Abstract
Background:
Patient responses to levothyroxine (LT4) monotherapy vary considerably. We sought to differentiate contributions of FT4 and FT3 in controlling pituitary thyroid stimulating hormone (TSH) secretion.
Methods:
We retrospectively assessed the relationships between TSH and thyroid hormones in 319 patients with thyroid carcinoma through 2914 visits on various LT4 doses during follow-up for 5.5 years (median, IQR 4.2, 6.9). We also associated patient complaints with the relationships.
Results:
Under varying dose requirements (median 1.84 µg/kg, IQR 1.62, 2.11), patients reached TSH targets below 0.4, 0.1 or 0.01 mIU/l at 73%, 54% and 27% of visits. While intercept, slope and fit of linearity of the relationships between lnTSH and FT4/FT3 varied between individuals, gender, age, LT4 dose and deiodinase activity influenced the relationships in the cohort (all p < 0.001). Deiodinase activity impaired by LT4 dose significantly affected the lnTSH–FT4 relationship. Dose increase and reduced conversion efficiency displaced FT3–TSH equilibria. In LT4-treated patients, FT4 and FT3 contributed on average 52% versus 38%, and by interaction 10% towards TSH suppression. Symptomatic presentations (11%) accompanied reduced FT3 concentrations (–0.23 pmol/l, p = 0.001) adjusted for gender, age and BMI, their relationships being shifted towards higher TSH values at comparable FT3/FT4 levels.
Conclusions:
Variation in deiodinase activity and resulting FT3 levels shape the TSH–FT4 relationship in LT4-treated athyreotic patients, suggesting cascade control of pituitary TSH production by the two hormones. Consequently, measurement of FT3 and calculation of conversion efficiency may identify patients with impaired biochemistry and a resulting lack of symptomatic control.
Keywords: deiodinase, levothyroxine treatment, set point, thyroid homeostasis, triiodothyronine, TSH feedback control
Introduction
Thyroid carcinoma is the most prevalent endocrine malignancy, and its incidence has increased in the developed world over the last decade.1–3 Prognosis is mostly favourable, with the majority of patients experiencing a normal life span.4 The main treatment modalities include surgery, radio iodine and levothyroxine (LT4) administration.5–7 The latter frequently invokes a lifelong replacement following thyroid ablative therapy, but is also used to suppress thyroid stimulating hormone (TSH) concentrations in order to prevent potential stimulation of remaining tumour cells.8,9 More recent guidelines have relaxed the TSH target for patients with prognostically favourable tumours.7–11 While LT4 treatment has been proven to be safe and well-manageable for many patients, recent studies have shown a higher level of variation and complexity in the treatment response than previously understood, challenging the widely held belief that thyroid replacement is a simple uncomplicated process.12 LT4 as the standard treatment for hypothyroidism is also increasingly viewed more critically. While large studies have attested to its improvement of the quality of life (QoL) in patients treated for overt hypothyroidism, this is not restored to a level found in the healthy population.13 LT4 monotherapy is at base an unphysiological approach, as the human thyroid gland produces and releases into the circulation both thyroid hormones, thyroxine (T4) and a lower proportion of triiodothyronine (T3). However, in randomized controlled trials (RCTs), combinations of T4 and T3 have not been consistently proven to be superior to regimes based on LT4 alone.14–16 This has left patients and doctors with a considerable dilemma.17 The question therefore arises as to whether free triiodothyronine (FT3) plays a differential role in controlling pituitary TSH production and symptomatic relief in athyreotic patients on LT4 medication.
In the present study, we sought to assess the dual roles of free thyroxine (FT4) and FT3 in the control of pituitary TSH secretion in a large cohort of patients with thyroid carcinoma that have been followed under long-term LT4 use. We also associated it with clinically symptomatic relief. This retrospective longitudinal analysis complements a prospective cross-sectional study that was reported earlier.18
Methods
Patient records
The present study comprises a retrospective analysis of records from 319 patients with differentiated thyroid carcinoma (DTC) who were treated and followed long-term from June 2008 to August 2016 in the Department of Nuclear Medicine of Klinikum Lüdenscheid, a teaching hospital of the University of Bonn. Patients with potentially interfering non-thyroidal comorbidities and pregnant women were excluded. Patient characteristics are reported under Results. The anonymized analysis was approved by the local authorities in data protection, not requiring an ethical vote.
The treatment strategy followed the German guidelines for DTC management.19 In patients with tumour stage pT1a according to TNM classification and presumably low risk of recurrence after initial surgery, radioiodine ablation and further diagnostic radioiodine scans were deemed unnecessary. Follow-up visits were carried out at 6-month intervals for 5 years and 12-month intervals thereafter. In contrast, patients with intermediate or high risk of tumour recurrence (stages pT1b to pT4) required radioiodine ablation of remnant tissue following surgery. Patients accordingly were hospitalized in a dedicated unit for 48 h after radioiodine administration, obeying German laws for radiation protection. Patients were followed every 6 months on an outpatient basis over a 5-year period, when another diagnostic radioiodine scan was carried out. In the absence of any evidence of tumour recurrence, follow-up intervals were changed to 12 months. For hormonal substitution, all patients received LT4 monotherapy administered in a single early-morning dose. To decrease the risk of tumour recurrence, thyroxine replacement aimed at TSH suppression in most patients during the first 5 years, except for low-risk stages or in cases of contraindications such as severe coronary heart disease. In the latter cases, the target was euthyroidism. After 5 years without suspicion of recurrence, treatment targets were adjusted to euthyroid levels within the reference range, and LT4 dose was reduced accordingly. To stimulate iodine uptake of potential DTC tissue, LT4 treatment was withdrawn 4 weeks before radioiodine application for diagnostic or treatment purposes, rendering the patient hypothyroid, or, alternatively, patients received two injections of recombinant human TSH (rhTSH, Thyrogen®, Sanofi Genzyme, Neu-Isenburg, Germany).
