Abstract
Background:
Women are at substantially greater risk for anterior cruciate ligament (ACL) injuries than are men.
Purpose:
To conduct a systematic review and meta-analysis of the literature to clarify the effect of the menstrual cycle and contraceptives on the laxity of and noncontact injuries to the ACL.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
Searches were conducted using MEDLINE (1946–August 2016), the Cochrane Library Database, clinical trial registries, and related reference lists. Search terms included athletic injuries, knee injuries, ligaments, joint instability, menstrual cycle, ovulation, hormones, and contraceptives. Investigators independently dually abstracted and reviewed study details and quality using predefined criteria and evaluated overall strength of evidence using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria.
Results:
Twenty-one studies totaling 68,758 participants were included: 5 on the menstrual cycle and ACL injury, 7 on hormonal contraceptives and ACL injury, as well as 13 on menstrual cycle and ligament laxity. Four of 5 studies of women not using hormonal contraception indicated that the luteal phase was the least associated with ACL injuries. The 2 largest and highest quality studies on hormonal contraceptives suggested that hormonal contraceptives may be protective against ACL injury. Six of 12 studies on ACL laxity provided quantitative data for meta-analysis, finding significantly increased laxity during the ovulatory phase compared with the follicular phase.
Conclusion:
The literature suggests an association between hormonal fluctuations and ACL injury. Recent studies have suggested that oral contraceptives may offer up to a 20% reduction in risk of injury. The literature on ACL injuries and the menstrual cycle has more than doubled over the past decade, permitting quantitative analysis for the first time. However, the overall strength of this evidence is low. Promising potential directions for future research include long-term observational studies with ongoing hormonal assays and large interventional trials of follicular suppression, including newer hormonal methods.
Keywords: anterior cruciate ligament, knee injury, hormonal contraceptives, female, human, menstrual cycle, systematic review, meta-analysis, sports medicine
As participation in sports has become increasingly popular among American youth, so too have anterior cruciate ligament (ACL) injuries, increasing 2.3% per year for children between the ages of 6 and 18 years in the United States.4 Overall, ACL injuries have steadily increased over the past 20 years, with a current annual incidence of 200,000.37 The short- and long-term consequences of ACL injuries—including reconstructive surgery, long-term rehabilitation, and premature osteoarthritis51—are costly, with an estimated financial burden of $7.6 billion per year when treated with surgical reconstruction and $17.7 billion per year when treated with rehabilitation in the United States.37 ACL injuries can be especially devastating and even career ending for athletes, with the rate of return to sports being less than 50%.8 Given the ramifications of ACL injuries to society, prevention is critical.
Women and girls are at particularly high risk for ACL injuries, with rates 3 to 6 times greater than men, leading some to suggest a hormonal effect.25 Estradiol, progesterone, and relaxin are the predominant hormones that have been studied in the menstrual cycle relating to ACL laxity. Estradiol and progesterone are at their lowest levels during menses at the beginning of the menstrual cycle (days 1-6). Estradiol reaches its peak concentration around the time of ovulation (days 12-14), with a second lower rise in the luteal phase (days 20-24). Progesterone begins a gradual rise in the late follicular phase just before ovulation, but its highest levels are reached in the mid-luteal phase (days 19-24). These periodic hormonal fluctuations in the menstrual cycle have been postulated to cause ligament laxity, increasing the risk for ACL injuries. Specifically, laboratory studies have found that exposure of the ACL to estradiol results in a dose-dependent reduction in fibroblast and collagen synthesis and that this effect is attenuated by the addition of progestins.56,57 Relaxin is a hormone produced by the ovary and placenta that contributes to laxity of the pubic symphysis in pregnancy and childbirth and has similarly been postulated to have an effect on the ACL in nonpregnant women.18 A small prospective study of elite female athletes playing National Collegiate Athletic Association (NCAA) Division 1 sports found that athletes with relaxin levels greater than 6.0 pg/mL were 4 times more likely to sustain an ACL tear.15
This systematic review examined the effect of the menstrual cycle and hormonal contraceptives on noncontact ACL injuries and laxity.
Methods
The protocol for this meta-analysis is registered with PROSPERO (CRD42016032794. Found at: https://www.crd.york.ac.uk/PROSPERO/). We conducted this review following Institute of Medicine, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidance for systematic reviews and meta-analyses.38,39,50
Data Sources and Searches
A research librarian, experienced in conducting systematic reviews, searched Ovid MEDLINE (1946 to July 29, 2016) and the Cochrane Library Database. Investigators (S.D.H., M.L.M., J-M.G.) supplemented electronic searches with hand-searching of reference lists of retrieved articles. Searches were peer reviewed by a second research librarian, as suggested by the Institute of Medicine, using the Peer Review of Electronic Search Strategies (PRESS) tool.43 The search included MeSH (Medical Subject Headings) terms and key words relating to athletic injuries, knee injuries, ligaments, joint instability, menstrual cycle, ovulation, hormones, and contraceptives.
Study Selection
Two independent reviewers (S.D.H., M.L.M.) identified relevant studies. Included in the review were randomized trials, cohort studies, case-control studies, and case series studies with more than 10 participants that evaluated the association between either menstrual cycle or hormonal contraceptives on either noncontact injury or laxity of the ACL. Discrepancies were resolved through discussion by the review team (S.D.H., M.L.M., J-M.G., W.L., J.B.).
