Abstract
Skull base lesions can be related to wide number of pathologies including infections, benign and malignant tumors. Accurate diagnosis and differentiation between these entities is important for prompt and appropriate treatment. However, computed tomography and routine magnetic resonance imaging techniques only provide information on the extent of the lesions, with limited ability to differentiate between benign and malignant lesions. Diffusion-weighted imaging can help in many such situations by providing additional information, including help in differentiating benign from malignant lesions, so that appropriate treatment can be initiated. In this review article, we illustrate the imaging findings of the spectrum of skull base lesions, emphasizing the role of diffusion-weighted imaging in this domain.
Keywords: Skull base, diffusion, benign, malignant, imaging
Introduction
The skull base may be involved primarily by intrinsic lesions or secondarily by systemic disease, and by extension from the intra- or extra-cranial head and neck region. Exact histopathological diagnosis, as well as benign versus malignant differentiation, of skull base lesions is crucial for management and prognosis. Clinical evaluation of skull base lesions is not directly accessible, so radiological imaging plays a critical role to identify important anatomic landmarks that are necessary for surgical planning and intra-operative guidance.1
Imaging also helps in a pivotal role in assessment of neoplastic and nonneoplastic skull base pathologies; however, none of the imaging findings are specific due to the inherent limitations of computed tomography (CT) and routine magnetic resonance (MR) techniques.1–3 CT is associated with beam hardening artifacts and poor soft tissue contrast resolution, whereas routine MR pulse sequences are also not reliable in the differentiation and characterization of skull base pathologies. Image-guided biopsies help in histopathological diagnosis: however, they are invasive, associated with complications, require operator skills, and are often denied by the patients.
With the advancement of magnetic resonance imaging (MRI), techniques like diffusion-weighted imaging (DWI) can be used as a non-invasive problem solving tool in the characterization of skull base lesions, evaluation of pathological grading, and monitoring treatment effects.4 DWI derives image contrast from the microscopic movement of water molecules called “Brownian motion” in tissues. The brain is a complex structure, which may and may not allow water to move freely.5 The purpose of this pictorial review is to describe the role of diffusion-weighted MR imaging in skull base lesions, which can serve as a substitute to positron emission tomography in evaluating malignant skull base lesion.
Technical considerations
The extent of translational diffusion of molecules measured in the human body is referred to as the apparent diffusion coefficient (ADC). The ADC is expected to vary according to the cellular density of the lesion and an ADC map can be generated by collecting a series of diffusion-weighted (DW) images with different b values. The ADC map depicts the magnitude of the ADC values in a voxel.6 When a fixed DW b-factor is used, tissue with a higher ADC value produces a lower signal intensity and vice versa. The degree to which a sequence is sensitive to diffusion is represented by the b-value. So, an increase in the b-value results in increased diffusion sensitivity of the image with reduced contribution of T1 and T2 images which in turn reduces the signal to noise ratio and increases time acquisition. DWI images due to T2 effects show artefactual hyperintensity and isointensity known as T2 shine-through and T2 wash-out effects respectively. The ADC map quantitatively eliminates any influence of T2-weighting and shows the true diffusion abnormality which can be calculated during post processing with the use of at least two different b values (b-0 and 1000 mm2/s).
Diffusion imaging has a well-established role in central nervous system (CNS) imaging, and is the subject of active research in imaging other parts of the body.7,8 DW imaging and the ADC have increasingly been used to characterize head and neck tumors and monitor response to treatment. Standard DWI is single shot echo-planar imaging (ss-EPI) which is prone to susceptibility artifacts and relatively low resolution at the tissue-air interface such as at the skull base.9,10 Multishot readout-segmented echo-planar imaging (rs-EPI) with parallel imaging and two-dimensional (2D) navigator-based reacquisition have been applied to generate high-resolution DWI images by decreasing the motion-induced errors which substantially improves the image quality, and accuracy for diagnosis compared to standard ss-EPI protocols.11,12
Benign lesions (Table 1)
Table 1.
Skull base lesions.
