnormal numbers of circulating myeloid cells, and this improved the patient T-cell responses.87 The nadir for such myeloid cells was seen at 2 weeks after the second cycle of chemotherapy, and this suggested an optimal point for vaccination.87 This was confirmed in patients who received a single dose of the HPV16-SLP vaccine at this time and generated very strong and sustained HPV16-specific T-cell responses.87 This important study has established that the carboplatin-paclitaxel therapy can reset the tumour associated abnormal myeloid cell composition in cervix cancer patients to normality thereby allowing for improved robust vaccine-induced T-cell responses when vaccination is given after chemotherapy. A clinical trial [ClinicalTrials.gov identifier: NCT02128126] is now in progress that is assessing the safety, tolerability and the HPV-specific immune responses of different doses of the long-peptide HPV16 vaccine with or without pegylated IFNα as combination therapy with carboplatin and paclitaxel
APC activity can be enhanced by the viral dsRNA analog Poly IC, which could act as an adjuvant for immunotherapy.88 It has been shown to act by induction of necroptotic cell death in cervical cancer, dependent on receptor-interacting protein kinase RIPK3, thus circumventing classical apoptotic cell death. The dying cells release IL-1α acting together with the dsRNA as a potent activator of DCs, which in turn produce IL-12, a key facilitator of effector T-cell responses.89 Interestingly, RIPK3 expression status in cervix cancer could influence outcome of Poly IC-based immunotherapy, suggesting assessment of this prior to treatment selection.90
Tumour MDSCs suppress local T-cell function via production of arginase (Arg), nitric oxide synthase (NOS), and possibly indoleamine 2,3-dioxygenase (IDO1).33 Inhibition or depletion of MDSCs could be feasible as inhibiting phosphodiesterase 5 reduces the number of peripheral MDSCs and enhances antigen-specific T-lymphocyte reactivity in patients with Head and Neck Squamous Cell Carcinomas (HNSCC).91 Tumour-associated macrophages and tumour-infiltrating regulatory T cells greatly hamper host-protective antitumour responses. Being able to functionally repolarize macrophages from a protumour M2 to an antitumour M1 phenotype,34 while reducing Tregs could limit tumour progression and support the efficacy of standard anticancer therapies.92
Combining checkpoint-inhibitor approaches aimed at enhancing the local antitumour immune microenvironment such as T-lymphocyte costimulatory agonists (CD27 agonist), chemokine receptor blockade (CXCR2, CSF1R and CCR4 blockade) and tumour-targeting agents (EGFR targeting mAb and STAT3 blockade are all being tested in HNSCC).93
Conclusion
In summary, high-risk HPV E6/E7 oncogene expression in an infected epithelium can act as a critical initiating factor for the development of a protumourigenic inflammatory milieu. This drives a succession of interactive and self-reinforcing events actioned through receptor, chemokine and cytokine responses on immune and other lesion-associated cells conspiring to promote the persistence and subsequent transformation of HPV lesions to overt cancer through immune deviation and subsequent additional genetic changes. Future progress in delivering successful immunotherapy will depend on the configuration of treatment protocols in an insightful and timely combination. Their key goal will be to rebalance the local immune or systemic suppressive factors to release existing, or provide opportunity to generate new and effective antitumour immunity. Avenues for intervention that are likely to contribute to this process should include targeting different immunosuppressive components in combination with therapeutic HPV vaccination.
Acknowledgments
PLS thanks University of Manchester and CRUK Manchester Institute for support.
Footnotes
Funding: This work was partly supported by a grant from the Deutsche Krebshilfe (grant no.109752) and the Saarland Staatskanzlei to S. Smola.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Sigrun Smola, Institute of Virology, Saarland University Medical Center, Germany.
Connie Trimble, Departments of Gynecology/Obstetrics, Oncology, and Pathology, The Johns Hopkins Hospital, USA.
Peter L. Stern, Division of Molecular and Clinical Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Paterson Building, Wilmslow Road, Manchester, M20 4BX, UK.