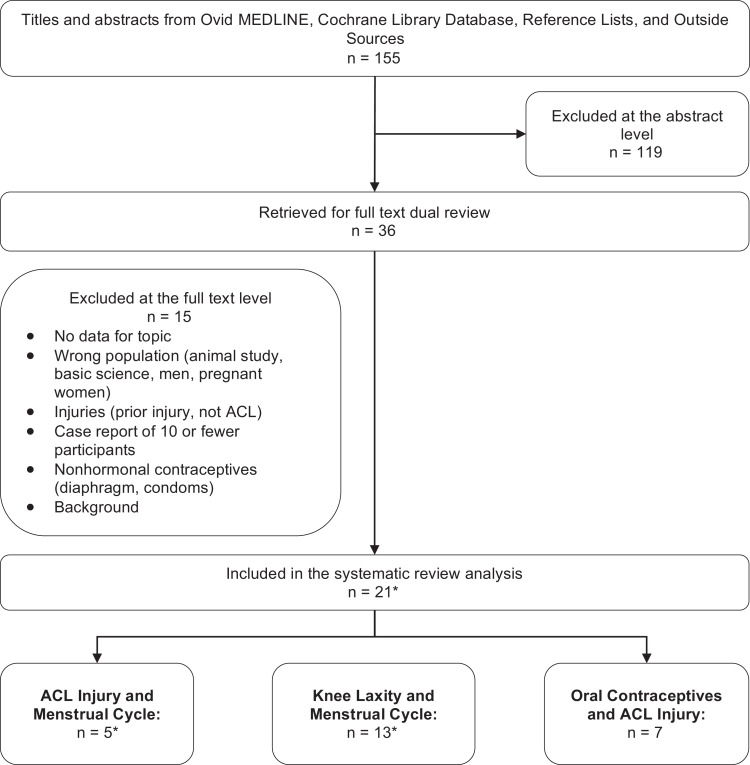
Studies were excluded if they were published exclusively as abstracts, had sample sizes less than 10, included pregnant women, or did not report results specific to phases of the menstrual cycle. Figure 1 provides details on the included and excluded studies at both the abstract and full-text levels.
Figure 1.
Literature flowchart. Background refers to articles that did not provide data but were helpful as background reading for the topic. *Studies overlap.
Data Extraction and Risk of Bias Assessment
Two independent reviewers extracted data, including study details, population, setting, results, potential confounders, follow-up, and analytic approach. Discrepancies were reconciled through discussion. The risk of bias (labeled as good, fair, or poor study quality) was assessed independently by 2 reviewers for each study using the United States Preventive Services Task Force (USPSTF) criteria.21 Discrepancies were resolved through discussion by the review team (S.D.H., M.L.M., J-M.G., W.L., J.B.). A “best evidence” approach was applied, in which studies with the highest quality and most rigorous design were emphasized.
Data Synthesis
Meta-analysis is useful to obtain precise summary estimates for outcomes. To determine the appropriateness of meta-analysis, we considered clinical and methodological diversity and assessed statistical heterogeneity. Only the outcome of knee laxity was eligible for meta-analysis. Means and standard deviations of knee laxity in each phase of the menstrual cycle were abstracted from included studies. In the KT-1000/2000 tests (MEDmetric Corp), knee laxity is commonly measured at 2 force levels: 134 and 89 N.13 If results from both levels were reported, we used the average in the primary analysis and separate levels in the sensitivity analysis. The pairwise mean differences among the menstrual cycle phases were calculated and combined across studies. The calculation of standard error for the mean difference assumed the correlation between menstrual phases to be 0.50. Additional sensitivity analyses were conducted assuming alternative values of the correlation (0.3 and 0.8).
The DerSimonian-Laird random-effects model was used to combine studies, given the minimally detectable between study heterogeneity.31 The calculation of 95% CI for the combined estimates was adjusted for the multiple comparisons across menstrual phases (ovulatory vs follicular, luteal vs ovulatory, and luteal vs follicular) using Bonferroni correction. Statistical heterogeneity among the studies was assessed using the standard Cochran chi-square test, and the magnitude of heterogeneity by using the I2 statistic.27 Results from the sensitivity analyses were similar and are not separately reported. All analyses were performed using Stata/IC 13.1 (StataCorp).
Overall Assessment of the Strength of Evidence
The quality of evidence for each finding was rated based on criteria established by the GRADE (Grading of Recommendations Assessment, Development and Evaluation) Working Group (GRADEWorkingGroup.org). The overall judgment of the strength of evidence was classified as high, moderate, low, or very low based on study design, magnitude of the association, and consistency of the results across studies.
Results
Of the 155 potentially relevant titles and abstracts, 36 met eligibility criteria for full-text review. A total of 21 articles met our inclusion criteria and were included in the final synthesis: 5 met inclusion the menstrual cycle and ACL injury, 7 for hormonal contraceptives and ACL injury, and 13 for ACL laxity and the menstrual cycle. Three studies overlapped across 2 categories, and 2 studies were published in multiple articles. The search and selection of studies are summarized in Figure 1.