| Benign lesions | Malignant lesions |
|---|---|
| Skull osteomyelitis | Chondrosarcoma |
| Cholesteatoma | Chordoma |
| Fibrous dysplasia | Nasopharyngeal carcinoma |
| Juvenile nasopharyngeal angiofibroma | Lymphoma |
| Aspergillus infection | Skull metastases |
| Craniopharyngioma | Rhabdomyosarcoma |
| Ecchordosis physaliphora | Malignant schwannoma |
| Posterior fossa epidermoid | |
| Meningioma |
Skull base osteomyelitis
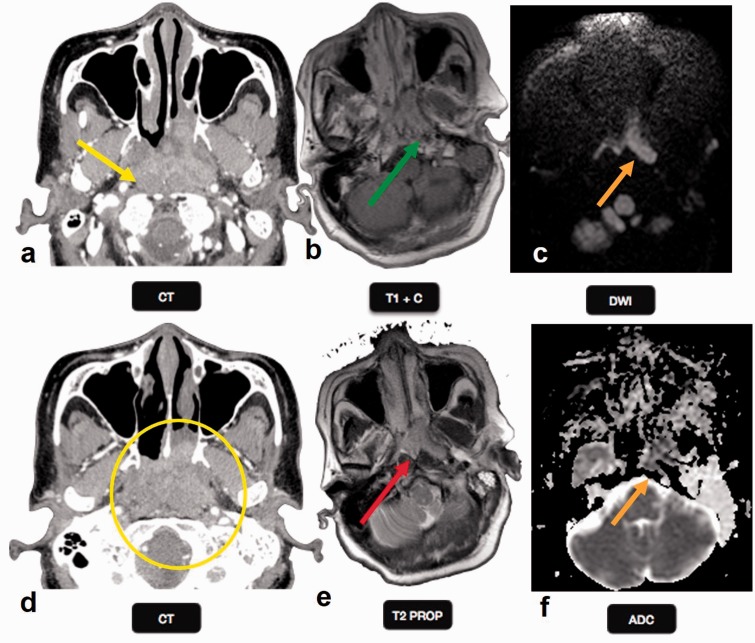
Skull base infections are uncommon aggressive infections of the skull base, seen predominantly in elderly diabetics and immunocompromised patients of all age groups. Skull base osteomyelitis is associated with high morbidity and mortality despite adequate treatment due to low clinical suspicion. MRI helps in showing the subtle early findings of altered signal intensity, enhancement, and bone marrow infiltration pattern which mimics the neoplastic pathology affecting the skull base such as lymphoma, metastasis, or nasopharyngeal carcinoma.13 DW imaging helps in evaluation of these patients to assist in differentiation from other pathologies (Figure 1). In a retrospective study, evaluation of MR imaging findings including DWI found a significantly high mean ADC value of 1.26 × 10−3 mm2/s for skull base osteomyelitis as compared to nasopharyngeal cancer and lymphoma.14
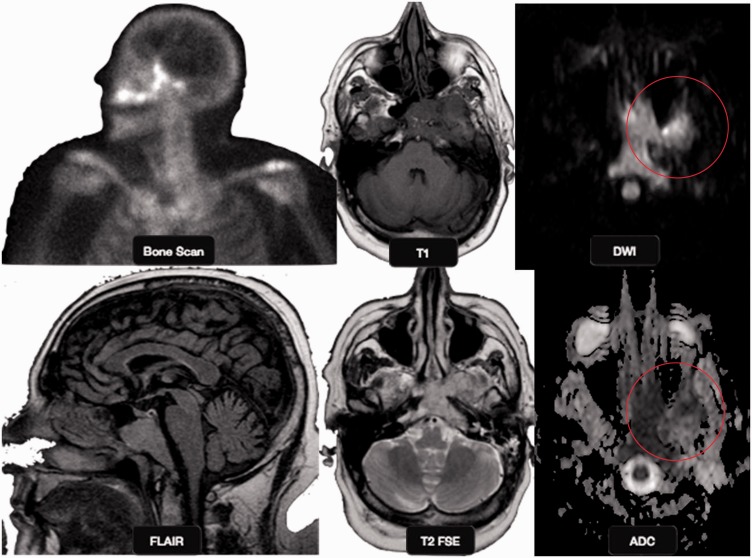
Figure 1.
Skull osteomyelitis: a 35-year-old female with skull bone osteomyelitis. Peripherally enhancing (yellow and red arrows) collections are noted along the left frontal bone extending to involve the skull base, clivus, greater wing and body of sphenoid (red and yellow circles). A breach is noted in the inner and outer skull tables with extension of the collection along the subgaleal region. DW images ((c), (g)) show brightness with low ADC ((d), (h)). DW: diffusion-weighted; ADC: apparent diffusion coefficient.
Cholesteatoma
Cholesteatoma may be a congenital or acquired lesion of the petrous apex arising from the ectopic ectodermal rest cells. The preferred mode of treatment is radical or modified radical mastoidectomy which is associated with high rate of residual and recurrent cholesteatoma of 35% and 18% respectively.15 It is difficult to clinically diagnose the recurrent or residual cholestatoma. MRI with DWI is considered an imaging modality of choice for confirming the diagnosis and extent of cholesteatomas. The recurrent or residual cholestatoma will show restricted diffusion while granulation tissue will not show restricted diffusion.15 However, it is important to interpret the DW images in combination with the conventional MR sequences to avoid false-positive findings. The cholestatoma will appear iso- to hypointense on T1, hyperintense on T2, bright on DWI, and dark on the ADC map (Figure 2). The multishot rs-EPI DW sequence offers an advantage over the ss-EPI DW sequence with a positive predictive value of 93–100% in cases of residual/recurrent or very small lesions due to fewer susceptibility artifacts, thinner sections, and high image resolution.16,17
Figure 2.