For the present evaluation, we included the reports of all DTC patients who entered our institution during the last 6 years, detailing the results of the below-mentioned examinations and the patient’s history, including prior surgery or radioiodine treatment and tumour classification. All visits on LT4 replacement therapy or after LT4 withdrawal were included. Only visits involving rhTSH stimulation were excluded from the present analysis, because this procedure affects the natural relationship between TSH and FT4 or FT3. A stable nonhypothyroid presentation was defined as no change of medication within the previous 6 weeks, an FT4 concentration above its lower reference limit and TSH below its upper reference boundary.
Patient complaints were presented in an open format. At all visits, a patient was seen by a specialist in nuclear medicine, discussing and documenting any patient complaints and adjusting dose as required. For the present analysis, the documented complaints were independently categorized into hypothyroid (e.g. tiredness, fatigue, lack of energy, cold intolerance, weight gain), hyperthyroid (e.g. nervousness, rapid pulse, palpitation, trembling, heat intolerance, unwanted weight loss) or thyroid-unrelated symptoms (e.g. back pain).
Neck ultrasound, thyroid tests and thyroglobulin measurement were carried out routinely at each visit. Peroxidase- or thyroglobulin-antibodies were included when clinically indicated.
Thyroid ultrasonography and scintigraphy
Thyroid volume, tissue echogenicity, nodularity and adjacent structures such as lymphatic nodes were routinely assessed in all subjects by ultrasonography (12 MHz transducer). While most thyroid carcinoma patients had no detectable residual thyroid volume, reference values for thyroid volumes are <18 ml for female and <25 ml for male subjects.
Radioiodine application for remnant tissue ablation, diagnostics in cases of suspected recurrence or treatment of proven tumour recurrence was performed according to the German guidelines of the Society of Nuclear Medicine.19
Laboratory methods
Thyroid function tests were performed by a single accredited institution, the Institute of Laboratory Medicine of Klinikum Luedenscheid. Blood samples were taken from morning to early afternoon. TSH was measured with an automated direct chemiluminescence method (third generation, TSH3-Ultra, ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). FT4 and FT3 were measured on the same platform (FT4- or FT3-ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). The methods used in this study had been extensively evaluated in the local population. Assay characteristics, verified reference ranges, methodological variation and biological variability have been reported in detail elsewhere.18,20,21
Briefly, the standard curve was calibrated with the 3rd International Standard of the World Health Organization for human TSH (IRP 81/565). It showed a functional sensitivity of 0.008 mIU/l. Intra-assay coefficients of variation (CVs) in pooled serum samples in the range from 0.52 mIU/l to 132.8 mIU/l (n = 20) were 1.4–2.4%. Inter-assay imprecision measured in duplicate over 10 consecutive days was 0.9–2.9%. At a TSH value of 0.52 mIU/l, the intra-assay CV was 1.4%, the inter- assay CV 2.2%, but at the functional sensitivity (TSH of 0.008 mIU/l) the inter-assay CV rose to 14.1%. Serum samples with FT3 concentrations ranging from 2.9 to 14.2 pmol/l showed intra-assay CVs from 2.4% to 3.1% and inter-assay CVs from 2.3% to 3.9%. Serum samples with FT4 concentrations in the range from 9.3 to 38.8 pmol/l showed intra-assay CVs from 2.2% to 3.3% and inter-assay CVs from 2.5% to 4.0%. Standard laboratory quality procedures were routinely employed, and regular participation in inter-laboratory tests was part of the quality-management strategy.
Laboratory-evaluated reference intervals were as follows: 0.4–4 mIU/l for TSH, 3.1–6.8 pmol/l for FT3, 10–23 pmol/l for FT4, for TPO Ab < 60 IU/ml and for TSH-R Ab < 2 IU/l.
Follow-up measurements obtained from 72 patients with TSH concentrations ranging from 0.2 to 8.8 mIU/l showed a biological variation (intra-individual CV) of 26% for logarithmically transformed TSH values.21 The bi- and trivariate CVs expressed by the Mahalanobis distance for the TSH–FT4 and TSH–FT4–FT3 combination were only slightly higher at 34% and 29%, respectively.21 Regression lines of the relationships between lnTSH and FT3, FT4 or deiodinase activity were also shown to closely concur in a control group during follow-up under unchanged conditions.18
Thyroglobulin was measured by RIA (Thermo Fisher Scientific BRAHMS, Henningsdorf, Germany). TPO Abs were measured with an automated competitive chemiluminescence method (Anti-TPO, ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany).
T3–T4 conversion and deiodinase activity
A simple estimate of the conversion of T4 to T3 was derived by dividing the molar serum concentrations of the two hormones, and expressing it as the FT3–FT4 ratio.