ACL Injury and the Menstrual Cycle
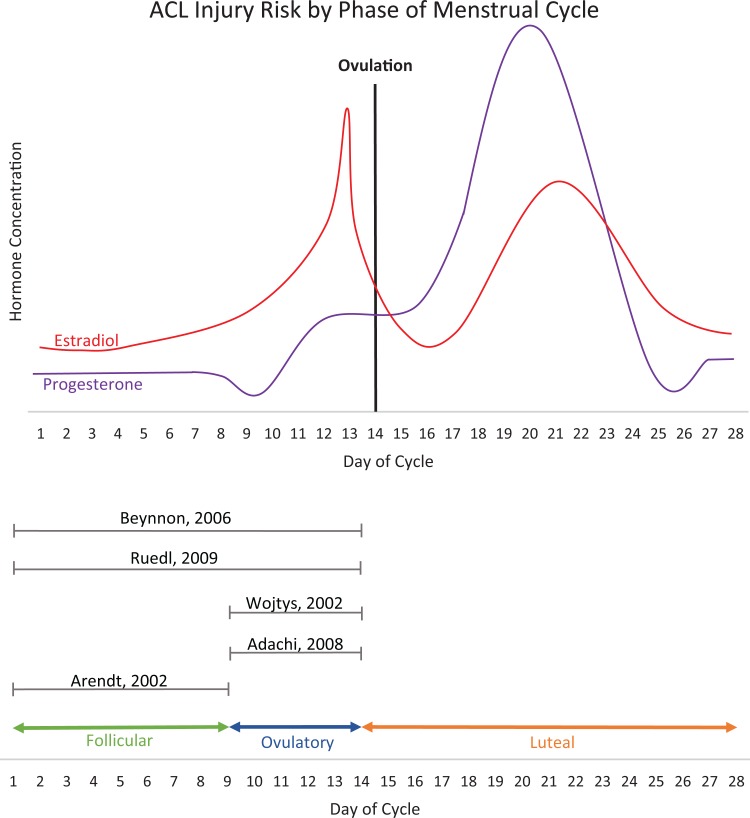
Five studies, 4 poor-quality and 1 fair-quality, examined the incidence of ACL injury and the menstrual cycle: 2 case-control studies7,44 and 3 case series1,3,54 (Table 1). Two studies separated the menstrual cycle into 2 phases, preovulatory and postovulatory.7,44 Both found the incidence of ACL injury was highest in the preovulatory phase. Three studies divided the menstrual cycle into 3 phases: follicular, ovulatory, and luteal. Two of those studies found the incidence of ACL injury was highest in the ovulatory phase,1,54 and 1 study found the highest incidence in the follicular phase.3 Figure 2 presents a schematic of the fluctuations in estradiol and progesterone levels and the classification of menstrual phase used in each of the 5 studies.49 Taken together, these studies suggest that the lowest risk time in the menstrual cycle for ACL injuries is in the luteal phase.
TABLE 1.
ACL Injury and the Menstrual Cyclea
| First Author, Date, Study Design (Quality) | Population (Sample Size, n) | Methods of Determining ACL Injury and Menstrual Cycle | Injury | Findings | ||
|---|---|---|---|---|---|---|
| Follicular | Ovulatory | Luteal | ||||
| Beynnon,7 2006, case-control (fair) | Cases (n = 46): skiers with ACL injuries Controls (n = 45): uninjured alpine skier, not a relative/friend of the injured skier | Questionnaire and serum samples | Based on history: 57% Based on progesterone levels: 73.9% | Based on history: 43% Based on progesterone level: 26.1% | Increased likelihood of ACL injury in preovulatory phase | |
| Ruedl,44 2009, case-control (poor) | Cases (n = 93): injured alpine skiers from a nearby clinic Controls (n = 93): uninjured skiers randomly selected from the lodge | MRI and questionnaire | Patients: 35 (57.4%) Controls: 25 (41.7%) | Patients: 26 (42.6%) Controls: 35 (58.3%) | Increased likelihood of ACL injury in preovulatory phase | |
| Wojtys,54 2002, case series, (poor) | Cases (n = 65): women with acute ACL injuries from local high schools, college, and 4 surrounding test sites | Questionnaire and urine assays | 13/51 (25%) | 24/51 (47%) | 14/51 (27%) | Increased likelihood for ACL injury in ovulatory phase |
| Adachi,1 2008, case series (poor) | Cases (n = 18): teenagers with noncontact ACL injuries | MRI and questionnaire | 2/18 (11%) | 13/18 (72%) | 3/17 (17%) | Increased likelihood for ACL injury in the ovulatory phase |
| Arendt,3 2002, case series (poor) | Cases (n = 83): athletes with noncontact ACL injuries from 1996 to 1998 | Reported by athletic trainer at the school | 26/58 (44.8%) | 10/58 (17.2%) | 22/58 (37.9%) | Increased likelihood for ACL injury in the follicular phase |
aACL, anterior cruciate ligament; MRI, magnetic resonance imaging.
Figure 2.
Fluctuations in estradiol and progesterone Levels. *Adapted from Speroff and Fritz.49
Hormonal Contraceptives and ACL Injury
As Table 2 shows, 7 studies investigated the effects of hormonal contraception on ACL injury. There were 4 studies conducted in the United States (US),20,22,23,26 1 in Austria,44 1 in France,33 and 1 in Denmark.42 The studies ranged in size from 65 to 51,348 participants. Because the question of relative reduction in injury by hormonal contraceptive use requires a joint distribution of ACL injuries and contraceptive users, the most reliable estimates came from the 2 largest and highest quality studies20,42; both suggested a nearly 20% reduced risk for ACL injury while on oral contraceptives (OCs).
TABLE 2.