Cholesteatoma: an 81-year-old male with left cholesteatoma. Left temporal bone shows abnormal lobulated T2 (a) and T1 (c) isointense enhancing lesion (d). The lesion was engulfing the semicircular canal on FIESTA (b) with sparing of the cochlea. The lesion is bright on DWI (blue circle) and the ADC map (red circle), suggesting T2-shine through phenomenon and benign etiology. FIESTA: fast imaging employing steady-state acquisition; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
Fibrous dysplasia (FD)
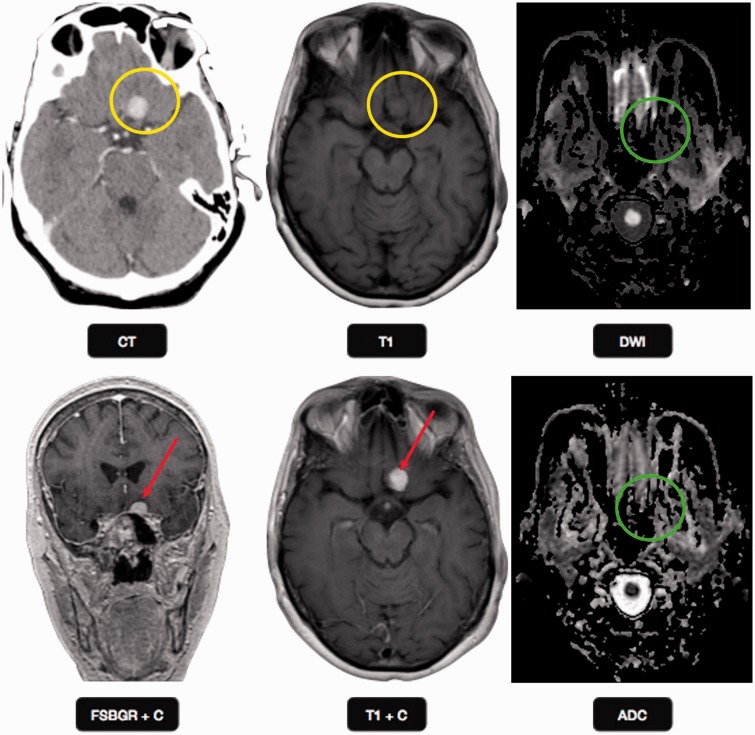
FD comprises 20% of bony skull lesions and can be monostotic or less commonly polyostotic.18 FD is characterized by replacement of the normal bone by dysplastic fibro-osseous tissue and displays various T2 signal intensities and enhancement pattern depending on the fibrous, cellular, cystic, and hemorrhagic changes. FD is best demonstrated on CT which shows an enlarged medullary space with ground-glass appearance (Figure 3). MRI shows low to intermediate T2 signal intensity with varied enhancement (Figure 3). The ADC was found to be high (2.0 × 10–3 mm2/s) in FD in a study of 20 T2 bright bone lesions in comparison to chondrosarcoma.18
Figure 3.
Fibrous dysplasia: a 69-year-old female presented with seizures. CT scan with bone windows through the skull base shows an expansile right greater wing of sphenoid with ground glass appearance (yellow oval), which is a characteristic location and appearance of fibrous dysplasia. T1W MR image shows an intermediate signal in the right lesser wing sphenoid lesion, with no enhancement on post gadolinium MR image (yellow oval). No diffusion restriction was identified on echo planar imaging (red ovals), suggesting benign etiology. CT: computed tomography; MR: magnetic resonance.
Juvenile nasopharyngeal angiofibroma (JNA)
This is a rare benign locally invasive highly vascular tumor of the nasopharynx seen in the adolescent males. JNA often originates in close relation to the sphenopalatine foramen and extends into pterygopalatine, infratemporal fossae, nasopharynx, nasal cavity, sphenoid sinus and intraorbital/intracranial extension with adjacent bony erosion in aggressive cases. Both CT and MRI help in accurate localization and the extension of the JNA which is crucial for the surgical planning. Bony erosion is better appreciated on CT while the extension of the lesion is better evaluated on MRI. JNA shows a characteristic MRI appearance with obliteration of pterygopalatine fossa fat and intralesional signal voids. On MRI, the lesions are mainly isointense to muscle on T1-weighted images and hyperintense on T2-weighted images (Figure 4). All lesions characteristically show internal signal-void and intense enhancement. Diffusion restriction is not an associated feature and a retrospective MR study of six histopathologically proven JNAs found high mean ADC values for these tumors (1.6 × 10–3 mm2/s), consistent with benign lesions.19 Similarly, another study by Razek et al., also found high ADC values (1.82 × 10–3).9
Figure 4.
Juvenile nasopharyngeal angiofibroma: a 17-year-old male with JNA. A large T1 hypo (a) and T2 heterogeneously hyperintense (b) enhancing lesion with necrotic areas ((c), (d)) is seen filling the nasal cavity, nasopharynx, pterygopalantine fossa, extending into ethmoid and sphenoid sinus, infratemporal fossa and eroding sella. The mass shows restricted diffusion on DWI (blue circle) with an a low ADC value (purple circle), suggesting malignant etiology. JNA: juvenile nasopharyngeal angiofibroma; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
Aspergillus infection
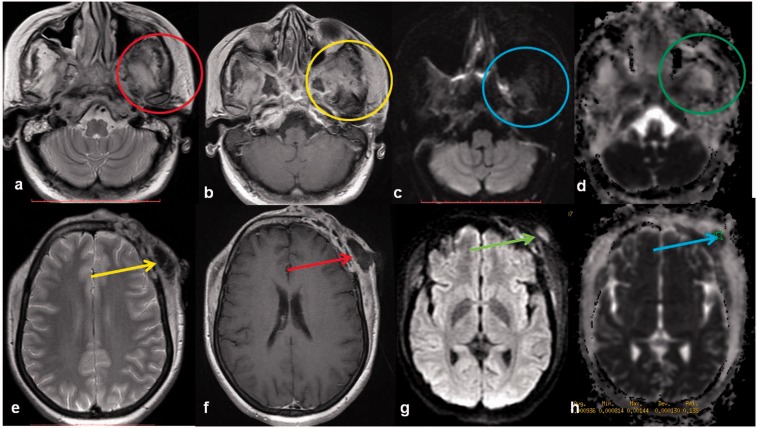
Craniocerebral aspergillosis of sinonasal origin is a rare clinical entity in immunocompetent individuals, and varies from the non-invasive to invasive variety resulting in high morbidity and mortality, warranting early diagnosis and appropriate management. Skull base and orbital erosion are seen in one-third of cases and orbital erosions are more common than skull base erosion.20
MRI in the clinical background may be helpful in early diagnosis and shows typical imaging features which include a mass lesion producing hypo-to-iso-intense signals on T1-weighted, extremely low signals on T2-weighted images, and homogenous enhancement (Figure 5). Sometimes, the conventional CT and MRI appearance of extraaxial fungal granulomas may mimic meningiomas.21 Aspergillus infections show restricted diffusion; however, intracerebral fungal abscesses have been found to have a higher ADC when compared with bacterial abscesses.22,23
Figure 5.
