Abstract
Background:
Injury to the distal musculotendinous T junction (DMTJ) of the biceps femoris is a distinct clinical entity that behaves differently from other hamstring injuries due to its complex, multicomponent anatomy and dual innervation. Injury in this region demonstrates a particularly high rate of recurrence, even with prolonged rehabilitation times.
Purpose:
To describe the anatomy of the DMTJ of the biceps femoris and analyze the injury patterns seen on magnetic resonance imaging (MRI) to aid prognosis and rehabilitation and minimize the risk of recurrence.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
Acute injury to the DMTJ of the biceps femoris was identified in 106 MRI examinations from 55 patients at a single institution. Each injury was classified as involving the long head, the short head, or both components of the DMTJ, with each component individually graded. Injuries were classified as recurrent if there was a previous MRI demonstrating an acute injury to the DMTJ or if there was scarring present at the site of an acute injury.
Results:
Of the 106 acute injuries to the DMTJ of the biceps femoris, isolated injury to the long head component was the most common (51%), with both components involved in [round 42.5% to 43%] of cases. Isolated injury to the short head component accounted for 7% of cases. The recurrence rate for reinjury to the DMTJ was 54% in this series. The date of prior injury was known in 45 of 57 recurrent cases, with 34 of these reoccurring within 3 months (76%) and 40 reoccurring within 12 months (89%). The recurrent injury was of a higher grade than the prior injury in 22 of 44 instances (50%), the same grade in 16 instances (36%), and a lower grade in 6 instances (14%). Thus, 86% of recurrent injuries were of the same or higher grade than prior injury.
Conclusion:
These results suggest that high-risk muscle injuries, such as that to the DMTJ of the biceps femoris, should be evaluated using MRI to determine the structural components involved and to assess the extent and severity of injury.
Keywords: magnetic resonance imaging, muscle injury, hamstring, biceps femoris
Magnetic resonance imaging (MRI) is increasingly being used to aid the diagnosis, management, and rehabilitation of acute and recurrent muscle injuries, particularly in professional sports. More than 90% of all muscle injuries in professional athletes affect 4 muscle groups (hamstrings, quadriceps, adductors, and calf muscles),13 but the injuries to each of these muscle groups behave differently. Muscles such as the biceps femoris that cross multiple joints and have complex anatomy are more susceptible to indirect injury.19 Hamstring strains are the most common injury in running and kicking sports, and the majority of these affect the biceps femoris, with reported rates of 57% to 87%.7,9,10,15,25,32 Recurrent hamstring injuries are common, with a reported incidence of 12% to 63%. Previous hamstring injury is one of the most important reinjury risk factors.‖
In our experience, injury to the distal musculotendinous T junction (DMTJ) of the biceps femoris is a distinct clinical entity that behaves differently from other hamstring injuries due to its complex, multicomponent anatomy and dual innervation. Injury in this region demonstrates a particularly high rate of recurrence, even with prolonged rehabilitation times. To our knowledge, this subset of hamstring injury has not been specifically reported in the literature. The aim of this study was to describe the anatomy of the DMTJ of the biceps femoris as well as the injury patterns and features seen on MRI in this region.
Methods
Between February 2011 and July 2015, approximately 1850 examinations of the hamstrings using 3.0-T MRI have been performed at our institution. Of these, 106 MRI examinations in 55 patients demonstrated an acute indirect injury to the DMTJ of biceps femoris, and these patients were included in this study. All patients gave prospective written informed consent at the time of their examination, and ethics approval for the study was obtained.
All MRIs were performed on Phillips Ingenia 3.0-T units with a 32-channel body coil and a skin marker placed over the region of clinical concern. Axial, coronal, and sagittal T2 SPAIR (spectral adiabatic inversion recovery) fat saturation and axial proton density sequences were routinely obtained. Coronal and sagittal T2 SPAIR fat saturation parameters were field of view (FOV) 320 × 200 mm, slice thickness 3.0 mm, 20 slices, slice gap 1.5 mm, repetition time/echo time (TR/TE) 3132 ms/60 ms, and voxel size 0.9 × 0.9 × 3.0 mm reconstructed to 0.45 × 0.45 × 3.0 mm. Axial proton density parameters were FOV 200 × 200 mm, slice thickness 3.5 mm, 36 slices, slice gap 2.5 to 3.5 mm, TR/TE 3570 ms/30 ms, and voxel 0.6 × 0.6 × 3.5 mm reconstructed to 0.35 × 0.35 × 3.5 mm. Axial T2 SPAIR fat saturation parameters were FOV 200 × 200 mm, slice thickness 3.5 mm, 36 slices, slice gap 2.5 to 3.5 mm, TR/TE 4300 ms/50 ms, and voxel 0.8 × 0.85 × 3.5 mm reconstructed to 0.42 × 0.42 × 3.5 mm.
Patient data and the reports of the MRI examinations were collated by a musculoskeletal radiology fellow (T.E.) and a medical student. The MRI examinations were then independently assessed and graded by the same fellow and an experienced musculoskeletal radiologist (T.E. and D.C.) with 1 and 20 years of experience, respectively. Disputes were resolved by consensus.