Hormonal Contraceptives and ACL Injurya
| First Author, Date, Study Design (Quality) | Population (Sample Size, n) | Methods of Determining Cycle Phase and OC Use | On Hormonal Contraception | Off Hormonal Contraception | Findings |
|---|---|---|---|---|---|
| Agel,2 2006, cohort (poor) | NCAA women’s soccer and basketball players (n = 3150) | Reported by athletic trainers to centralized injury surveillance system | Injury: 16/1124 (1.42%) | Injury: 29/2026 (1.43%) | Hormonal contraceptive use had no effect on ACL injury |
| Rahr-Wagner,42 2014, case-control (good) | Women with operatively treated ACL injury registered in the Danish Knee Ligament Reconstruction Registry (n = 4497 cases; 8858 controls) | Danish Knee Ligament Reconstruction Registry linked to Danish Prescription Registry records for 5 years preceding date of injury | Cases: 2047/4497 (45.5%) Controls: 4218/8858 (47.6%) | Cases: 2450/4497 (54.5%) Controls: 4640/8858 (52.4%) | OC use decreased risk of operatively treated ACL injury for long term RR 0.80 (95% CI, 0.74-0.91) and recent OC users RR 0.81 (95% CI, 0.72-0.89) |
| Ruedl,44 2009, case-control (poor) | Female recreational alpine skiers (n = 93 cases; 93 controls) | MRI confirmed ACL, with questionnaire for OC use and cycle phase | Cases: 32/93 (34.4%) Controls: 33/93 (35.5%) | Cases: 61/93 (65.6%) Controls: 60/93 (64.5%) | OC use had no effect (0.95, 95% CI, 0.52-1.74) |
| Wojtys,54 2002, case series (poor) | High school or college-aged women with ACL injuries (n = 65) | Urine assays and questionnaire | 14/65 (21.5%) | 51/65 (78.5%) | Women not taking OCs sustained more injuries during the ovulatory phase (χ2 = 29.8; P < .0001). Women taking OCs demonstrated no change in risk (χ2 = 2.38; P = .7) |
| Lefevre,33 2013, case series (poor) | Female skiers with ACL injuries (n = 172) | Questionnaire | 53/172 (30.8%) | 119/172 (69.2%) | OC use had no effect on ACL injury rates (85/119 [71.4%] OC vs 36/53 no OC, OR, 1.18; 95% CI, 0.59-2.38; P = 0.64]) |
| Arendt,3 2002, cohort (poor) | Female collegiate athletes (n = 83) | Reported by athletic trainers to centralized surveillance system | 20/25 (80%) | 54/58 (93%) | OC users had a greater difference between high- and low-risk periods of ACL injury, but OC use did not change the period of high risk (follicular) |
| Gray,20 2015, case-control (fair) | Females with ACL injury, aged 15-39 years (n = 12,891 cases; 38,457 controls) | ICD-9-CM procedural codes applied to a national insurance claim database | Cases: <90 d of use: 598/12,891 (4.6%) >90 d of use: 2408/12,891 (4.7%) Controls: <90 d of use: 1911/38,457 (5.0%) >90 d of use: 6864/38,457 (17.8%) | Cases: 9885/12,891 (76.7%) Controls: 29,682/38,457 (77.2%) | OC use decreased risk of ACL reconstructions in women 15-19 y (adjusted OR, 0.82; 95% CI, 0.75-0.91; P = .0001) OC use higher in older age groups 25-29 and 30-34 y (adjusted OR, 1.15; 95% CI, 1.02-1.30; P < 0.05 and 1.16; 95% CI, 1.04-1.31; P < .05) |
aACL, anterior cruciate ligament; ICD-9-CM, International Classification of Diseases Ninth Revision Clinical Modification; MRI, magnetic resonance imaging; NCAA, National Collegiate Athletic Association; OC, oral contraceptive; OR, odds ratio; RR, relative risk.
The first was a population-based case-control study from Denmark,42 where all citizens are registered in medical and administrative databases and are given a unique ID number that allows linkage across these databases. The investigators obtained information about ACL injuries through the Danish Knee Ligament Reconstruction Register and Danish National Registry of Patients and obtained information about exposure to OC from the Danish Prescription Registry. This study identified 4497 women with an ACL injury requiring surgery and 8858 age-matched controls with no ACL injury and found an 18% reduction in risk of ACL injury requiring surgery among OC users (relative risk [RR], 0.82; 95% CI, 0.75-0.90). The second was a US population–based case-control study using a large commercial insurance database (Clinformatics Data Mart).20 This study identified 12,819 cases of women sustaining ACL injuries requiring surgery and a randomly selected sample of 38,457 age-matched controls. Similar to findings in the Denmark study, the US analysis found a nearly 20% reduction in risk for OC users (adjusted odds ratio, 0.82; 95% CI, 0.75-0.91).
Knee Laxity and the Menstrual Cycle
Twelve studies examined knee laxity across the menstrual cycle.†† Of these, 4 fair-quality9,24,26,40 and 2 poor-quality5,14 studies provided quantitative data for the meta-analysis; the remaining 6 studies6,28,30,32,47,48 with fair-quality cohort study findings are reported in Table 3.
TABLE 3.
Knee Laxity and the Menstrual Cyclea
| First Author, Date, Study Design (Quality) | Sample Size, n | Methods of Determining ACL Laxity and Menstrual Phase | Data | Findings |
|---|---|---|---|---|
| Beynnon,6 2005, cohort (fair) | 17 | KT-1000 and Ovuquick | Early follicular: 3.1 mm (0.98) Late follicular: 3.1 mm (0.95) Mid-luteal: 3.2 mm (0.86) Late luteal: 3.3 mm (1.27) | No change in laxity across cycle |
| Hoffman,28 2008, cohort, (fair) | 28 | KT-2000 with compu-KT and self-report of phase in cycle with saliva measurements of hormones | Unable to determine summary measures of laxity, and statistical contrast | No change in laxity across cycle |
| Khowailed,30 2015, cohort (fair) | 12 | KT-2000 and blood assays | Early follicular: 4.18 mm (0.27) Ovulatory: 5.75 mm (0.47) (P < .001) | Increased laxity in the ovulatory phase |
| Lee,32 2013, cohort (fair) | 19 | KT-2000 and self-report | Menses: 5.1 mm (1.5) Follicular: 5.4 mm (1.7) Ovulatory: 5.9 mm (1.7) Luteal: 5.7 mm (1.7) (P < .01) | Increased laxity in the ovulatory and luteal phases |
| Shultz,47 2005, cohort (fair) | 22 | KT-2000 and CVS One-Step Ovulation Predictor | Unable to determine summary measures of laxity and statistical contrast | Inconsistent findings of increased laxity in late follicular phase near ovulation and early luteal, relative to menses (significant) |
| Shultz,48 2010, cohort (fair) | 74 | KT-2000 and CVS One-Step Ovulation Predictor | 14-16 visits but only tested twice T1 (menses): 6.7 mm (1.9) T2 (early luteal phase): 7.4 mm (2.1) (P < .001) | Increased laxity in the early luteal phase |
aACL, anterior cruciate ligament.