References
- 1. Garland SM, Kjaer SK, Muñoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016; 63: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect 2016; 144: 449–468. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Research UK: cancerresearchuk.org; Cervical cancer Survival by stage at diagnosis. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/survival#heading-Three
- 4. German Cancer Registry (Bavaria): awmf.org; S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervoxkarzinom. http://www.awmf.org/uploads/tx_szleitlinien/032-033OLl_S3_Zervixkarzinom_2014-10.pdf
- 5. American Cancer Society: Cancer.org; Survival rates for Cervical Cancer by stage. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html
- 6. Wiebe E, Denny L, Thomas G. Cancer of the cervix uteri. Int J Gynaecol Obstet 2012; 119(Suppl. 2): S100– S109. [DOI] [PubMed] [Google Scholar]
- 7. Kelly JR, Husain ZA, Burtness B. Treatment de-intensification strategies for head and neck cancer. Eur J Cancer 2016; 68: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maldonado L, Teague JE, Morrow MP, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 2014; 6: 221ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015; 386: 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015; 33: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Draper LM, Kwong ML, Gros A, et al. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin Cancer Res 2015; 21: 4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med 2016; 8: 327ra26. [DOI] [PubMed] [Google Scholar]
- 15. Lu YC, Robbins PF. Targeting neoantigens for cancer immunotherapy. Int Immunol 2016; 28: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124: 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevanović S, Pasetto A, Helman SR, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017; 356: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcon N, Stern P, Cunningham T, et al. Understanding modern vaccines. Elsevier, 2011, http://www.sciencedirect.com/science/journal/22107622 [Google Scholar]
- 19. Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 2014; 17: 335–345. [DOI] [PubMed] [Google Scholar]
- 20. Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol 2015; 237: 273–281. [DOI] [PubMed] [Google Scholar]
- 21. Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 2001; 1: 220–228. [DOI] [PubMed] [Google Scholar]
- 22. Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19: 5300–5309. [DOI] [PubMed] [Google Scholar]
- 23. Walker LSK, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol 2015; 36: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci 2011; 1217: 45–59. [DOI] [PubMed] [Google Scholar]
- 25. Walker LSK. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun 2013; 45: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother 2016; 65: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trimble CL. HPV infection-associated cancers: next-generation technology for diagnosis and treatment. Cancer Immunol Res 2014; 2: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016; 16: 219–233. [DOI] [PubMed] [Google Scholar]
- 29. Denny LA, Franceschi S, de Sanjosé S, et al. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012; 30(Suppl. 5): F168– F174. [DOI] [PubMed] [Google Scholar]
- 30. Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361: 1838–1847. [DOI] [PubMed] [Google Scholar]
- 31. Daayana S, Elkord E, Winters U, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 2010; 102: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu AA, Drake V, Huang HS, et al. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015; 4: e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Draghiciu O, Lubbers J, Nijman HW, et al. Myeloid derived suppressor cells – an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 2015; 4: e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011; 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marshall EA, Ng KW, Kung SH, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer 2016; 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016; 4. pii: E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015; 35(Suppl.): S185–S198. [DOI] [PubMed] [Google Scholar]
- 38. Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases – elimination, equilibrium and escape. Curr Opin Immunol 2014; 27: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37: 430–439. [DOI] [PubMed] [Google Scholar]
- 40. Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 2017; 66: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map. J Immunother Cancer 2017; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Offringa R, de Jong A, Toes RE, et al. Interplay between human papillomaviruses and dendritic cells. Curr Top Microbiol Immunol 2003; 276: 215–240. [DOI] [PubMed] [Google Scholar]
- 43. Comerford I, Harata-Lee Y, Bunting MD, et al. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 2013; 24: 269–283. [DOI] [PubMed] [Google Scholar]
- 44. Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J Virol 2005; 79: 14852–14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karim R, Meyers C, Backendorf C, et al. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One 2011; 6: e17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sperling T, Ołdak M, Walch-Rückheim B, et al. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog 2012; 8: e1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schröer N, Pahne J, Walch B, et al. Molecular pathobiology of human cervical high-grade lesions: paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Cancer Res 2011; 71: 87–97. [DOI] [PubMed] [Google Scholar]
- 48. Walch-Rückheim B, Pahne-Zeppenfeld J, Fischbach J, et al. STAT3/IRF1 pathway activation sensitizes cervical cancer cells to chemotherapeutic drugs. Cancer Res 2016; 76: 3872–3883. [DOI] [PubMed] [Google Scholar]
- 49. Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol 2004; 14: 149–154. [DOI] [PubMed] [Google Scholar]
- 50. Zijlmans HJ, Fleuren GJ, Baelde HJ, et al. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol 2006; 208: 507–517. [DOI] [PubMed] [Google Scholar]
- 51. Venuti A, Paolini F, Nasir L, et al. Papillomavirus E5: the smallest oncoprotein with many functions. Mol Cancer 2011; 10: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bottley G, Watherston OG, Hiew YL, et al. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 2008; 27: 1794–1799. [DOI] [PubMed] [Google Scholar]