Aspergillus infection: a 70-year-old male patient with throbbing left mandible and maxillary pain. Bone scan performed for unrelated indication showed increased uptake in the left maxillary and paranasal regions. MR images show a T1 intermediate intensity and T2 hyperintense lobulated mass involving the clivus, with extension into the left anterolateral and inferior left orbit. The mass shows restricted diffusion on DWI (red circles) with an intermediate ADC value. MR: magnetic resonance imaging; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
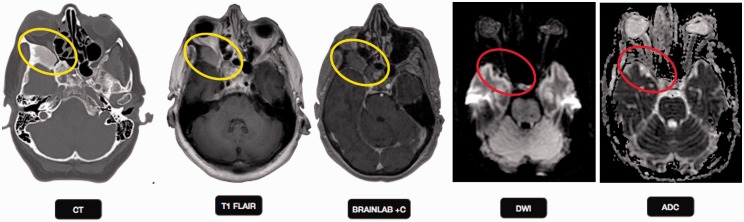
Cranipharyngioma
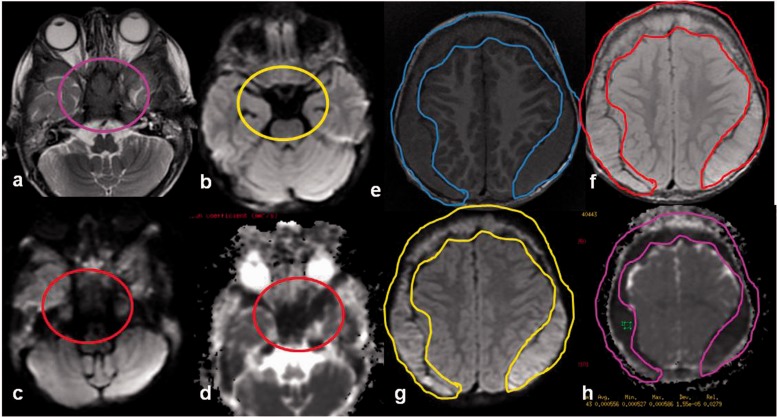
Craniopharyngioma is a benign tumor comprising 1–5% of primary brain tumors. It arises from Rathke's cleft epithelium, typically located in the suprasellar region in 95%, both the suprasellar and intrasellar spaces in 75%, purely suprasellar in 20% and purely intrasellar in <5%. Histologically, craniopharyngiomas have two subtypes; adamantinomatous and papillary accounting for the various imaging features. Adamantinomatous craniopharyngiomas are most common (90%), and typically have a lobulated contour with multiple components and calcification, while papillary craniopharyngiomas are more spherical in outline and usually have more solid than cystic component with less calcification.24 Craniopharyngiomas often show no restricted diffusion (Figure 6). DWI in histopathologically proven 19 craniopharyngioma and 24 germ cell tumors found significantly high ADC values in craniopharyngiomas, consistent with a benign etiology.25
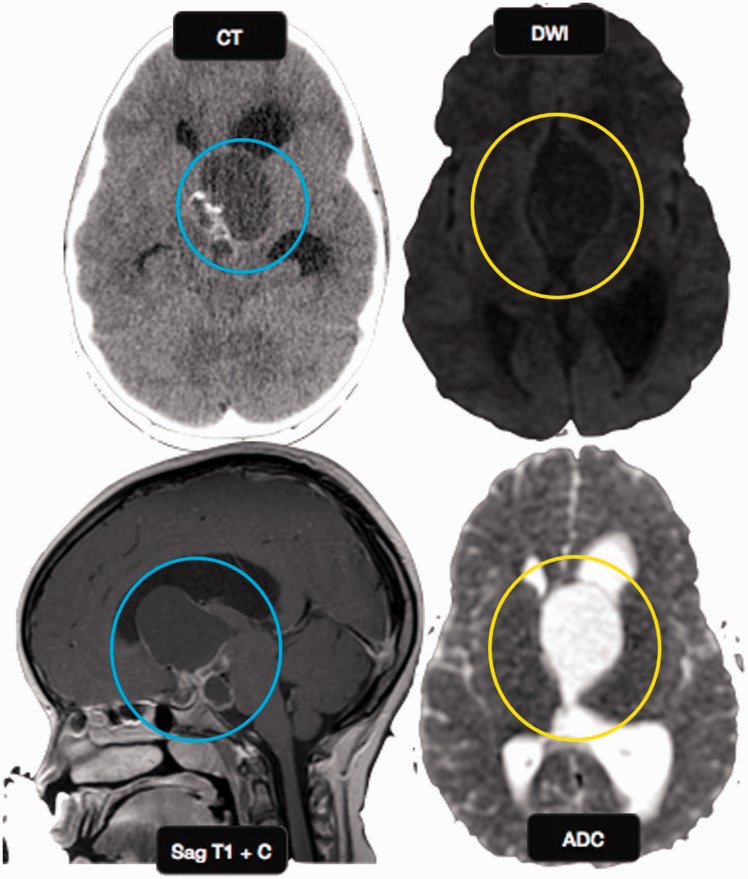
Figure 6.