In addition, global deiodinase activity (SPINA-GD) was calculated, using a mathematical model of thyroid hormone homeostasis, as has been previously described.22–24 SPINA-GD is more precise than the simple FT3–FT4 ratio, as it accounts for non-linear enzyme saturation kinetics, although it does not further differentiate global activity by type of deiodinase. The SPINA parameters can be calculated with free open source software (SpinaThyr 4.0), available from sourceforge.net (http://spina.sf.net). They have been recently reviewed,25 and were extensively validated in several large studies.22–24,26–29 SPINA-GD estimates the maximum global activity of peripheral deiodinases per unit of time (termed deiodinase activity, nmol/s) from equilibrium levels of FT3, FT4 and estimated constants for plasma protein binding, distribution and elimination.22–26
Statistical methods
Descriptive data are reported as median and interquartile range (IQR), because they were mostly non-normally distributed. TSH values were natural logarithmically transformed for that reason. Wilcoxon’s rank-sum test was used for between-group comparisons of continuous variables, and chi-squared test with Yates’ correction for continuity for categorical variables. Variables were considered explanatory and therefore no adjustments were made for multiple comparisons. Generalized linear mixed models were used to account for non-independence of repeated measurements in the same subject, individual variations in slope and non-linearity of the relationships. The models were fitted using natural splines with four knots and a Gaussian link function for continuous variables or a binomial link for categories, and relied on restricted maximum likelihood estimators (REML).30 Interaction terms were tested in the models to assess and, if significant, to account for gradient change. We also estimated the relative importance of FT3 and FT4 – which were strongly correlated with each other – on the TSH response by averaging of the sequential sum-of-squares obtained from all possible orderings of the predictors, thereby accounting for collinearity.31 All tests were two-sided with p < 0.05 denoting statistical significance. We used the R statistical software base package (version 3.3.1 for Mac) together with JGR 1.1-18, Deducer 0.7-9, lme4 1.1-12 and relaimpo 2.2-2.30–33
Results
Three hundred and nineteen patients with thyroid carcinoma were followed for at median 5.5 years (IQR 4.2, 6.9). The median number of visits per patient was 9 (IQR 6, 12), resulting in 2914 patient visits. Three hundred and five patients had thyroidectomy, 14 hemithyroidectomy, and 295 (92.5%) underwent radioiodine treatment. Patient characteristics are summarized in Table 1. All patients achieved a biochemically nonhypothyroid state on thyroid hormone replacement with LT4, defined by FT4 >10 pmol/l and TSH <4 mIU/l. Excluding periods of drug withdrawal for radioiodine treatment, 64% of the patients had no complaints about symptoms over follow-up treatment. Thirty-six per cent of the patients expressed complaints about hypothyroid (26%) or hyperthyroid (10%) symptoms at any one visit and were then adjusted for dosage. While patients were asymptomatic most of the time, there were 7% presentations with complaints about hypothyroid symptoms, compared to 2% with hyperthyroid symptoms. The latter is expressed as the average symptom reporting over all patients and with several presentations per patient. A TSH target below 0.4, 0.1 or 0.01 mIU/l was reached at 73%, 54% and 27% of presentations, respectively, and by 94%, 84% and 56% of patients for any one visit. Six per cent of patients were biochemically euthyroid at all visits, whereas a TSH-suppressive regime was maintained throughout follow-up in 25% (<0.4 mIU/l) or 10% (<0.1 mIU/l).
Table 1.
Characteristics of the study group.
| Parameter | Median (IQR)1
n = 319 |
|---|---|
| Gender (female/male) | 230 (72%) / 89 (28%) |
| Age at initial presentation (years) | 50.1 (41.1; 62.0) |
| Body mass index (kg/m2) | 28.2 (24.3; 31.3) |
| Tumour type | Papillary 69%, follicular 19%, other 12% |
| Tumour stage at initial presentation | pT1 46%, pT2 20%, pT3 12%, pT4 3%, N1 12%, M1 4% |
| Ablative treatment | surgery 100%, plus radioiodine 92.5% |
| LT4 dose (µg/day) | 150 (125; 175) |
| Weight-adjusted LT4 dose (µg/kg BW/day) | 1.84 (1.62; 2.14) |
| FT3 (pmol/l) | 5.15 (4.60; 5.80) |
| FT4 (pmol/l) | 22.3 (19.6; 25.4) |
| TSH (mIU/l) | 0.07 (0.01; 0.46) |
| FT3–FT4 ratio | 0.23 (0.20; 0.26) |
| Deiodinase activity (nmol/s) | 21.6 (18.8; 24.2) |
Values shown are under nonhypothyroid (FT4 >10 pmol/l, TSH <4 mIU/l) stable conditions, excluding temporary LT4 withdrawal for radioiodine treatment.
BW, body weight; FT3, free triiodothyronine; FT4, free thyroxine; LT4, levothyroxine; TSH, thyroid stimulating hormone.
At stable nonhypothyroid presentations, LT4 dose requirements varied widely from 50 to 375 µg/day (median 150, IQR 125,175), corresponding to 0.73 to 4.1 µg/kg BW (median 1.84, IQR 1.62, 2.14). Weight-adjusted LT4 dose during follow-up was significantly associated with the level of TSH suppression (–0.08 per log unit, p < 0.001), adjusted for gender (p = 0.35) and age (0.12 µg/kg lower per 10-year increase, p < 0.001). Calculated deiodinase activity was non-linearly and inversely related with weight-adjusted LT4 dose (–1.5 nmol/s for a dose increase from 1.5 to 2 µg/kg BW, p < 0.001), independently of gender (1.7 nmol/s higher in men, p < 0.001), age (–0.4 nmol/s per 10 years, p < 0.001) and TSH (0.11 nmol/s per log unit, p = 0.02).
An FT3–TSH mismatch – defined by FT3 concentrations below 5 pmol/l (the median level of normal controls) despite achieved TSH suppression (<0.4 mIU/l) – occurred in 31% of the presentations. This was strongly associated with deiodinase activity (OR 0.80, 95% CI: 0.77; 0.83, p < 0.001). It remained independently significant (OR 0.79, 95% CI: 0.75, 0.83, p < 0.001) in the presence of other important influences such as gender (p < 0.001), age (p < 0.001) and weight-adjusted dose (p < 0.001).
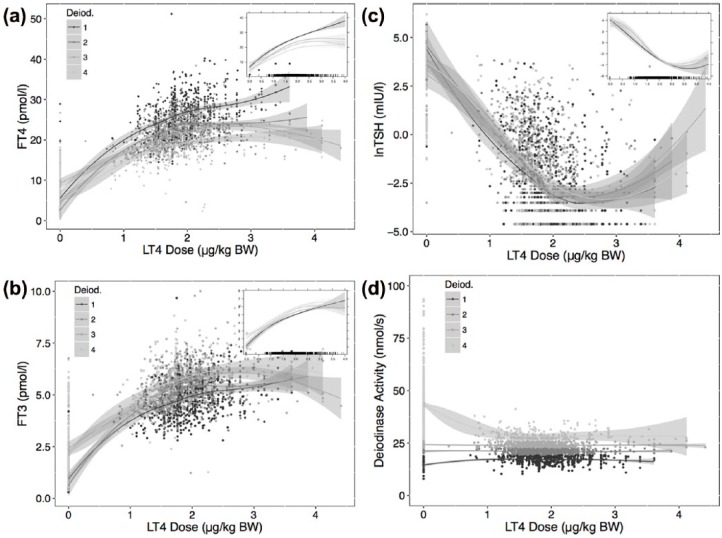
Relationships between FT4, FT3 or TSH and weight-adjusted LT4 dose were stratified by determining quartiles of deiodinase activity (<19, 19–22.5, 22.5–26, >26 nmol/s) over the full dose range, including times of temporary LT4 withdrawal, as shown in Figure 1. The influence of the deiodinase categories proved significantly non-linear (p < 0.001) and non-parallel (interactive, p < 0.001) (Figure 1). The associations remained significant when using deiodinase activity as a continuous variable and restricted to the nonhypothyroid range (FT4 p < 0.001, FT3 p < 0.001, lnTSH p = 0.02), but only the interaction with FT4 was significant (p < 0.001).