- 53. Li S, Labecque S, Gauzzi, et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene 1999; 18: 5727–5737. [DOI] [PubMed] [Google Scholar]
- 54. Hess S, Smola H, Sandaradura De, Silva U, et al. Loss of IL-6 receptor expression in cervical carcinoma cells inhibits autocrine IL-6 stimulation: abrogation of constitutive monocyte chemoattractant protein-1 production. J Immunol 2000; 165: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 55. Srivani R, Nagarajan B. A prognostic insight on in vivo expression of interleukin-6 in uterine cervical cancer. Int J Gynecol Cancer 2003; 13: 331–339. [DOI] [PubMed] [Google Scholar]
- 56. Pahne-Zeppenfeld J, Schröer N, Walch-Rückheim B, et al. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int J Cancer 2014; 134: 2061–2073. [DOI] [PubMed] [Google Scholar]
- 57. Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J 2004; 18: 1439–1441. [DOI] [PubMed] [Google Scholar]
- 58. Walch-Rückheim B, Mavrova R, Henning M, et al. Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res 2015; 75: 5248–5259. [DOI] [PubMed] [Google Scholar]
- 59. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou F, Chen J, Zhao KN. Human papillomavirus 16-encoded E7 protein inhibits IFN-γ-mediated MHC class I antigen presentation and CTL-induced lysis by blocking IRF-1 expression in mouse keratinocytes. J Gen Virol 2013; 94: 2504–2514. [DOI] [PubMed] [Google Scholar]
- 61. Um SJ, Rhyu JW, Kim EJ, et al. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett 2002; 179: 205–212. [DOI] [PubMed] [Google Scholar]
- 62. Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012; 30: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bontkes HJ, Walboomers JM, Meijer CJ, et al. Specific HLA class I down-regulation is an early event in cervical dysplasia associated with clinical progression. Lancet 1998; 351: 187–188. [DOI] [PubMed] [Google Scholar]
- 64. Trimble CL, Clark RA, Thoburn C, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol 2010; 185: 7107–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim TJ, Jin HT, Hur SY, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun 2014; 5: 5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stern PL, van der Burg SH, Hampson IN, et al. Therapy of human papillomavirus-related disease. Vaccine 2012; 30(Suppl.): F71–F82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cory L, Chu C. ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother 2014; 10: 3190–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wallecha A, Carroll KD, Maciag PC, et al. Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Adv Appl Microbiol 2009; 66: 1–27. [DOI] [PubMed] [Google Scholar]
- 69. Sacco JJ, Evans M, Harrington KJ, et al. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001. Hum Vaccin Immunother 2016; 12: 1085–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Witte CJ, van de Sande AJ, van Beekhuizen HJ, et al. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review. Gynecol Oncol 2015; 139: 377–384. [DOI] [PubMed] [Google Scholar]
- 71. Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol 2002; 27: 571–577. [DOI] [PubMed] [Google Scholar]
- 72. Winters U, Daayana S, Lear JT, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res 2008; 14: 5292–5299. [DOI] [PubMed] [Google Scholar]
- 73. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 74. Kourie HR, Klastersky J. Immune checkpoint inhibitors side effects and management. Immunotherapy 2016; 8: 799–807. [DOI] [PubMed] [Google Scholar]
- 75. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol 2016; 2: 1217–1222. [DOI] [PubMed] [Google Scholar]
- 77. Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016; 29: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Esch EM, van Poelgeest MI, Trimbos JB, et al. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer 2015; 136: E85– E94. [DOI] [PubMed] [Google Scholar]
- 79. Heeren AM, de Boer E, Bleeker MC, et al. Nodal metastasis in cervical cancer occurs in clearly delineated fields of immune suppression in the pelvic lymph catchment area. Oncotarget 2015; 6: 32484–32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Heeren AM, Koster BD, Samuels S, et al. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res 2015; 3: 48–58. [DOI] [PubMed] [Google Scholar]
- 81. Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 2013; 73: 4791–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oguejiofor K, Hall J, Slater C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer 2015; 113: 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73: 128–138. [DOI] [PubMed] [Google Scholar]
- 84. Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73: 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oguejiofor K, Galletta-Williams H, Dovedi SJ, et al. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV–oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017; 8: 14416–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Melief CJ, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest 2015; 125: 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Welters MJ, van der Sluis TC, van Meir H, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016; 8: 334ra52. [DOI] [PubMed] [Google Scholar]
- 88. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015; 16: 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schmidt SV, Seibert S, Walch-Rückheim B, et al. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1α release, and efficient paracrine dendritic cell activation. Oncotarget 2015; 6: 8635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Smola S. RIPK3-a predictive marker for personalized immunotherapy? Oncoimmunology 2015; 5: e1075695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Califano JA, Khan Z, Noonan KA, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015; 2: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Banerjee S, Halder K, Ghosh S, et al. The combination of a novel immunomodulator with a regulatory T cell suppressing antibody (DTA-1) regress advanced stage B16F10 solid tumor by repolarizing tumor associated macrophages in situ. Oncoimmunology 2015; 4: e995559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Allen CT, Clavijo PE, Van Waes C, et al. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel) 2015; 7: 2397–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]