Craniopharyngioma: an 8-year-old girl with headache, photophobia, and increased urination. Axial CT image shows a cystic suprasellar mass with incomplete rim calcification (blue circle). Post gadolinium T1W sagittal MR image shows rim enhancement. No diffusion restriction was identified on echo planar imaging (yellow circles), suggesting benign etiology. CT: computed tomography; MR: magnetic resonance.
Ecchordosis physaliphora (EP)
EP is a rare (0.4–2% of autopsies) congenital asymptomatic, small benign gelatinous lesion originating from notochordal remnants, located along the midline from the clivus to coccyx region, intradural in the prepontine cistern and attached to the clivus via a small pedicle.26 CT is of limited help in demonstrating EP which appears as single or multiple low-attenuation lesions in the cistern with an osseous stalk. MRI is the best modality in precisely localizing EP in the prepontine cistern as T1 hypointense and T2 hyperintense non-enhancing lesions with a distinct T2 hypointense pedicle hallmark of EP. On DWI, no diffusion restriction is seen, which suggests benign etiology (Figure 7). EP shares the same histopathology with notochordal origin chordoma which is extradural in location and a more aggressive malignant lesion with soft tissue and bone destruction.27
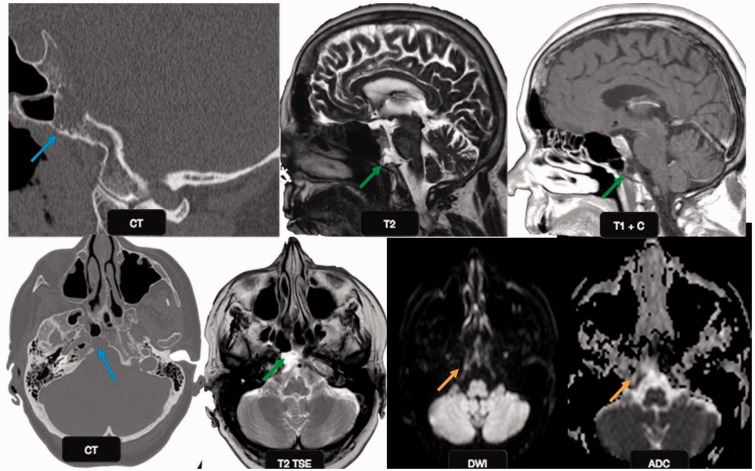
Figure 7.
Ecchordosis physaliphora: a 66-year-old male patient with vertigo and syncope. Magnified sagittal and axial CT scan images show an erosive lesion in the clivus (blue arrows). The lesion appears hypointense on T1 and hyperintense on T2W images (green arrows). No diffusion restriction was identified on echo planar imaging (orange arrows), suggesting benign etiology. This was a case of ecchordosis physaliphora, a benign notochord remnant. CT: computed tomography.
Posterior fossa epidermoid