Figure 1.
Relationships between FT4 (a), FT3 (b), and TSH (c) and weight-adjusted LT4 dose, stratified by quartiles of deiodinase activity. The main panels show crude relationships obtained by locally weighted scatterplot smoothing in each strata. The inserts are derived by a linear mixed model (see Methods), fitting natural splines to the interaction of deiodinase activity with LT4 dose. The interaction term was significant (p < 0.001) for all relationships, indicating a change in slope, not a strictly parallel shift. The distribution of the deiodinase strata is shown in (d). The shaded area surrounding the curves indicates 95% confidence interval in this and other figures.
FT3, free triiodothyronine; FT4, free thyroxine; LT4, levothyroxine; TSH, thyroid stimulating hormone; BW, body weight.
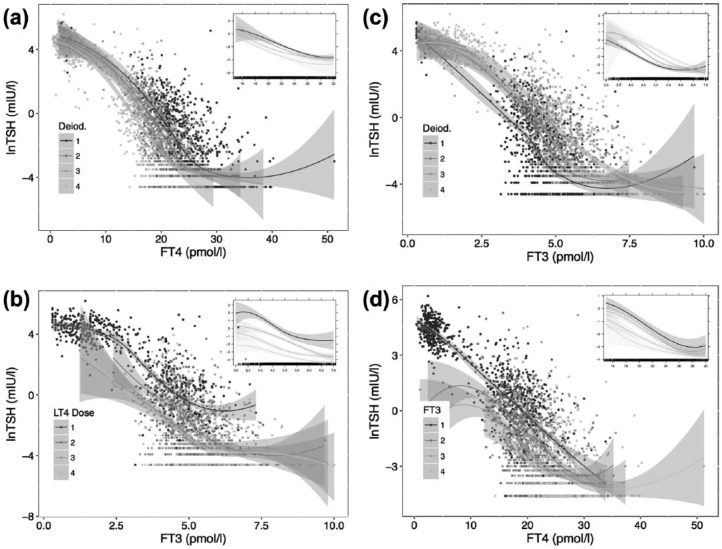
While the lnTSH–FT4 and lnTSH–FT3 relationships displayed considerable variation among individual patients in slope, intercept and fit of linearity, various influences such as gender, age, weight-adjusted LT4 dose and deiodinase activity all significantly (all p < 0.001) influenced the relationship. Compared to the preoperative level, deiodinase activity had declined from 31.3 nmol/s (IQR 28.9, 35.0) to 23.1 nmol/s (IQR 21.0, 24.9, p < 0.001) after thyroidectomy in a small series of 35 patients that had been referred prior to surgery. Deiodinase activity was of particular interest, as it interacted (p < 0.001) with FT4 to alter the gradient of the lnTSH–FT4 relationship in both univariable (not shown) and multivariable models adjusted for gender, age and BMI (Figure 2a), and was itself altered by the LT4 dose administered (as described above).
Figure 2.
Relationship between TSH and FT4 or FT3, stratified by quartiles of deiodinase activity (a, c), administered LT4 dose (b) or FT3 concentrations (d).
The main panel shows the crude relationship obtained by locally weighted scatterplot smoothing in each strata over the full range. The inserts are restricted to the nonhypothyroid range, fitted by a mixed model using natural splines for the independent variable and its interaction with the respective strata after adjusting for gender, age and body mass index (except if dose was already weight-adjusted). The curves shown are significantly different (see Results).
FT3, free triiodothyronine; FT4, free thyroxine; LT4, levothyroxine; TSH, thyroid stimulating hormone.
With both escalation of LT4 dose and declining conversion efficiency, FT3 became increasingly dissociated from TSH, as shown by stratifying the relationships by quartiles of LT4 dose (Figure 2b) or deiodinase activity (Figure 2c). Quartile-based FT3 groups (<4.2, 4.2–4.9, 4.9–5.6, >5.6 pmol/l) displayed a shift in their corresponding lnTSH–FT4 relationships, which was not entirely parallel (p for interaction < 0.001) (Figure 2d). This means that at any given FT4 concentration the level of TSH suppression was additionally dependent on circulating FT3 concentrations (p < 0.001 between groups, Figure 2d). As can be seen, the influences of FT3 and FT4 were supra-additive, the two hormones interacting multiplicatively over both the full and nonhypothyroid spectrum.
Taking collinearity between FT3 and FT4 into account (see Methods), the relative importance of FT3 and FT4 for TSH suppression in the nonhypothyroid range was on average estimated to be 52% (95% CI 47–57%) for FT4, 38% (95% CI 32–42%) for FT3 and 10% (95% CI 8–15%) for the interaction of the two hormones.
The differential influences of FT3 and FT4 can be illustrated by comparing two quartile-based groups of patients from our panel, high FT4/low FT3 versus low FT4/high FT3 (Table 2). At a comparable level of TSH suppression, the extent of global deiodinase and T3 involvement differed between the groups, suggesting that FT3 can effectively suppress TSH in the presence of low T4 substrate, but high global deiodinase activity.
Table 2.
Thyroid parameters in two groups of patients with high FT4/low FT3 versus low FT4/high FT3 serum concentrations.
| Parameter | High FT4/low FT3 median (IQR) n = 24 |
Low FT4/high FT3 median (IQR) n = 33 |
p-value |
|---|---|---|---|
| Weight-adjusted LT4 dose (µg/kg BW/day) | 2.02 (1.68; 2.31) | 1.63 (1.40; 2.26) | 0.10 |
| FT3 (pmol/l) | 3.84 (3.65; 4.00) | 5.93 (5.74; 6.20) | <0.001 |
| FT4 (pmol/l) | 27.2 (26.3; 28.9) | 18.1 (17.0; 18.8) | <0.001 |
| TSH (mIU/l) | 0.12 (0.02; 0.23) | 0.11 (0.02; 0.43) | 0.81 |
| Deiodinase activity (nmol/s) | 12.5 (11.7; 13.7) | 31.2 (29.2; 33.5) | <0.001 |
BW, body weight; FT3, free triiodothyronine; FT4, free thyroxine; LT4, levothyroxine; TSH, thyroid stimulating hormone.