Only acute injuries involving the DMTJ of the biceps femoris, with the scan needing to be performed within 1 week of the incident, were included in the study. Excluded were subacute and healing injuries and MRIs demonstrating features of overuse/delayed-onset muscle soreness and contusion.
Each injury was classified as involving the long head, short head, or both components of the DMTJ, with each component independently graded using a 3-point MRI classification system as described by Chan et al.6 The 4-point classification system put forth by Mueller-Wohlfahrt et al26 and Pollock et al30 was not used because of the difficulty in applying it to the 2-component, 3-arm anatomy of the DMTJ of the biceps femoris.
According to the Chan et al6 classification, a grade 1 injury demonstrates peritendinous “feathery” edema and blood-fluid between the muscle fibers without disruption of any portion of the adjacent musculotendinous junction. A grade 2 injury demonstrates a partial-thickness split, defect, or tear in any the components of the musculotendinous junction. A grade 3 injury involves a complete tear of either the long or short head components of the DMTJ. We noted whether the defect or tear involved the adjacent epimysium. Retraction distance was documented for grade 3 tendon ruptures.
A recurrence is defined as a subsequent injury of the same type and at the same site as the index injury after returning to full training. If occurring within 2 months, it is referred to as an “early recurrence”; between 2 and 12 months, a “late recurrence”; and greater than 12 months, a “delayed recurrence.”18 In this study, an injury was classified as recurrent if there was a previous MRI demonstrating an acute injury to the distal musculotendinous junction of the biceps femoris or if there was evidence of scarring at the site of an acute injury. If known, the date of the prior injury and the interval between the 2 injuries was documented.
The presence of scarring in the distal portion of the biceps femoris was noted and classified as involving the DMTJ, the proximal intramuscular tendon, or the adjacent epimysium. The presence of scarring in any of the other thigh muscles was also recorded. Note was also made of other acute thigh muscle findings, such as overuse/delayed-onset muscle soreness and contusion.
MRI Anatomy
The biceps femoris is the most lateral hamstring muscle, and it has 2 heads of origin (Figures 1 and 2). The proximal tendon of the long head arises with the tendon of semitendinosus as the conjoint tendon from the posteroinferior aspect of the ischial tuberosity. The short head arises as a muscular attachment from the line aspera on the posterior aspect of the femoral diaphysis, the lateral supracondylar ridge, and the lateral intermuscular septum. The 2 muscles are in contact in the posterolateral aspect of the distal half of the thigh.
Figure 1.
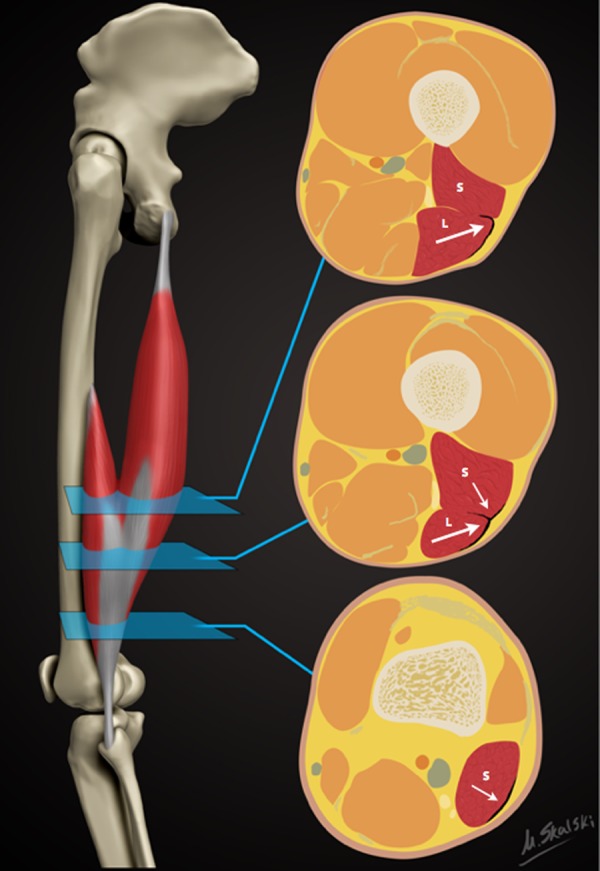
Schematic demonstrating the sequential axial anatomy of the distal musculotendinous T junction (DMTJ) of the biceps femoris. Proximally, the anterolateral epimysial surface of the long head (L) condenses to form the proximal portion of the DMTJ and appears L-, C-, or U-shaped (large arrow). In the midportion, the opposing epimysial condensations at the anterolateral aspect of the long head and the posterolateral aspect of the short head (S, small arrow) form the DMTJ that appears as a T-shaped structure. Distally, the posterolateral epimysial condensation of the short head forms the DMTJ and appears as a shallow convex structure.
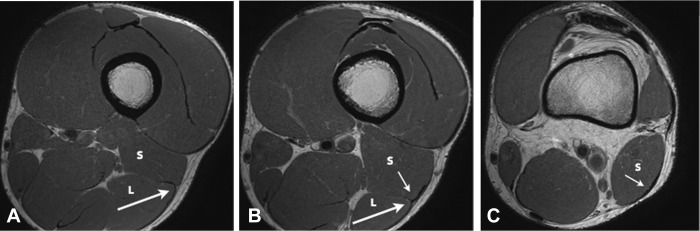
Figure 2.