An intracranial epidermoid tumor is a benign, slow growing extra-axial congenital neoplasm, comprising 1% of all intracranial tumors, arising from accumulation of keratin and cholesterol from normal epithelium lining desquamation.28 Most commonly located in the cerebellopontine angles (CPAs), parasellar cisterns, and the fourth ventricles, and rarely inraparenchymal and intradiplic with a tendency to encase the neurovascular structures in cisterns. On CT, epidermoids appear as non-enhancing, lobulated hypoattenuating lesions. On MR, epidermoids are of either similar or of slightly higher signal intensity than cerebrospinal fluid (CSF) and often give a heterogeneous marbled appearance. Fluid attenuated inversion recovery and DWI are the best MR sequences in differentiation of epidermoids from similar lesions such as arachnoid and confirmation of residual post-operative tumour (Figure 8). Epidermoids on DWI show hyperintensity with a high ADC value due to T2 shine-through effect which is not because of diffusion restriction.29
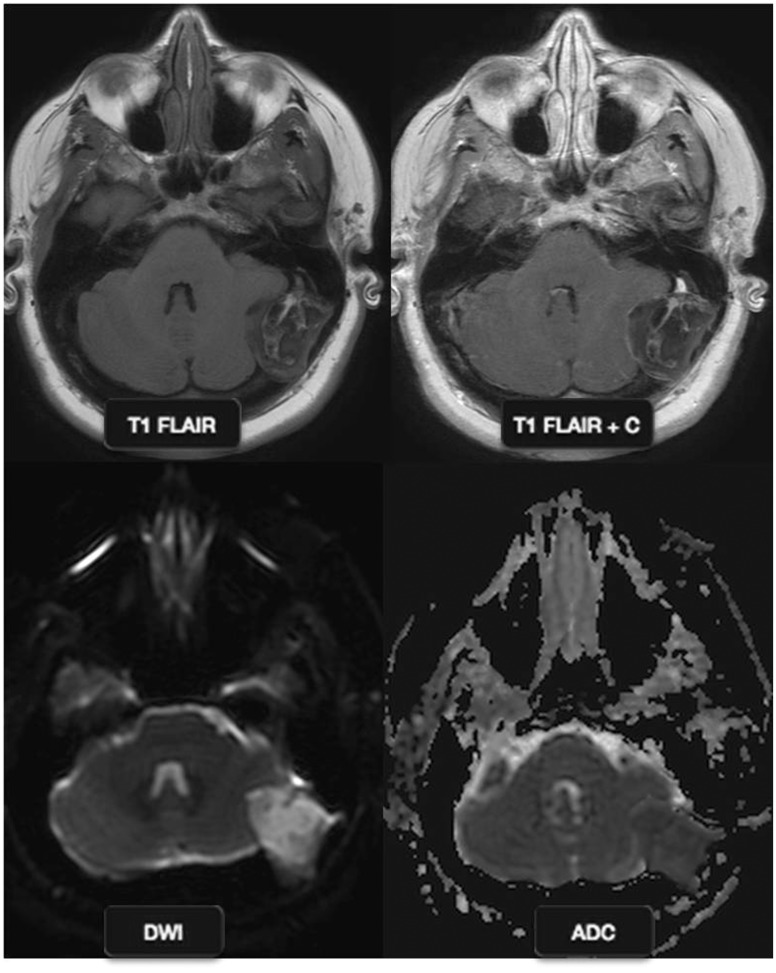
Figure 8.
Posterior fossa epidermoid: a 47-year-old female with right cerebellar ataxia. MR images show an extra-axial mass with heterogeneous signal, abutting the left cerebellar cortex, without significant enhancement. The mass shows diffusion restriction with a high ADC value. This is a typical case of posterior fossa epidermoid. MR: magnetic resonance; ADC: apparent diffusion coefficient.
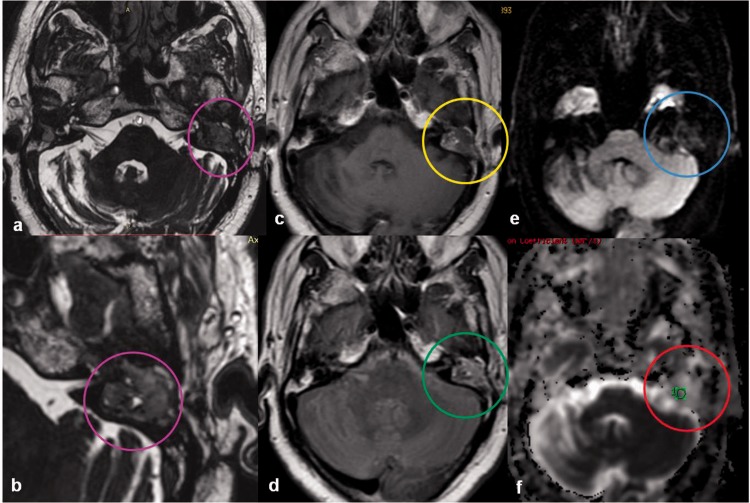
Jugular foramen glomus tumor
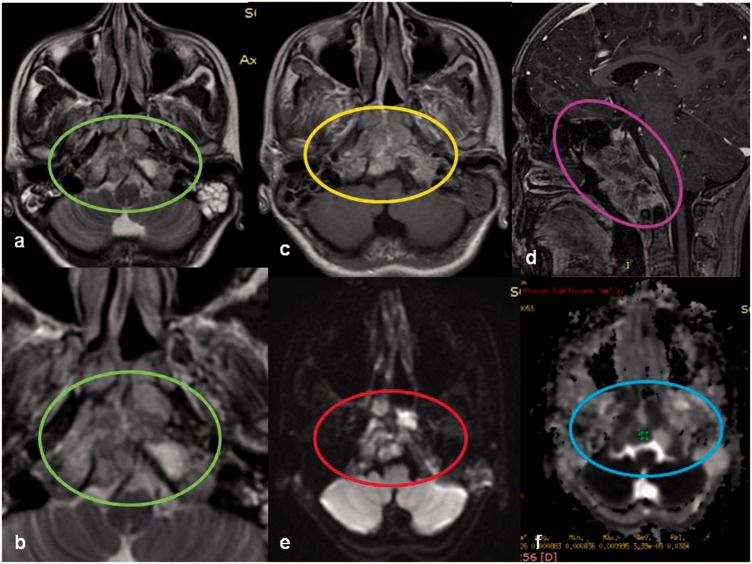
Paragangliomas are highly vascular soft tissue tumors originating from the jugulotympanicum region. These are classically associated with a “salt and pepper” appearance on MRI because of multiple areas of signal void interspersed with hyperintense foci due to slow flow or hemorrhage and permeative bony destruction on CT. Razek et al.,9 found a significant difference in the ADC value between benign highly vascular tumors such as glomus tumors and angiofibromas and other benign solid lesions of the skull base (Figure 9). Paragangliomas tend to display a high ADC value of 1.83 × 10–3 mm2/s owing to excess extracellular spaces with free diffusion within the vascular lesions.9
Figure 9.