When comparing symptomatic and asymptomatic presentations under biochemically stable nonhypothyroid conditions, the lnTSH–FT4 relationship was significantly (p < 0.001) shifted towards higher TSH values at the same FT4 concentration in the presence of hypothyroid symptoms (Figure 3a), as was the lnTSH–FT3 relationship (p < 0.001) (Figure 3b). FT3 levels at symptomatic presentations during follow-up were significantly reduced, –0.19 pmol/l, p = 0.005 and –0.23 pmol/l, p = 0.001 after adjusting for gender (p < 0.001), age (p < 0.001) and BMI (p = 0.38), compared to the levels at asymptomatic presentations (Table 3). Associated hypothyroid complaints decreased from 5.7% at 3 pmol/l to 2.3% at 6 pmol/l FT3 (p = 0.002), and, similarly, from 3.9% to 1.9% at a TSH of 1 mIU/l versus 0.05 mIU/l (p < 0.001). The presence of hypothyroid symptoms was associated with deiodinase activity (p = 0.050), but the relationship weakened after adjusting for covariates (Table 3). Hyperthyroid symptoms were below 0.1% at TSH 0.05 mIU/l, and increased from 0.01% at 5 pmol/l FT3 to 0.5% at 7 pmol/l FT3 (p < 0.001) during follow-up.
Figure 3.
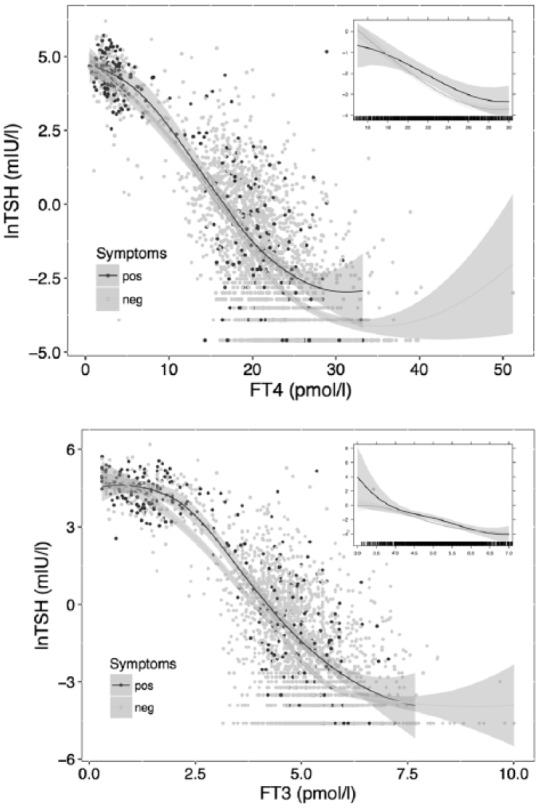
Relationships between TSH and FT4 or FT3 at symptomatic versus asymptomatic presentations.
The main panel shows the crude relationships obtained by locally weighted scatterplot smoothing in each group over the full range. The inserts are restricted to the nonhypothyroid range, fitted by a mixed model adjusted for FT4/FT3, gender, age and BMI. Responses in symptomatic patients were significantly shifted to the right (FT4: p = 0.03, FT3: p = 0.007).
BMI, body mass index; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone.
Table 3.
Thyroid parameters at symptomatically hypothyroid versus asymptomatic presentations.
| Parameter | Asymptomatic mean (95% CI)1 |
Symptomatic mean (95% CI)1 |
p-value |
|---|---|---|---|
| Visits (n) | 2270 | 152 | |
| Gender (female/male) | 71%/29% | 64%/36% | 0.08 |
| Age | 53.3 [51.6; 55.0] | 53.7 [51.9; 55.4] | 0.08 |
| BMI | 27.2 [26.7; 27.7] | 27.2 [26.8; 27.6] | 1.0 |
| Weight-adjusted LT4 dose (µg/kg BW/day) | 1.94 [1.89; 2.00] | 1.86 [1.79; 1.93] | <0.001 |
| FT3 (pmol/l) | 5.43 [5.35; 5.52] | 5.20 [5.05; 5.36] | 0.001 |
| FT4 (pmol/l) | 22.8 [22.4; 23.2] | 21.4 [20.6; 22.1] | <0.001 |
| TSH (mIU/l) | 0.10 [0.08; 0.12] | 0.17 [0.12; 0.23] | <0.001 |
| Deiodinase activity (nmol/s) | 22.1 [21.7; 22.5] | 22.5 [21.9; 23.2] | 0.11 |
This excludes temporary LT4 withdrawal for radioiodine treatment. Thyroid parameters were obtained under nonhypothyroid stable conditions, and adjusted for gender, age and BMI (except for LT4 dose) in a multivariable mixed model. TSH was logarithmically transformed.
BW, body weight; FT3, free triiodothyronine; FT4, free thyroxine; LT4, levothyroxine; TSH, thyroid stimulating hormone.