Axial proton density images demonstrating the anatomy of the distal musculotendinous T junction (DMTJ) of the biceps femoris. (A) The anterolateral epimysial surface of the long head (L) condenses to form the proximal portion of the DMTJ and appears L-, C-, or U-shaped (large arrow). (B) The opposing epimysial condensations at the anterolateral aspect of the long head (L, large arrow) and the posterolateral aspect of the short head (S, small arrow) form the midportion of the DMTJ that appears T-shaped. (C) The posterolateral epimysial condensation of the short head (S, small arrow) forms the distal portion of the DMTJ and appears as a shallow convex structure.
The anatomy of the DMTJ of the biceps femoris is complex and variable. The anterolateral epimysial surface of the distal long head condenses to form the proximal portion of the DMTJ and appears L-, C-, or U-shaped on axial images (Figure 2A). As the long head muscle tapers distally, the opposing posterolateral epimysial surface of the short head condenses, thus forming a “T-shaped” structure on axial images (Figure 2B). The long head component and the deep arm of the T junction taper as they pass distally. Inferior to the distal extent of the long head muscle belly, the short head epimysial condensation is the major component of the DMTJ and has a shallow convex appearance on axial images (Figure 2C). The structure of the DMTJ is quite variable. Any of the 3 arms can be small or hypoplastic relative to the other components.
The distal extent of the long head muscle belly is located on average 5 cm above the knee joint space, ranging from 1.5 to 10 cm.37 The distal extent of the short head muscle belly is located at the level of the knee joint space in 51% of cases, with 21% extending above the level of the joint and 28% extending below37 (Figure 1).
The anatomy of the distal tendon insertion of the biceps femoris has been well described in cadaveric and MRI studies.3,33,34,37 The common free distal tendon splits around the lateral collateral ligament and has 4 separate insertions onto the proximal, distal, and medial footprints of the fibular head as well as a tibial footprint on the lateral tibial plateau behind the Gerdy tubercle.3
The muscle unit as a whole therefore has complex anatomy, with the long head crossing both the hip and knee joint and the short head only crossing the knee joint. The different sites of origin give rise to different force vectors during contraction that converge on, and are transmitted through, the DMTJ. The innervation of the 2 heads also differs, with the long head innervated by the tibial component of the sciatic nerve and the short head innervated by the common peroneal nerve.
Results
The average age of the 55 study patients was 26.4 years; all patients were male. The breakdown of the sport played during injury was Australian Football (AFL), 37 (67%); soccer, 4 (7%); cricket, 3 (5%); rugby league, 1 (2%); athletics, 1 (2%); and hockey, 1 (2%). The sporting activity at the time of injury was unknown in 8 (15%) patients. Professional athletes comprised 78% of the study population.
The distribution of the 106 total injuries is shown in Table 1. There were 54 (50.9%) isolated injuries to the long head component, of which 27 (50.0%) were recurrent. Grade 1 injuries to the long head component (Figure 3) were most common, accounting for 31 of the 54 cases (57.4%), with 9 of these being recurrent (29.0%). Grade 2 injuries (Figure 4) comprised 22 of the 54 cases (40.7%), of which 16 were recurrent (72.7%). There was only 1 grade 3 injury isolated to the long head component (Figure 5), and this was recurrent.
TABLE 1.
Injury Patterns to the Distal Musculotendinous T Junction of Biceps Femoris
| Injury | Grade 1 | Recurrent | Grade 2 | Recurrent | Grade 3 | Recurrent |
|---|---|---|---|---|---|---|
| Overall, n/N | 48/106 | 17/48 | 44/106 | 30/44 | 14/106 | 10/14 |
| % | 45.3 | 35.4 | 41.5 | 68.2 | 13.2 | 71.4 |
| Component | Long Head | Recurrent | Both Heads | Recurrent | Short Head | Recurrent |
| Overall, n/N | 54/106 | 27/54 | 45/106 | 26/45 | 7/106 | 5/7 |
| % | 50.9 | 50.0 | 42.5 | 57.8 | 6.6 | 71.4 |
| Grade 1, n/N | 31/54 | 9/31 | 13/45 | 6/13 | 4/7 | 2/4 |
| % | 57.4 | 29.0 | 28.9 | 46.2 | 57.1 | 50.0 |
| Grade 2, n/N | 22/54 | 16/22 | 19/45 | 11/19 | 3/7 | 3/3 |
| % | 40.7 | 72.7 | 42.2 | 57.9 | 42.9 | 100.0 |
| Grade 3, n/N | 1/54 | 1/1 | 13/45 | 9/13 | 0/7 | 0 |
| % | 1.9 | 100.0 | 28.9 | 69.2 | 0.0 | 0.0 |
Figure 3.