Jugular foramen glomus tumor: a 62-year-old-female presented with right sided pulsatile tinnitus. Sagittal and axial MR images show a lobulated enhancing mass in the right jugular foramen (green arrows), with extension into the neck along the carotid sheath. No diffusion restriction was identified on echo planar imaging (orange arrows), suggesting benign etiology which is consistent with most cases of jugular foramen glomus tumors. MR: magnetic resonance.
Meningioma
Primary intraosseous meningiomas (IOMs) of cranial and spheno-orbital region are rare and comprise <2% of all brain meningiomas. Bony involvement by the menigiomas may be either primary or secondary and difficult to differentiate (Figure 10). Low grade IOMs show hyperostosis while higher-grade IOMs present with a mixed hyperostotic/lytic pattern with radial bony spiculations and scalp mass.30 Although no DWI study in IOMs is currently available in the literature, however, a low ADC can be appreciated in these lesions. Primary atypical or malignant intracranial meningiomas have shown lower ADC values compared with benign meningiomas.31
Figure 10.
Meningioma: a 69-year-old female presented with seizures (same patient as in Figure 3). CT scan with contrast at the level of suprasellar cistern shows a left sided well-circumscribed round enhancing extraaxial mass anterior to the suprasellar cistern (yellow circle), consistent with meningioma. The mass is isointense to gray matter on T1-weighted images (yellow circle) and shows intense enhancement of post gadolinium imaging (red arrows). No diffusion restriction was identified on echo planar imaging (green circles), suggesting benign etiology. CT: computed tomography.
Malignant lesions (Table 1)
Chondrosarcoma
Chondrosarcoma arises from malignant transformation of chondromas which are rare, slowly growing tumors arising from the cartilaginous portion of bones. It is predominantly seen in the male, comprises 0.15% of intracranial tumors and 6% of skull base tumors. It usually arises in the parasellar area, cerebellopontine angle, or paranasal sinuses and in the clivus. Histologically, chondrosarcomas can be divided into conventional, clear cell, mesenchymal, and dedifferentiated variants. The lower-grade tumors are less aggressive with minimal malignant potential. The absence of epithelial markers and oncofetal antigens helps in distinguishing them from chordoma. CT is favorable over MR to detect the subtle intralesional calcification (Figure 11), however, these lesions due to high water content appear as low attenuation on CT and very high signal intensity on MRI.32
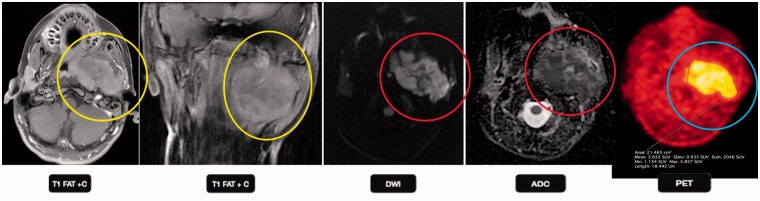
Figure 11.
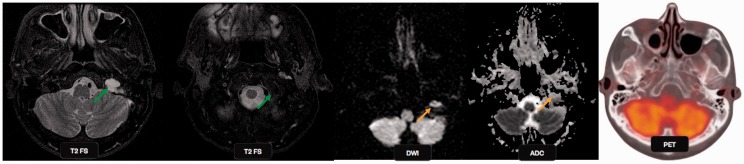
Chondrosarcoma: a 57-year-old male presented with dysarthria, dysphonia, rightward deviation of tongue and visual changes over the last three days. T2-weighted images show increased signal intensity within the left jugular foramen (green arrows), with an associated increased signal in the left sigmoid and anterior transverse sinuses. The lesion shows restricted diffusion on DWI (orange arrows) with a low ADC value, suggestive malignant mass. PET-CT failed to show tracer uptake in the mass. DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; PET-CT: positron emission tomography computed tomography.
DWI plays an important role in differentiation of low-grade chondrosarcomas from chordoma, having a markedly elevated ADC due to the presence of hyaline cartilage with myxoid ground substance and relatively sparse cellularity.10 In a retrospective study of DW imaging in 19 pathologically confirmed chordoma and chondrosarcoma cases, the authors found a significantly high mean ADC value of 2.0 × 10–3 mm2/s in chondrosarcoma versus 1.4 × 10–3 mm2/s in classic chordoma.33 DWI can also be used to differentiate high grade from low grade chondrosarcoma, with the former showing greater restricted diffusion.
PET-CT complements standard imaging and the maximum standardized uptake value range of 2.0–2.2 can be of value in deciding the best treatment for patients with cartilaginous lesions in long bones.34
Chordoma
Chordoma most commonly arises from notochordal remnants in the midline of clivus and may be confused with chondrosarcoma which is mesenchymal in origin and is typically centered on the spheno-occipital synchondrosis. Conventional imaging features cannot always reliably differentiate chordoma from chondrosarcoma despite distinct histological features. Chordoma occurs more commonly in the later age group, and is generally slow growing with subtle clinical presentation. MRI is the best modality of choice for lesion detection and its extent which helps in presurgical planning and follow-up. Histologically, chondrosarcoma is divided into conventional, chondroid, and dedifferentiated varieties. Poorly differentiated chordoma shows low T2 signal intensity and a low ADC value due to high mitotic activity and cellularity (Figure 12). Yeom et al., 2013, reported a low ADC value of 0.8 × 10–3 mm2/s) in poorly differentiated chordoma than classic chordoma (1.4 × 10−3 mm2/s).33,35
Figure 12.