Discussion
While LT4 monotherapy remains the standard treatment for hypothyroid patients, concerns have been raised about the implications of its non-physiological nature.34–38 In particular, relatively low FT3 concentrations accompanied by elevated FT4 levels have long been recognized in patients treated with LT4.24,39–41 In more recent and detailed analyses, we and others found profound alterations in both the mechanisms of feedback and feedforward control by thyroid hormones in LT4-treated patients that were aggravated by the absence of a functioning thyroid gland.18,42–44 In the present retrospective analysis, we further examined the dual and differential roles of FT3 and FT4 in controlling pituitary TSH secretion. This has demonstrated a substantial influence of conversion efficiency and the resulting FT3 concentrations on the TSH–FT4 relationship in LT4-treated athyreotic patients. The FT3–FT4 interplay introduces a considerable amount of variation in the treatment response between individual patients. Because LT4 monotherapy supplies only the largely inactive pro-hormone T4, in the absence of a functioning thyroid gland, the generation of T3 becomes dependent entirely on enzymatic T4 to T3 conversion, which is achieved by two types of deiodinases, type 1 and type 2.45 However, conversion efficiency may vary widely among LT4-treated patients, and is less efficient in the absence of remnant thyroid tissue.12 This was reconfirmed by the present analysis, as, in a small series with preoperative measurements, deiodinase activity significantly declined by at median 26% after thyroidectomy. In the present study, we demonstrated a strong influence of deiodinase activity on the TSH–FT4 relationship, poor T4 to T3 converters displaying a much flatter TSH response curve at the same FT4 concentrations. Interestingly, deiodinase activity and conversion efficiency – albeit already reduced in athyreotic patients – are not exclusively genetically predetermined, but correlated inversely with LT4 dose in our study. This may counteract and limit the success of LT4 dose escalation in terms of raising circulating FT3 concentrations, as has been discussed previously.12,43
This raises the question of whether patients may still gain satisfaction of treatment despite the less efficient conversion process and lower circulating FT3 concentrations on high-dose LT4, potentially achieving sufficient compensation at the tissue levels. To a degree, this was apparently true for a large proportion of patients, as hypothyroid complaints were only reported at 11% of presentations. However, the proportion of presentations with hypothyroid complaints was significantly and inversely associated with circulating FT3 concentration, after correcting for independently significant non-specific influences of gender, age and weight-adjusted dose. In symptomatic patients, the TSH–FT4 relationship was slightly, but significantly, shifted to higher TSH values. Hyperthyroid complaints were less frequent, and associated with higher FT3 concentrations. Hence, measurement of FT3 and determination of conversion rate may identify patients with impaired biochemistry and displaced equilibria that are at higher risk of unsatisfactory treatment outcomes and residual complaints.
While the study size of more than 300 patients and the available long-term follow-up over at median 5.5 years and nine visits per patient are a strength of the study, some limitations relate to its retrospective and uncontrolled design. Although the use of equilibrium dialysis or tandem liquid chromatography mass spectrometry was not practicable in such a large cohort, the immunometric assays employed in this study have been extensively evaluated in the local population.18,20,21 This is particularly relevant because FT3 and FT4 assays may differ in quality and suffer from a lack of standardization among different manufacturers.46,47 We also note that our analysis was done on a sufficiently large sample using the same instrument at a single institution. Apart from assay performance, the clinical relevance of additional FT3 measurements was corroborated by demonstrating a significant, non-linear, U-shaped relationship with the Hospital Anxiety and Depression Score (HADS) plus subscales in an unselected sample of more than 1000 outpatients, whereas no association with mood change was observed for FT4 and TSH in the same sample.49
It should further be cautioned that the true nature of the TSH–FT4 relationship in the untreated condition may not be inferred from this study, because the relationship is profoundly altered by the LT4 treatment itself. Unlike the damped response seen in untreated euthyroid patients, in this LT4-treated cohort, the gradient of the TSH–FT4 relationship was much steeper, confirming our previous findings from a prospective cross-sectional study and earlier reports by others.18,41–44 Although clustering of set points may have contributed to flattening the relationship in the euthyroid range in cross-sectional studies, pronounced alterations were recognized in LT4-treated patients, irrespective of study design.18,42,43,49 Relationships showed a high degree of individuality, and were influenced by demographic factors such as gender, age and BMI, as noted before.18 Direct treatment-related influences were additionally apparent, LT4 showing a dose-dependent influence on the TSH response curve. Drug-related variations in deiodinase activity reduced the attainment of effective circulating FT3 concentrations, thereby impairing T3 stability at the peripheral and central level of control. Unlike in healthy subjects where FT3 levels are uncorrelated with TSH, and remain stable over the entire euthyroid spectrum, FT3 concentrations become unstably correlated with TSH concentrations in LT4-treated athyreotic patients.29,42,43 The demonstrated severe perturbations of TSH control in athyreotic patients under LT4 monotherapy affect both the central feedback regulation by thyroid hormones and the peripheral feedforward motif on deiodinase activity. They are consistent with accumulating and recently reviewed36 experimental evidence suggesting that LT4 monotherapy largely fails to restore euthyroidism at the tissue level.50–52
A differential influence of FT3 and FT4 was most apparent when comparing subgroups from the panel with high FT4/low FT3 versus low FT4/high FT3 serum concentrations. This suggested that FT3 can effectively suppress TSH to a comparable level in the presence of low T4 substrate, but high global deiodinase activity. The effect of T3 may be partly due to direct action, with an estimated pharmacological equivalence ratio of 1 to 3 for LT3 compared to LT4 substitution.53 In addition, it may indirectly modulate the FT4 response at the hypothalamic–pituitary level (as suggested by the significant statistical interaction), as in T3-infused rats pituitary type 2 deiodinase activity was elevated compared to euthyroid controls.51 While we could only estimate global deiodinase activity in this clinical study, the differential expression of type 1 or type 2 deiodinase by peripheral and central organs together with differing enzyme characteristics such as a higher T4 sensitivity of deiodinase type 2 may have contributed to the central–peripheral disequilibrium observed in LT4 treatment.24,36,50–52,54 However, the situation is more complex. Ubiquitination has been experimentally shown to be a substrate-limiting factor.52 In extremis, set point control may even be sufficiently efficient without the involvement of deiodinases to maintain adequate serum and tissue thyroid hormone concentrations, as has been reported in triple deiodinase-deficient mice.55
Although this study was neither intended nor designed to evaluate patient symptoms in a detailed standardized way – relying on outcomes of a neutral non-leading communication between patient and doctor routinely documented at each visit and independent review – it should be noted that the observed impairment in the TSH control and inefficiencies in the conversion process were significantly associated with reduced patient satisfaction. It is well known and was also recognized in this study that hypothyroid symptoms are partly non-specific and heavily confounded by age and BMI and may be masked by statistical averaging.56–61 However, the association between FT3 and the presence of hypothyroid complaints was highly significant, and remained so after adjusting for other independently significant influences such as gender, age and weight-adjusted dose in multivariable models.