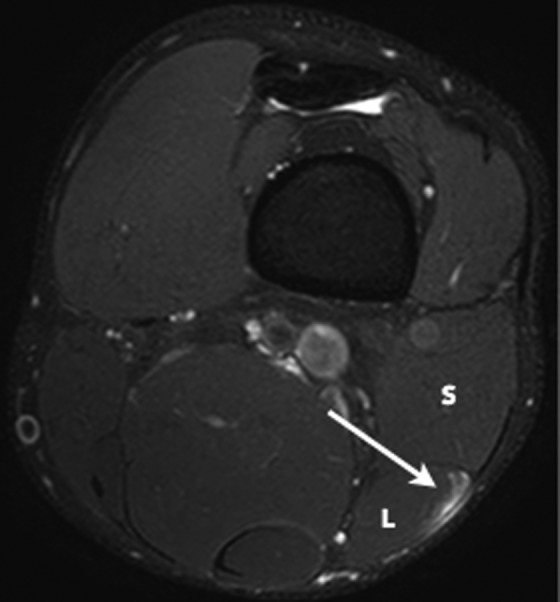
Axial T2 SPAIR (spectral adiabatic inversion recovery) image of a grade 1 strain of the long head component (L) of the distal musculotendinous T junction of the biceps femoris. Note the intact T junction tendon structure with peritendinous edema (arrow) at the myotendinous interface of the long head. The short head component (S) is normal.
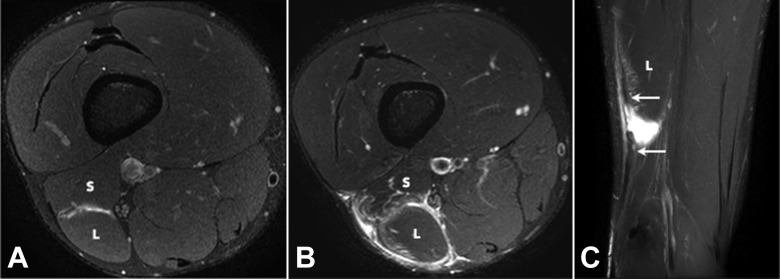
Figure 4.
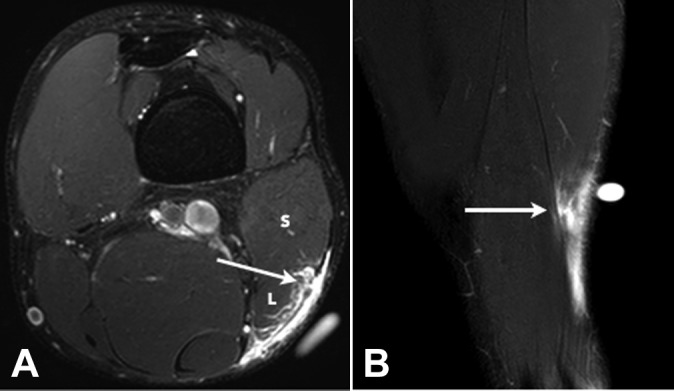
(A) Axial and (B) coronal T2 SPAIR (spectral adiabatic inversion recovery) images of a grade 2 tear of the long head component of the distal musculotendinous T junction of the biceps femoris. There is a partial-thickness tear of the long head component (L) of the T junction tendon structure (arrows) and peritendinous edema and blood fluid.
Figure 5.
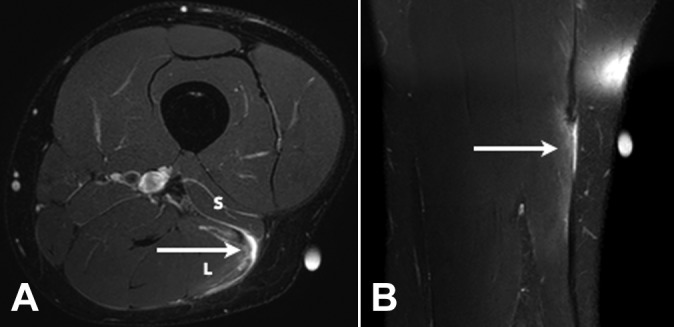
(A) Axial and (B) coronal T2 SPAIR (spectral adiabatic inversion recovery) images of a grade 3 tear of the long head component (L) of the distal musculotendinous T junction of the biceps femoris. There is a full-thickness tear with retraction of the long head component of the T junction tendon structure (arrows) and peritendinous edema and blood fluid present. The short head component (S) is normal.
Of the 106 injuries, 45 (42.5%) involved both the long and short head components, with 26 of these recurrent (57.8%). Grade 1 injuries accounted for 13 of the 45 injuries (28.9%), of which 6 were recurrent (46.2%). Grade 2 partial-thickness tears involving both components (Figure 6) comprised 19 of the 45 cases (42.2%), with 11 of these being recurrent (57.9%). Grade 3 ruptures involving both components comprised 13 of the 45 injuries (28.9%), and 9 of these were recurrent (69.2%).
Figure 6.