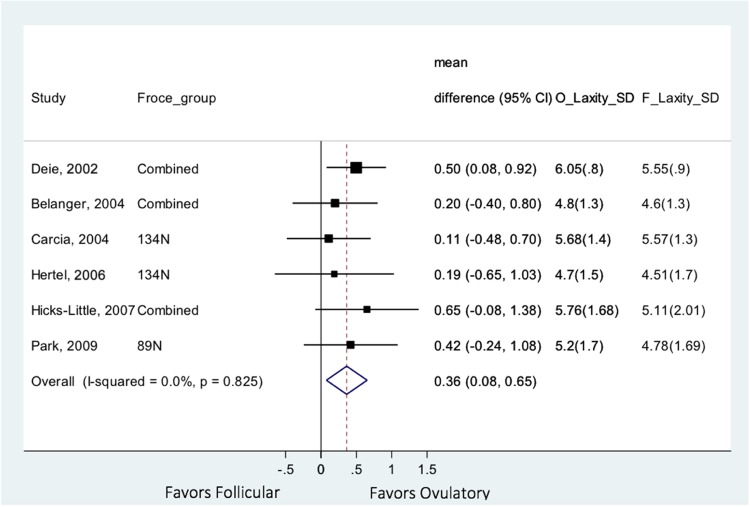
Results and 95% CIs for the 6 studies5,9,14,24,26,40 with data suitable for meta-analysis are presented in Figure 3. Looking across studies and menstrual phases, knee laxity was significantly increased in the ovulatory phase compared with the follicular phase (mean difference, 0.36 mm; 95% CI, 0.08-0.65). Knee laxity did not differ between luteal and ovulatory phases or between luteal and follicular phases. It is unclear whether these measured differences are clinically significant. Of the remaining 6 fair-quality studies that did not report quantitative summary measures appropriate for inclusion in the meta-analysis, 3 reports similarly found increased laxity in the ovulatory phase,30,32,47 1 found the highest laxity in the luteal phase,48 and 2 reported no variation in laxity across the menstrual cycle.6,28
Figure 3.
Meta-analysis: knee laxity in follicular versus ovulatory phases.
These same 12 studies†† related ACL laxity to estradiol levels. Because self-reported cycle length is notoriously inaccurate, we focused on the 5 fair-quality studies6,28,30,32,47 that provided quantitative data for both estradiol concentration and ACL laxity. To understand whether the estradiol peak or rapid decline affects laxity, measurements across studies at select times were examined: day 1 (menses; lowest estradiol concentration), days 8 to 10 (follicular), days 13-15 (ovulation), and day 22 (luteal) among these 5 studies (Table 4). Four of the 5 studies28,30,32,47 reported highest ligament laxity when estradiol concentration was highest, suggesting that estradiol may be contributing to increased laxity. While 3 studies30,32,47 reported the highest estradiol concentration in the follicular phase as expected, 1 study28 reported the highest peak at day 22 (late luteal phase). Although there was a second estradiol peak in the luteal phase, the highest occured in the follicular phase. Finally, it was unclear why the ACL laxity measurements in 1 study6 were nearly double that of the others and why estradiol levels in 1 study28 were lower than physiological levels.
TABLE 4.
Estradiol Levels and Laxity Measurements
| Menses, d | ||||||
|---|---|---|---|---|---|---|
| First Author, Date | 1 | 8-10 | 13-15 | 22 | Testing Methods | |
| Khowailed,30 2015 | Estradiol, pg/mL | 34 | — | 207.74 | — | ClearBlue ovulatory test; KT-2000 |
| Laxity, mm | 4.18 | — | 5.74 | — | ||
| Hoffman,28 2008 | Estradiol, pg/mL | 1.52 | 2.23-2.41 | 2.31 | 3.6 | Self-report; KT-2000 |
| Laxity, mm | 5.29 | 5.16-5.25 | 5.44 | 5.72 | ||
| Shultz,47 2005 | Estradiol, pg/mL | 50 | — | 175 | 129-138 | Ovulatory test; KT-2000 |
| Laxity, mm | 5.0 | — | 5.4 | 5.4-5.3 | ||
| Beynnon,6 2005 | Estradiol, pg/mL | 50 | — | 200 | 175 | Ovuquick; bloodwork; KT-1000 |
| Laxity, mm | 9.1 | — | 8.9 (11.13) | 8.7 | ||
| Lee,32 2013 | Estradiol, pg/mL | 51.4 | 82.5 | 175 | 130 | Ovulatory test; KT-2000 |
| Laxity, mm | 5.1 | 5.4 | 5.9 | 5.7 | ||
Overall Strength of Evidence
Following the GRADE approach, the evidence for this review was classified as “very low” in that “We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect” (GRADEWorkingGroup.org).
Discussion
Studies to date suggest that knee ligament laxity and risk of ACL injury may be increased during the ovulatory phase of the menstrual cycle and that suppression of follicular development and ovulation with hormonal contraceptives may reduce this risk. This finding differs from the last systematic review on this subject, published almost a decade ago.25 Since that time, the number of published studies has more than doubled and the total number of included participants increased from 382 to 68,758. Thus, one of the strengths of this review is a much larger body of evidence, allowing the use of quantitative meta-analysis to provide summary measures. Although the literature has increased substantially, the overall strength of this evidence, and therefore confidence in the findings, remains low.