These findings may hint at possible mechanisms that could explain why according to recent QoL studies patients treated for hypothyroidism with the LT4 standard treatment cannot expect full symptomatic relief.13 This should provide further directives for prospective investigation and potential improvements towards a more personalized treatment approach. Despite TSH-suppressive targets that were maintained over 5 years in many patients before being relaxed in the absence of tumour recurrence, high LT4 doses were well tolerated and presentations with hyperthyroid complaints were infrequent in this study.
The present findings have several practical implications. (1) Conversion efficiency and the resulting FT3 play an important regulatory role in shaping the TSH–FT4 relationship in LT4-treated athyreotic patients. The data suggest a dual role of both thyroid hormones, FT4 and FT3, on hypothalamic–pituitary TSH control. This type of controlling of the controller is technically known under the term of cascade control. If the need arises, this provides mechanisms for the efficient adjustment of controlling elements. Clinically, the relatively strong additional direct and indirect influences of FT3 on TSH control demonstrate an important element in determining a suitable set point for adequate treatment. (2) The findings identify wide individual variations in both the biochemical and symptomatic treatment responses that demand more flexible treatment modalities. (3) Measurement of FT3 and determination of the conversion rate or global deiodinase activity may aid in identifying subgroups of patients on LT4 with impaired biochemistry and displaced relationships responding less satisfactorily to the drug. (4) In view of the heterogeneity in the treatment responses, averaged data from previous RCTs on T3/T4 combination treatment and broadly applied recommendations should be reconsidered in order to better identify and satisfy the variable needs of individuals.
The current retrospective analysis cannot solve the open questions, but may provide novel insights into pituitary control that are at the core of lingering issues with the current standard treatment and a testable approach for persisting symptoms in a minority of patients.
Acknowledgments
The authors wish to thank Hans Günther Wahl, Institute of Laboratory Medicine, Klinikum Luedenscheid, for measurement of thyroid hormones.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: JWD received funding and personal fees by Sanofi-Henning, Hexal AG and Pfizer, and is co-owner of the intellectual property rights for the patent “System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual” (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940-5, WIPO number WO/2014/088516). All other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Contributor Information
Rudolf Hoermann, Department of Nuclear Medicine, Klinikum Lüdenscheid, Paulmannshoeher Str 14, D-58515 Luedenscheid, Germany.
John E. M. Midgley, North Lakes Clinical, Ilkley, UK
Johannes W. Dietrich, Medical Department I, Endocrinology and Diabetology, Bergmannsheil University Hospitals, Ruhr University of Bochum, Bochum, Germany Ruhr Center for Rare Diseases (CeSER), Ruhr University of Bochum and Witten/Herdecke University, Bochum, Germany.
Rolf Larisch, Department of Nuclear Medicine, Klinikum Lüdenscheid, Lüdenscheid, Germany.
References
- 1. Sassolas G, Hafdi-Nejjari Z, Remontet L, et al. Thyroid cancer: is the incidence rise abating? Eur J Endocrinol 2009; 160: 71–79. [DOI] [PubMed] [Google Scholar]
- 2. Amphlett B, Lawson Z, Abdulrahman GO, et al. Recent trends in the incidence, geographical distribution, and survival from thyroid cancer in Wales, 1985–2010. Thyroid 2013; 23: 1470–1478. [DOI] [PubMed] [Google Scholar]
- 3. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016; 12: 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Joint Committee on Cancer. Thyroid. In: Edge DR, Byrd C, Compton C, et al. (eds) AJCC cancer staging manual, 7th edn New York: Springer, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Durante C, Costante G, Filetti S. Differentiated thyroid carcinoma: defining new paradigms for postoperative management. Endocr Relat Cancer 2013; 20: R141–R154. [DOI] [PubMed] [Google Scholar]
- 6. Trigo JM, Capdevila J, Grande E, et al. Thyroid cancer: SEOM clinical guidelines. Clin Translat Oncol 2014; 16: 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001; 86: 1447–1463. [DOI] [PubMed] [Google Scholar]
- 9. Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nature Clin Pract Endocrinol Metab 2005; 1: 32–40. [DOI] [PubMed] [Google Scholar]
- 10. Carhill AA, Litofsky DR, Ross DS, et al. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987–2012. J Clin Endocrinol Metab 2015; 100: 3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nieto HR, Boelaert K. Thyroid stimulating hormone in thyroid cancer: does it matter? Endocr Relat Cancer 2016. DOI: 10.1530/ERC-16-0328. [DOI] [PubMed] [Google Scholar]
- 12. Midgley JEM, Larisch R, Dietrich JW, et al. Variation in the biochemical response to L-thyroxine therapy and relationship with peripheral thyroid hormone conversion. Endocr Connect 2015; 4: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winther KH, Cramon P, Watt T, et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One 2016; 11: e0156925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grozinsky-Glasberg S, Fraser A, Nahshoni E, et al. Thyroxine–triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2006; 91: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 15. Wiersinga WM. Do we need still more trials on T4 and T3 combination therapy in hypothyroidism? Europ J Endocrinol 2009; 161: 955–959. [DOI] [PubMed] [Google Scholar]
- 16. Biondi B, Wartofsky L. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? J Clin Endocrinol Metab 2012; 97: 2256–2271. [DOI] [PubMed] [Google Scholar]
- 17. Wiersinga WM, Duntas L, Fadeyev V, et al. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyr J 2012; 1: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoermann R, Midgley JEM, Giacobino A, et al. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014; 81: 907–915. [DOI] [PubMed] [Google Scholar]
- 19. Dietlein M, Eschner W, Grünwald F, et al. Radiojodtherapie beim differenzierten Schilddrüsenkarezinom. Nuklearmedizin 2016; 55: 77–89. [PubMed] [Google Scholar]
- 20. Larisch R, Giacobino A, Eckl WA, et al. Reference range for thyrotropin. Post hoc assessment. Nuklearmedizin 2015; 54: 112–117. [DOI] [PubMed] [Google Scholar]