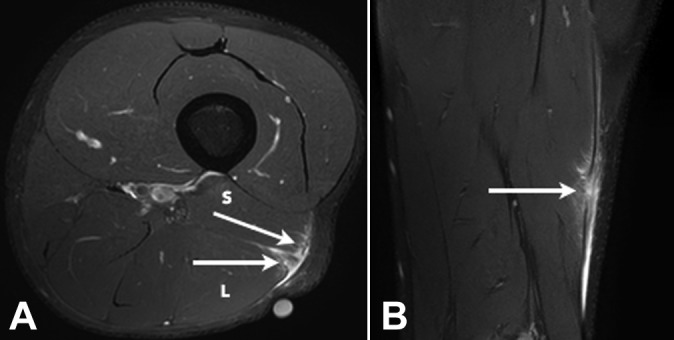
(A) Axial and (B) coronal T2 SPAIR (spectral adiabatic inversion recovery) image of a grade 2 tear of both the long (L) and short (S) head components of the distal musculotendinous T junction of the biceps femoris with several partial-thickness defects in both the long and short head components of the T junction tendon structure (arrows). Peritendinous edema and blood fluid are noted.
Isolated injury to the short head component was uncommon, with 7 cases identified (6.6%). Five of these were recurrent (71.4%). There were 4 grade 1 injuries (57.1%), with 2 of these recurrent (50%). Grade 2 injuries accounted for 3 cases (42.9%), of which all were recurrent (100%). There were no isolated grade 3 ruptures to the short head component.
In 4 patients, a complex pattern of injury was identified that involved both the distal-most aspect of the proximal intramuscular tendon of the long head and the DMTJ (Figure 7).
Figure 7.
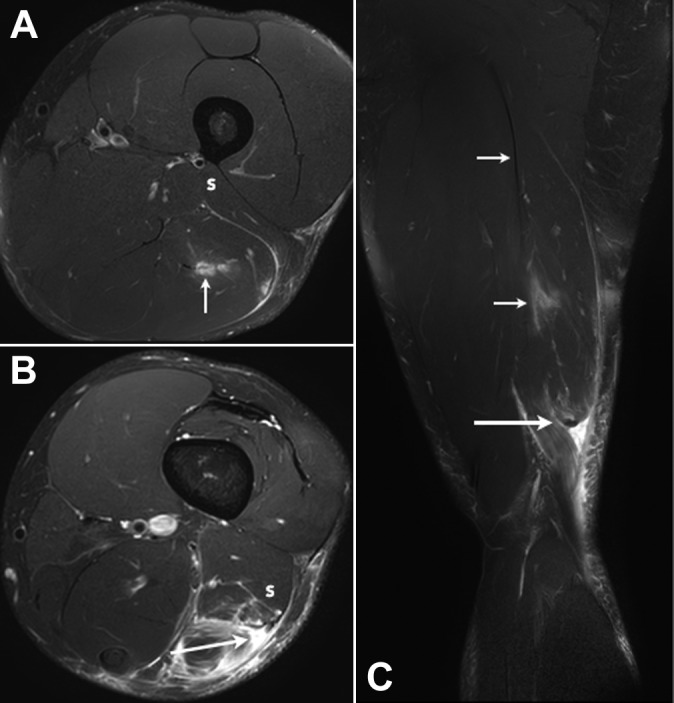
(A, B) Axial and (C) coronal T2-weighted T2 SPAIR (spectral adiabatic inversion recovery) images of a complex multicomponent tear in the distal aspect of the biceps femoris muscle. There is a grade 3 full-thickness tear with retraction of the long head component of the distal musculotendinous T junction (DMTJ) (large arrows). Partial-thickness tears of the short head component of the DMTJ (S) and the distal-most aspect of the proximal intramuscular tendon of the long head (small arrows) are also present.
The overall recurrence rate for reinjury to the DMTJ was 53.8% (57 of 106 injuries). These recurrent injuries were grade 1 in 17 (29.8%) cases, grade 2 in 30 (52.6%) cases, and grade 3 in 10 (17.5%) cases.
There were 44 recurrent injuries in 27 patients where the pattern of recurrences was able to be analyzed. The recurrent injury was of a higher grade than the prior injury in 22 of the 44 cases (50.0%), the same grade in 16 (36.4%), and a lower grade in 6 (13.6%). Thus, 86.4% of recurrent injuries were of the same or higher grade than the prior injury. Furthermore, 68.2% of grade 2 injuries and 71.4% of the grade 3 injuries were recurrent. The date of the prior injury was known in 45 of the 57 recurrent cases, with 34 reoccurring within 3 months (75.6%) and 40 reoccurring within 12 months (88.9%). Two or more recurrences in the same muscle were seen on 11 occasions, with 7 of these demonstrating 3 or more recurrences at the same site (Figure 8).
Figure 8.
(A) Axial T2 SPAIR (spectral adiabatic inversion recovery) image of an initial grade 1 strain of the long (L) and short (S) head components of the distal musculotendinous T junction (DMTJ) of the biceps femoris muscle. (B) Axial and (C) coronal T2 SPAIR images of a subsequent grade 3 tear of the long (L) and short (S) head components of the DMTJ of the biceps femoris muscle in the same patient as in (A). Blood fluid fills the interval between the retracted ends of the tendon (arrows).
Areas of chronic scarring were identified in the distal-most aspect of the biceps femoris muscle in 24 of 63 scanned hamstring muscles (38.1%), in keeping with this being a major risk factor for reinjury. Of these, 14 involved the DMTJ (58.3%), 7 involved the distal portion of the proximal intramuscular tendon (29.2%), and 3 involved the distal epimysium at the level of the DMTJ (12.5%).