Studies to date suggest a potential connection between hormonal levels in the menstrual cycle and knee laxity and/or injury. Several theories exist regarding the higher incidence of ACL rupture in women versus men, including anatomic differences,‡‡ dynamic factors such as jumping and landing mechanics,11,19,36,58 and ligamentous laxity. Fluctuations in hormones have been hypothesized to potentially play a role in the latter two. Hormones have been hypothesized to influence ACL injury by exerting a direct effect of ligament laxity or stiffness through collagen synthesis and tensile properties given estrogen, progesterone, testosterone, and relaxin receptors on the ACL or through neuromuscular changes affecting knee alignment.16,35,45 ACLs exposed to increased estradiol in cell culture show decreased fibroblast proliferation and alterations in collagen synthesis, whereas progesterone is associated with increased fibroblast proliferation and increased collagen synthesis.56,57
Hormones have also been hypothesized to exert an effect through neuromuscular changes that are thought to affect knee alignment. Two included studies24,30 looked at the effect of hormonal fluctuations on neuromuscular activity. Both used electromyography (EMG) to measure the neuromuscular activity of the quadriceps and hamstrings. One study found no difference,24 while the other30 found increased activity in the follicular phase, with most pronounced effect in the lateral quadriceps (as measured by EMG activity) whereas hamstring activity was increased in the ovulatory compared with the follicular phase. Specifically, the medial hamstring had the greatest activity before impact.30 Khowailed et al30 suggested that these differences result in misalignment of the knee prior to impact and may contribute to increased ACL injuries observed in the ovulatory period.
Several authors§§ have investigated the possibility of the hormonal variations in the menstrual cycle playing a role in joint laxity and possibly predisposition to instability and resultant ligament rupture. The higher rate of noncontact ACL ruptures in women has raised particular interest in ligamentous laxity as a potential contributor. The classic noncontact ACL injury mechanism involves a combination of change of direction, internal rotation, and valgus across the knee. The tibia internally rotates and translates anteriorly, creating a “pivot shift” and placing the ACL in excessive tension. Any increase in ligamentous laxity, therefore, might allow more tibiofemoral motion and increase the risk of injury.
If the hormonal fluctuations involved in ovulation cause an increase in ACL rupture risk, suppression of follicular development and ovulation could be protective. While smaller studies found no effect, larger studies20,42 powered to detect a difference in the joint association between hormonal contraception and ACL injury suggested a potential 20% reduction in risk. It is possible that the relative progesterone dominance of hormonal contraceptives mitigates the effect of estradiol. However, the 2 studies that suggested this association20,42 were based on administrative data from patients undergoing surgery. While the studies attempted to adjust for important confounders such as age, it is impossible to assign cause and effect due to the retrospective nature of these studies. These interesting findings, however, suggest that a large long-term cohort study of female elite athletes on and off OCs or an interventional trial of hormonal contraceptives among elite athletes (to control for healthy user effect) would be highly valuable.
An important finding of our systematic review is our conclusion that the current quality of evidence is very low. Despite 21 studies published to date, including 68,758 participants, methodological shortcomings limited the confidence in their findings. Our review identified problems with the assembly and maintenance of the cohort (lack of clarity or not including all participants who met eligibility criteria), lack of blinding of outcome assessors (eg, people performing Lachman test) to exposure, and considering and adjusting for important confounders such as excluding people with prior ACL injury, and/or those with a history of smoking, known collagen disorders, and diabetes; confirming ACL injury by magnetic resonance imaging; accounting for athletic status, age, and body mass index; and adjusting for medications such as corticosteroids. Addressing these important population, outcome measurement, and analytic factors is feasible in our judgment.
One of the continued and important challenges faced by investigators is the accurate assessment of menstrual cycle phase and hormonal evaluation. A recent population-based study revealed that more than one-third of women with normal cycle length were anovulatory.41 Methods used to obtain hormone levels varied from serum, urine, and saliva and varied in timing of evaluations; in addition, for some studies the levels were not consistent with expected peak levels in threshold or timing, suggesting that some women might not have had ovulatory cycles (this would not be unusual for elite athletes) or were in a different phase than expected. The ideal study would evaluate serum hormonal levels on a regular basis over longer than a single cycle in order to account for cycle differences.
There are additional factors to consider for laxity assessment. Studies have suggested that Lachman testing measurements vary based on temperature and repeated measurements, with the least laxity being reported in the first examination and plateauing after repeated measures (thought possible because of learned relaxation after initial test). Extending the evaluation over a longer time frame would help account for these effects. Finally, studies of hormonal contraception focused exclusively on combined OCs and not newer progesterone-only options. Given that the timing of injury and laxity appears to coincide with the estradiol peak, it is possible that the reason studies have not found a protective effect is because the contraceptives used contain estradiol. Studies comparing injury rates and/or laxity among girls and women using progesterone-only contraceptives are needed.
Our systematic review has several limitations. First, there are few fair- or good-quality studies in this field. Additionally, it is recognized that electronic searches miss up to half of potential studies. While this systematic review followed recommended strategies to identify additional studies, including searching reference lists of prior reviews, some studies may have been erroneously excluded. Because only published studies were included, publication bias could also have potentially affected our results. However, only 1 study published exclusively as an abstract was identified during our search, and including this report would not have changed the results.