- 21. Hoermann R, Larisch R, Dietrich JW, et al. Derivation of a multivariate reference range for pituitary thyrotropin and thyroid hormones: diagnostic efficiency compared with conventional single-reference method. Eur J Endocrinol 2016; 174: 735–743. [DOI] [PubMed] [Google Scholar]
- 22. Dietrich JW, Tesche A, Pickardt CR, et al. Thyrotropic feedback control: evidence for an additional ultrashort feedback loop from fractal analysis. Cybern Syst 2004; 35: 315–331. [Google Scholar]
- 23. Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyr Res 2012; 2012: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoermann R, Midgley JEM, Larisch R, et al. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment? Eur J Endocrinol 2013; 168: 271–280. [DOI] [PubMed] [Google Scholar]
- 25. Dietrich JW, Landgrafe-Mende G, Wiora E. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol 2016; 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dietrich JW. Der Hypophysen-Schilddrüsen-Regelkreis. In: Schardt F. (ed.) Spektrum medizinischer Forschung. Berlin: Logos Verlag, 2002. [Google Scholar]
- 27. Dietrich JW, Tesche A, Pickardt CR, et al. Fractal properties of the thyrotropic feedback control implications of a nonlinear model compared with empirical data. Proc Cybern System 2002; 329–334. [Google Scholar]
- 28. Dietrich JW, Stachon A, Antic B, et al. The AQUA-FONTIS study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome. BMC Endocr Disord 2008; 8: 13. DOI: 10.1186/1472-6823-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoermann R, Midgley JEM, Larisch R, et al. Relational stability of thyroid hormones in euthyroid subjects and patients with autoimmune thyroid disease. Eur Thyr J 2016; 5: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 31. Grömping U. Estimators of relative importance in linear regression based on variance decomposition. Am Stat 2007; 61: 139–147. [Google Scholar]
- 32. Fellows I. Deducer: a data analysis GUI for R. J Stat Softw 2012; 49: 1–15. [Google Scholar]
- 33. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, www.R-project.org (2016, accessed 21 June 2016). [Google Scholar]
- 34. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24: 1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev 2014; 35: 433–512. [DOI] [PubMed] [Google Scholar]
- 36. McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol 2015; 3: 756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dietrich JW, Midgley JEM, Larisch R, et al. Of rats and men: thyroid homeostasis in rodents and human beings. Lancet Diabetes Endocrinol 2015; 3: 932–933. [DOI] [PubMed] [Google Scholar]
- 38. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med 2016; 164: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Investig 2002; 25: 106–109. [DOI] [PubMed] [Google Scholar]
- 40. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. New Engl J Med 1987; 316: 764–770. [DOI] [PubMed] [Google Scholar]
- 41. Gullo D, Latina A, Frasca F, et al. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 2011; 6: e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoermann R, Midgley JEM, Larisch R, et al. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm Metab Res 2015; 47: 674–680. [DOI] [PubMed] [Google Scholar]
- 43. Hoermann R, Midgley JEM, Larisch R, et al. Homeostatic control of the thyroid–pituitary axis: perspectives for diagnosis and treatment. Front Endocrinol 2015; 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ito M, Miyauchi A, Kang S, et al. Effect of the presence of remnant thyroid tissue on the serum thyroid hormone balance in thyroidectomized patients. Europ J Endocrinol 2015; 173: 1–8. [DOI] [PubMed] [Google Scholar]
- 45. Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23: 38–89. [DOI] [PubMed] [Google Scholar]
- 46. Midgley JEM. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem 2001; 47: 1353–1363. [PubMed] [Google Scholar]
- 47. Thienpont LM, Van Uytfanghe K, Beastall G, et al. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; part 2: free thyroxine and free triiodothyronine. Clin Chem 2010; 56: 912–920. [DOI] [PubMed] [Google Scholar]
- 48. Larisch R, Schulte S, Hildenbrand G, et al. The role of thyroid hormones in anxiety and depression. Nuklearmedizin 2015; 53: V162 (Abstract). [Google Scholar]
- 49. Rothacker KM, Brown SJ, Hadlow NC, et al. Reconciling the log-linear and non-log-linear nature of the TSH-free T4 relationship: intra-individual analysis of a large population. J Clin Endocrinol Metab 2016; 101: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 50. Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, et al. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Investig 1995; 96: 2828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Escobar-Morreale HF, del Rey FE, Obregón MJ, et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 1996; 137: 2490–2502. [DOI] [PubMed] [Google Scholar]
- 52. Werneck de, Castro JP, Fonseca TL, Ueta CB, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Investig 2015; 125: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Celi FS, Zemskova M, Linderman JD, et al. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf) 2010; 72: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Escobar-Morreale HF, Obregón MJ, Hernandez A, et al. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 1997; 138: 2559–2568. [DOI] [PubMed] [Google Scholar]
- 55. Galton VA, de Waard E, Parlow AF, et al. Life without the iodothyronine deiodinases. Endocrinology 2014; 155: 4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saravanan P, Chau W-F, Roberts N, et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002; 57: 577–585. [DOI] [PubMed] [Google Scholar]
- 57. Wekking EM, Appelhof BC, Fliers E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 2005; 153: 747–753. [DOI] [PubMed] [Google Scholar]
- 58. Watt T, Groenvold M, Rasmussen ÅK, et al. Quality of life in patients with benign thyroid disorders: a review. Europ J Endocrinol 2006; 154: 501–510. [DOI] [PubMed] [Google Scholar]
- 59. Watt T, Cramon P, Hegedüs L, et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab 2014; 99: 3708–3717. [DOI] [PubMed] [Google Scholar]
- 60. Kelderman-Bolk N, Visser TJ, Tijssen JP, et al. Quality of life in patients with primary hypothyroidism related to BMI. Europ J Endocrinol 2015; 173: 507–515. [DOI] [PubMed] [Google Scholar]
- 61. Massolt ET, van der Windt M, Korevaar TIM, et al. Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2016; 85: 781–788. [DOI] [PubMed] [Google Scholar]