Discussion
Complex muscle anatomy appears to be an independent risk factor for injury, longer return to full training, and higher incidence of reinjury.5,8,19,25 The 2 heads of the biceps femoris are innervated separately and have differing force vectors due to their separate sites of origin. The different force vectors converge across the DMTJ and likely contribute to the increased rate of injury and recurrence seen in this region.24
Injury can involve the long head, short head, or both components of the DMTJ of the biceps femoris. In the current study, the long head component was injured in isolation in 50.9% of cases, and the long and short head components were both involved in 42.5% of cases. Isolated injury to the short head component was uncommon (6.6% of cases), in keeping with the notion that muscles crossing a single joint are less susceptible to indirect injury. This study shows that injury can affect the 2 components of the T junction structure with differing severity (Figures 3 –5, 9, and 10).
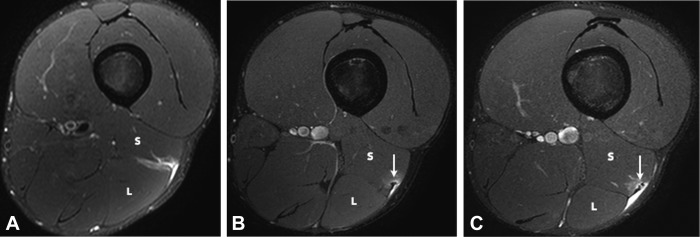
Figure 9.
Axial T2 SPAIR (spectral adiabatic inversion recovery) images of 3 separate injuries involving the distal musculotendinous T junction of the biceps femoris over a 5-week period. The initial injury (A) was a grade 1 strain of both the long (L) and short (S) head components. There is a small amount of muscle fiber disruption at the myotendinous interface of the short head component but the T junction tendon structure was intact throughout. The first recurrent injury (B) was an isolated grade 1 strain of the short head component. A small focus of developing scarring is present at the epicenter of the strain (arrow). The long head component (L) is normal. The second recurrent injury (C) was a grade 2 tear of the short head component, where there is a small longitudinal split/delamination evident (arrow). The long head component (L) is normal.
Figure 10.
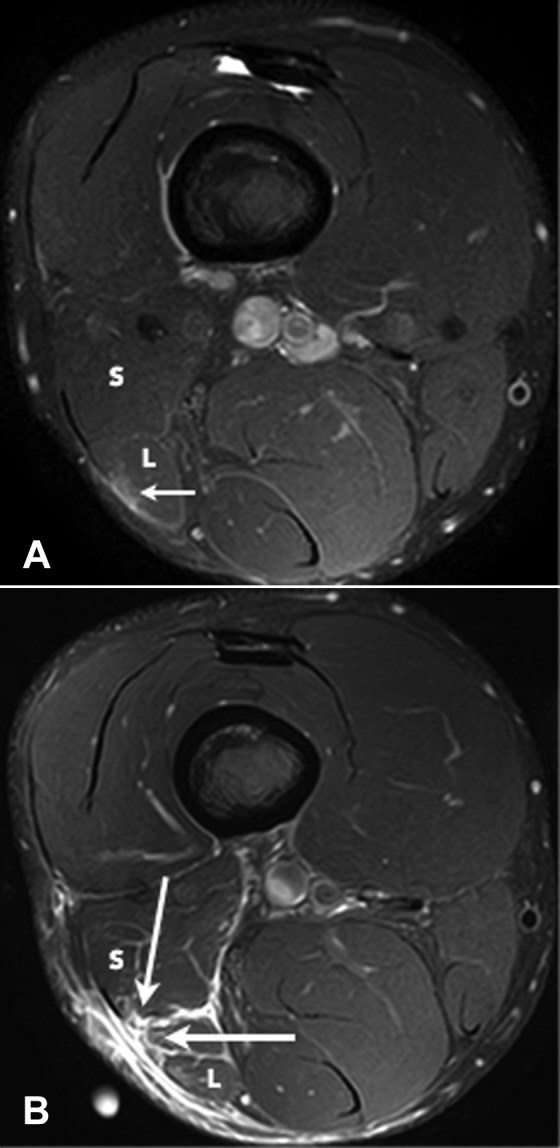
Axial T2 SPAIR (spectral adiabatic inversion recovery) images of 2 separate injuries involving the distal musculotendinous T junction of the biceps femoris over a 4-week period. The initial injury (A) was a grade 1 strain of the long head component (L) and the adjacent posterior epimysium (small arrow). The T junction tendon structure was intact. The recurrent injury (B) demonstrates a full-thickness grade 3 tear with retraction of the long head component and a grade 2 partial-thickness tear of the short head component (large arrows).
The recurrence rate for reinjury to the DMTJ of the biceps femoris in this study was 53.8%, which is at the upper limits of that reported in the literature (12%-63%).¶ This suggests that the DMTJ is one of the most, if not the most, frequently reinjured muscle(s).
Of the 63 biceps muscles scanned, 24 (38.1%) had signs of chronic scarring, and thus evidence of prior injury, at the site of the acute injury. Scarring can be seen on MRI 14 days after the initial injury and appears as spiculated or nodular areas of low signal on the proton density and the T2 SPAIR sequences.24 It usually involves, or is located adjacent to, a connective tissue structure such as the tendon or epimysium.