In conclusion, the literature suggests that ACL laxity and risk of injury may be increased in the ovulatory phase of the menstrual cycle. However, given the rating of very low strength of evidence, additional studies are needed to address the concerns of bias and confounding identified in this review and meta-analysis.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Adachi N, Nawata K, Maeta M, Kurozawa Y. Relationship of the menstrual cycle phase to anterior cruciate ligament injuries in teenaged female athletes. Arch Orthop Trauma Surg. 2008;128:473–478. [DOI] [PubMed] [Google Scholar]
- 2. Agel J, Bershadsky B, Arendt EA. Hormonal therapy: ACL and ankle injury. Med Sci Sports Exerc. 2006;38:7–12. [DOI] [PubMed] [Google Scholar]
- 3. Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. J Gend Specif Med. 2002;5(2):19–26. [PubMed] [Google Scholar]
- 4. Beck NA, Lawrence JT, Nordin JD, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents over 20. Pediatrics. 2017;139(3):E20161877. [DOI] [PubMed] [Google Scholar]
- 5. Belanger MJ, Moore DC, Crisco JJ, 3rd, Fadale PD, Hulstyn MJ, Ehrlich MG. Knee laxity does not vary with the menstrual cycle, before or after exercise. Am J Sports Med. 2004;32:1150–1157. [DOI] [PubMed] [Google Scholar]
- 6. Beynnon BD, Bernstein IM, Belisle A, et al. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33:1298–1304. [DOI] [PubMed] [Google Scholar]
- 7. Beynnon BD, Johnson RJ, Braun S, et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: a case-control study of recreational alpine skiers. Am J Sports Med. 2006;34:757–764. [DOI] [PubMed] [Google Scholar]
- 8. Bjordal JM, Arnøy F, Hannestad B, Strand T. Epidemiology of anterior cruciate ligament injuries in soccer. Am J Sports Med. 1997;25:341–345. [DOI] [PubMed] [Google Scholar]
- 9. Carcia CR, Shultz SJ, Granata KP, Gansneder BM, Perrin DH. Knee ligament behavior following a controlled loading protocol does not differ by menstrual cycle day. Clin Biomech (Bristol, Avon). 2004;19:1048–1054. [DOI] [PubMed] [Google Scholar]
- 10. Chandrashekar N, Slauterbeck J, Hashemi J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. Am J Sports Med. 2005;33:1492–1498. [DOI] [PubMed] [Google Scholar]
- 11. Chappell JD, Yu B, Kirkendall DT, Garrett WE. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am J Sports Med. 2002;30:261–267. [DOI] [PubMed] [Google Scholar]
- 12. Cheung EC, Boguszewski DV, Joshi NB, Wang D, McAllister DR. Anatomic factors that may predispose female athletes to anterior cruciate ligament injury. Curr Sports Med Rep. 2015;14:368–372. [DOI] [PubMed] [Google Scholar]
- 13. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13:401–407. [DOI] [PubMed] [Google Scholar]
- 14. Deie M, Sakamaki Y, Sumen Y, Urabe Y, Ikuta Y. Anterior knee laxity in young women varies with their menstrual cycle. Int Orthop. 2002;26:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dragoo JL, Castillo TN, Braun HJ, Ridley BA, Kennedy AC, Golish SR. Prospective correlation between serum relaxin concentration and anterior cruciate ligament tears among elite collegiate female athletes. Am J Sports Med. 2011;39:2175–2180. [DOI] [PubMed] [Google Scholar]
- 16. Dragoo JL, Lee RS, Benhaim P, Finerman GAM, Hame SL. Relaxin receptors in the human female anterior cruciate ligament. Am J Sports Med. 2003;31:577–584. [DOI] [PubMed] [Google Scholar]
- 17. Everhart JS, Flanigan DC, Chaudhari AM. Anteromedial ridging of the femoral intercondylar notch: an anatomic study of 170 archival skeletal specimens. Knee Surg Sports Traumatol Arthrosc. 2014;22:80–87. [DOI] [PubMed] [Google Scholar]
- 18. Faryniarz DA, Bhargava M, Lajam C, Attia ET, Hannafin JA. Quantitation of estrogen receptors and relaxin binding in human anterior cruciate ligament fibroblasts. In Vitro Cell Dev Biol Anim. 2006;42:176–181. [DOI] [PubMed] [Google Scholar]
- 19. Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35:1745–1750. [DOI] [PubMed] [Google Scholar]
- 20. Gray AM, Gugala Z, Baillargeon JG. Effects of oral contraceptive use on anterior cruciate ligament injury epidemiology. Med Sci Sports Exerc. 2015;48:648–654. [DOI] [PubMed] [Google Scholar]
- 21. Harris R, Atkins D, Berg A, Best D, Eden K, Feightner J. US Preventive Services Task Force Procedure Manual. Rockville, MD: Agency for Healthcare Research and Quality; 2001. [Google Scholar]
- 22. Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashemi J, Mansouri H, Chandrashekar N, Slauterbeck JR, Hardy DM, Beynnon BD. Age, sex, body anthropometry, and ACL size predict the structural properties of the human anterior cruciate ligament. J Orthop Res. 2011;29:993–1001. [DOI] [PubMed] [Google Scholar]
- 24. Hertel J, Williams NI, Olmsted-Kramer LC, Leidy HJ, Putukian M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:817–822. [DOI] [PubMed] [Google Scholar]
- 25. Hewett TE, Zazulak BT, Myer GD. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35:659–668. [DOI] [PubMed] [Google Scholar]
- 26. Hicks-Little C, Thatcher J, Hauth J, Goldfuss A, Cordova M. Menstrual cycle stage and oral contraceptive effects on anterior tibial displacement in collegiate female athletes. J Sports Med Phys Fitness. 2007;47:255–260. [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffman M, Harter RA, Hayes BT, Wojtys EM, Murtaugh P. The interrelationships among sex hormone concentrations, motoneuron excitability, and anterior tibial displacement in women and men. J Athl Train. 2008;43:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hudek R, Fuchs B, Regenfelder F, Koch P. Is noncontact ACL injury associated with the posterior tibial and meniscal slope? Clin Orthop Relat Res. 2011;469:2377–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khowailed IA, Petrofsky J, Lohman E, Daher N, Mohamed O. 17β-Estradiol induced effects on anterior cruciate ligament laxness and neuromuscular activation patterns in female runners. J Womens Health (Larchmt). 2015;24:670–680. [DOI] [PubMed] [Google Scholar]