Given the reduced tensile strength of immature scars, the prolonged time for scar tissue to reach full maturity in active muscles is another factor that contributes to re-injury.22,26 The fibrotic process of scar formation results in tissue contraction, reduced compliance, and altered local biomechanics, all of which have been postulated to contribute to the increased risk of recurrent injury.22,23
We found that 86.4% of recurrent injuries were of the same or higher grade than the prior injury and that 68.2% of grade 2 injuries were recurrent and 71.4% of the grade 3 injuries were recurrent. These results support the idea that recurrent injuries tend to be of greater severity than the index injury.4,13,21,38 This may be due to an underestimation of the extent of injury, an early return to full training, and/or inadequate rehabilitation.
In our experience, injury to the DMTJ becomes relatively symptom free 3 to 4 weeks after the initial injury (pain-free full range of motion and with sport-specific activities), but the scarring at the epicenter of the injury remains as an intermediate-to-low signal on MRI, suggesting immaturity. Scar tissue initially appears ill-defined and with intermediate signal on MRI, then becomes progressively well circumscribed and low signal, and ultimately black, as it matures. MRI is beneficial in the assessment of the extent and severity of the initial injury and the structural muscle components involved, as well as to monitor scar formation and maturation during rehabilitation.
Strengths and Limitations
This study is the largest in the literature focusing on the MRI findings of acute indirect injury to the DMTJ of the biceps femoris. All MRI scans were performed at a single center on 3.0-T units. The study population was relatively homogeneous, as all patients were male and 78% were professional athletes.
The AFL has a higher prevalence and incidence of hamstring muscle injuries relative to other codes. The high proportion of AFL players in our study population (67%) may, in part, have contributed to the high rate of recurrence seen in this study. Hamstring muscle injuries account for 21% of all injuries in the AFL,28 6% to 15% in rugby, 12% in soccer, 11% in cricket, and 6% in basketball.4 Hamstring reinjury rates have been reported at 12% in soccer14 and rugby league,27 16.5% in the National Football League,16 and 23% in Rugby Union,4 compared with 34% in the AFL.28 The increased rate of hamstring injury and recurrent injury in the AFL is likely due to its requiring a combination of high-intensity running, kicking, jumping, and body contact/impact.
The reinjury rate in this study may also have been overstated due to bias in the patient population, where higher-grade injuries clinically, as well as recurrent injuries, are more likely to be scanned with MRI. Further to this, clinical data were lacking, with the time to return to full training an important parameter that would have added significant weight to the results.
Conclusion
Injuries to the DMTJ of the biceps femoris involved the long head component in 51% of cases, both the long and short head components in 43% of cases, and the short head component in 7% of cases in this study. The recurrence rate of injury to the DMTJ of the biceps femoris in this series was 54%, suggesting it is one of the most commonly reinjured muscles. The recurrent injury occurred within 3 months in 76% of cases, with the subsequent injury of the same or higher grade than the prior injury in 86% of cases. These results suggest that high-risk muscle injuries, such as to the DMTJ of the biceps femoris, should be evaluated with MRI to determine the structural components involved and to assess the extent and severity of injury.
Acknowledgment
The authors would like to acknowledge Mr Marcus Davenport in obtaining ethical approval for the study and for his work with the collection of patient data. We would also like to thank Dr Matt Skalski of Radiopaedia for his schematic illustration of the anatomy of the distal musculotendinous T junction. Finally, we recognize the excellent work of the MRI and all other staff at Imaging @ Olympic Park in Melbourne.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from Monash University Human Research Ethics Committee (Project No. CF13/2997-2013001608).
References
- 1. Ahmad CS, Redler LH, Ciccotti MG, Maffulli N, Longo UG, Bradley J. Evaluation and management of hamstring injuries. Am J Sports Med. 2013;41:2933–2947. [DOI] [PubMed] [Google Scholar]
- 2. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(suppl 1):5S–16S. [DOI] [PubMed] [Google Scholar]
- 3. Branch EA, Anz AW. Distal insertions of the biceps femoris: a quantitative analysis. Orthop J Sports Med. 2015;3:2325967115602255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34:1297–1306. [DOI] [PubMed] [Google Scholar]
- 5. Brukner P. Hamstring injuries: prevention and treatment—an update. Br J Sports Med. 2015;49:1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan O, Del Buono A, Best TM, Maffulli N. Acute muscle strain injuries: a proposed new classification system. Knee Surg Sports Traumatol Arthrosc. 2012;20:2356–2362. [DOI] [PubMed] [Google Scholar]
- 7. Cohen SB, Towers JD, Zoga A, et al. Hamstring injuries in professional football players: magnetic resonance imaging correlation with return to play. Sports Health. 2011;3:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Comin J, Malliaras P, Baquie P, Barbour T, Connell D. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med. 2013;41:111–115. [DOI] [PubMed] [Google Scholar]
- 9. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: association between anatomical location and extent of injury—a large single-center MRI report. J Sci Med Sport. 2016;19:317–322. [DOI] [PubMed] [Google Scholar]
- 10. De Smet AA, Best TM. MR imaging of the distribution and location of acute hamstring injuries in athletes. AJR Am J Roentgenol. 2000;174:393–399. [DOI] [PubMed] [Google Scholar]
- 11. de Visser HM, Reijman M, Heijboer MP, Bos PK. Risk factors of recurrent hamstring injuries: a systematic review. Br J Sports Med. 2012;46:124–130. [DOI] [PubMed] [Google Scholar]
- 12. De Vos RJ, Reurink G, Goudswaard GJ, Moen MH, Weir A, Tol JL. Clinical findings just after return to play predict hamstring re-injury, but baseline MRI findings do not. Br J Sports Med. 2014;48:1377–1384. [DOI] [PubMed] [Google Scholar]
- 13. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39:1226–1232. [DOI] [PubMed] [Google Scholar]
- 14. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45:553–558. [DOI] [PubMed] [Google Scholar]
- 15. Ekstrand J, Healy JC, Walden M, Lee JC, English B, Hagglund M. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med. 2012;46:112–117. [DOI] [PubMed] [Google Scholar]
- 16. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39:843–850. [DOI] [PubMed] [Google Scholar]
- 17. Freckleton G, Pizzari T. Risk factors for hamstring muscle strain injury in sport: a systematic review and meta-analysis. Br J Sports Med. 2013;47:351–358. [DOI] [PubMed] [Google Scholar]
- 18. Fuller CW, Molloy MG, Bagate C, et al. Consensus statement on injury definitions and data collection procedures for studies of injuries in rugby union. Br J Sports Med. 2007;41:328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrett WE., Jr Muscle strain injuries. Am J Sports Med. 1996;24(suppl 6):S2–S8. [PubMed] [Google Scholar]
- 20. Hagglund M, Walden M, Ekstrand J. Previous injury as a risk factor for injury in elite football: a prospective study over two consecutive seasons. Br J Sports Med. 2006;40:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagglund M, Walden M, Ekstrand J. Risk factors for lower extremity muscle injury in professional soccer: the UEFA Injury Study. Am J Sports Med. 2013;41:327–335. [DOI] [PubMed] [Google Scholar]
- 22. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. [DOI] [PubMed] [Google Scholar]
- 23. Jonhagen S, Nemeth G, Eriksson E. Hamstring injuries in sprinters. The role of concentric and eccentric hamstring muscle strength and flexibility. Am J Sports Med. 1994;22:262–266. [DOI] [PubMed] [Google Scholar]
- 24. Koulouris G, Connell D. Hamstring muscle complex: an imaging review. Radiographics. 2005;25:571–586. [DOI] [PubMed] [Google Scholar]
- 25. Koulouris G, Connell DA, Brukner P, Schneider-Kolsky M. Magnetic resonance imaging parameters for assessing risk of recurrent hamstring injuries in elite athletes. Am J Sports Med. 2007;35:1500–1506. [DOI] [PubMed] [Google Scholar]
- 26. Mueller-Wohlfahrt HW, Haensel L, Mithoefer K, et al. Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med. 2013;47:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Connor D. NRL injury report 2011. Sport Health. 2012;30:12–22. [Google Scholar]
- 28. Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997-2000. Br J Sports Med. 2002;36:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29:300–303. [DOI] [PubMed] [Google Scholar]
- 30. Pollock N, James SL, Lee JC, Chakraverty R. British athletics muscle injury classification: a new grading system. Br J Sports Med. 2014;48:1347–1351. [DOI] [PubMed] [Google Scholar]
- 31. Prior M, Guerin M, Grimmer K. An evidence-based approach to hamstring strain injury: a systematic review of the literature. Sports Health. 2009;1:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR Am J Roentgenol. 2002;179:1621–1628. [DOI] [PubMed] [Google Scholar]
- 33. Sneath RS. The insertion of the biceps femoris. J Anat. 1955;89:550–553. [PMC free article] [PubMed] [Google Scholar]
- 34. Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotatory instability. Am J Sports Med. 1996;24:2–8. [DOI] [PubMed] [Google Scholar]
- 35. Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Esterman A. Assessment of physical examination and magnetic resonance imaging findings of hamstring injury as predictors for recurrent injury. J Orthop Sports Phys Ther. 2006;36:215–224. [DOI] [PubMed] [Google Scholar]
- 36. Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Spriggins AJ. Clinical risk factors for hamstring muscle strain injury: a prospective study with correlation of injury by magnetic resonance imaging. Br J Sports Med. 2001;35:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vieira RL, Rosenberg ZS, Kiprovski K. MRI of the distal biceps femoris muscle: normal anatomy, variants, and association with common peroneal entrapment neuropathy. AJR Am J Roentgenol. 2007;189:549–555. [DOI] [PubMed] [Google Scholar]
- 38. Walden M, Hagglund M, Ekstrand J. Injuries in Swedish elite football—a prospective study on injury definitions, risk for injury and injury pattern during 2001. Scand J Med Sci Sports. 2005;15:118–125. [DOI] [PubMed] [Google Scholar]
- 39. Woods C, Hawkins RD, Maltby S, et al. The Football Association Medical Research Programme: an audit of injuries in professional football—analysis of hamstring injuries. Br J Sports Med. 2004;38:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]