- 31. Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res. 2012;21:409–426. [DOI] [PubMed] [Google Scholar]
- 32. Lee H, Petrofsky JS, Daher N, Berk L, Laymon M, Khowailed IA. Anterior cruciate ligament elasticity and force for flexion during the menstrual cycle. Med Sci Monit. 2013;19:1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lefevre N, Bohu Y, Klouche S, Lecocq J, Herman S. Anterior cruciate ligament tear during the menstrual cycle in female recreational skiers. Orthop Traumatol Surg Res. 2013;99:571–575. [DOI] [PubMed] [Google Scholar]
- 34. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu SH, al-Shaikh R, Panossian V, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14:526–533. [DOI] [PubMed] [Google Scholar]
- 36. Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech (Bristol, Avon). 2001;16:438–445. [DOI] [PubMed] [Google Scholar]
- 37. Mather RC, 3rd, Koenig L, Kocher MS, et al. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 39. Morton S, Levit L, Berg A, Eden J. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: Institute of Medicine; 2011. [PubMed] [Google Scholar]
- 40. Park SK, Stefanyshyn DJ, Ramage B, Hart DA, Ronsky JL. Relationship between knee joint laxity and knee joint mechanics during the menstrual cycle. Br J Sports Med. 2009;43:174–179. [DOI] [PubMed] [Google Scholar]
- 41. Prior JC, Naess M, Langhammer A, Forsmo S. Ovulation prevalence in women with spontaneous normal-length menstrual cycles—a population-based cohort from HUNT3, Norway. PLoS One. 2015;10:E0134473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahr-Wagner L, Thillemann TM, Mehnert F, Pedersen AB, Lind M. Is the use of oral contraceptives associated with operatively treated anterior cruciate ligament injury? A case-control study from the Danish Knee Ligament Reconstruction Registry. Am J Sports Med. 2014;42:2897–2905. [DOI] [PubMed] [Google Scholar]
- 43. Relevo R, Paynter R. Peer Review of Search Strategies. Methods Research Report (Prepared by the Oregon Evidence-based Practice Center under Contract No. 290-2007-100572). AHRQ Publication No. 12-EHC068-EF Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 44. Ruedl G, Ploner P, Linortner I, et al. Are oral contraceptive use and menstrual cycle phase related to anterior cruciate ligament injury risk in female recreational skiers? Knee Surg Sports Traumatol Arthrosc. 2009;17:1065–1069. [DOI] [PubMed] [Google Scholar]
- 45. Sciore P, Frank CB, Hart DA. Identification of sex hormone receptors in human and rabbit ligaments of the knee by reverse transcription-polymerase chain reaction: evidence that receptors are present in tissue from both male and female subjects. J Orthop Res. 1998;16:604–610. [DOI] [PubMed] [Google Scholar]
- 46. Shelbourne KD, Davis TJ, Klootwyk TE. The relationship between intercondylar notch width of the femur and the incidence of anterior cruciate ligament tears. A prospective study. Am J Sports Med. 1998;26:402–408. [DOI] [PubMed] [Google Scholar]
- 47. Shultz SJ, Sander T, Kirk S, Perrin D. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45:594–603. [PMC free article] [PubMed] [Google Scholar]
- 48. Shultz SJ, Schmitz RJ, Beynnon BD. Variations in varus/valgus and internal/external rotational knee laxity and stiffness across the menstrual cycle. J Orthop Res. 2010;29:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed New York, NY: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 50. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 51. Sugimoto D, Alentorn-Geli E, Mendiguchía J, Samuelsson K, Karlsson J, Myer GD. Biomechanical and neuromuscular characteristics of male athletes: implications for the development of anterior cruciate ligament injury prevention programs. Sports Med. 2015;45:809–822. [DOI] [PubMed] [Google Scholar]
- 52. Todd MS, Lalliss S, Garcia ES, DeBerardino TM, Cameron KL. The relationship between posterior tibial slope and anterior cruciate ligament injuries. Am J Sports Med. 2010;38:63–67. [DOI] [PubMed] [Google Scholar]
- 53. Van Eck CF, Martins CA, Vyas SM, Celentano U, van Dijk CN, Fu FH. Femoral intercondylar notch shape and dimensions in ACL-injured patients. Knee Surg Sports Traumatol Arthrosc. 2010;18:1257–1262. [DOI] [PubMed] [Google Scholar]
- 54. Wojtys EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30:182–188. [DOI] [PubMed] [Google Scholar]
- 55. Wolters F, Vrooijink SH, Van Eck CF, Fu FH. Does notch size predict ACL insertion site size? Knee Surg Sports Traumatol Arthrosc. 2011;19:17–21. [DOI] [PubMed] [Google Scholar]
- 56. Yu WD, Liu SH, Hatch JD, Panossian V, Finerman GA. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop Relat Res. 1999;366:229–238. [DOI] [PubMed] [Google Scholar]
- 57. Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001;383:268–281. [DOI] [PubMed] [Google Scholar]
- 58. Zeller BL, McCrory JL, Kibler WB, Uhl TL. Differences in kinematics and electromyographic activity between men and women during the single-legged squat. Am J Sports Med. 2003;31:449–456. [DOI] [PubMed] [Google Scholar]