A clival chordoma in a 12-year-old male child. Large lobulated T2 heterogenously hyperintense ((a), (b)) enhancing ((c), (d)) extraaxial lesion involving the basisphenoid with extension into the sphenoid sinus, nasopharynx, reteropharangeal, and prevertebral space. Lesion shows DWI restriction (red circle) with low ADC (blue circle) suggesting malignant etiology. DWI: diffusion weighted imaging; ADC: apparent diffusion coefficient.
Nasopharyngeal carcinoma (NPC)
NPCs are aggressive tumors, arise within the fossa of Rosenmueller and may directly breach the pharyngobasilar fascia with further extension to sinus of Morgagni or directly invade the clivus. MRI is a very sensitive technique for identifying NPC, which appears isointense on T1, iso- to hyperintense on T2, and diffusely enhances on post gadolinium T1W images (Figure 13). NPC has a tendency to invade skull base or paranasal sinuses in 60% of patients.36
Figure 13.
Nasopharyngeal carcinoma: a 66-year-old male with nasopharyngeal mass and vertigo. (a) CT scan shows a heterogeneously enhancing mass in the posterior nasopharynx more on the left side (yellow arrow in image a and yellow circle in image d), with extension into the pterygopalatine fossa. On MRI the mass showed a lobular T2 hyperintense nasopharyngeal mass (red arrow in image e) showing uniform post gadolinium enhancement (green arrow in image b). The mass was identified extending into the Meckel's cave. The mass shows restricted diffusion on DWI (orange arrow in f) with a low ADC value, suggesting malignant etiology. CT: computed tomography; MRI: magnetic resonance imaging; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
NPC showed low diffusivity with a mean ADC value of 0.74 × 10–3 mm2/s in comparison with skull base osteomyelitis. Compared with lymphoma, NPC exhibited higher ADC and intravoxel incoherent motion and DWI is useful for differentiating lymphoma from NPC.14,37
Pretreatment mean ADCs were significantly lower for responders than for non-responders. Mean percentage increases in ADC were higher for responders than for non-responders. The optimal pretreatment primary lesion and metastatic adenopathy ADCs for differentiating responders from non-responders were 0.897 × 10−3 mm2/s and 1.031 × 10−3 mm2/s, respectively. NPC patients with low pretreatment ADCs tend to respond better to neoadjuvant chemotherapy. Pretreatment ADCs could be used as a new pretreatment imaging biomarker of response to neoadjuvant chemotherapy.38
Lymphoma
Primary non-Hodgkin's lymphoma of the cranium is a rare condition. Lymphomas are highly cellular tumors with a low cytoplasmic ratio (Figure 14). They exhibit a lower ADC than NPC with a cut-off value of 0.761 × 10–3 mm2/s, suggesting a lower level of pure diffusion and making DWI a useful imaging marker.37 The mean ADC values were 1.26 × 10–3 mm2/s for SBO, 0.74 × 10–3 mm2/s for NPC, 0.59 × 10–3 mm2/s for lymphoma, and 0.99 × 10–3 mm2/s for metastatic disease, respectively.
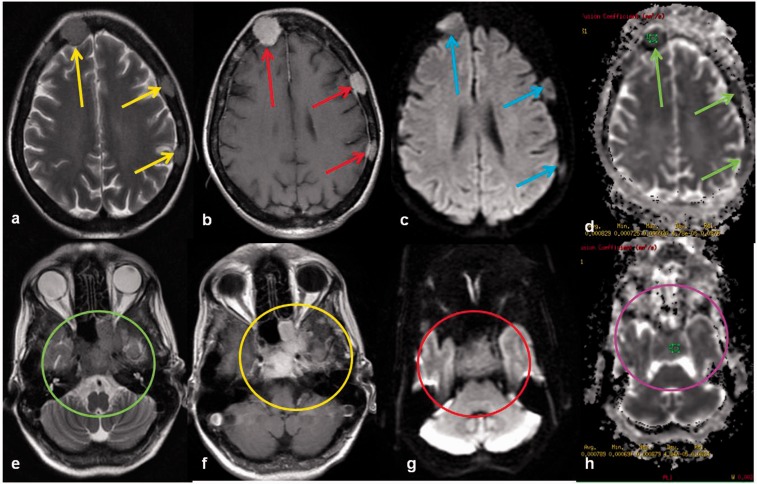
Figure 14.
A 4-year-old female child with metastatic small round cell tumour. Extraaxial lobulated T2 (a) and T1 (b) isointense soft tissue is seen along the inner table of the bony calvarium causing compression of the underlying brain parenchyma. DW image (c) shows diffusion restriction with low ADC (d). Similar lesions are also seen the skull base with low ADC (e–h). DW: diffusion-weighted; ADC: apparent diffusion coefficient.
An ADC value equal to or higher than 1.08 × 10−3 mm2/s was used to rule out lymphoma and NPC with 96% accuracy.14 Recently, Sumi and Nakamura also reported that the mean ADC value of lymphomas is lower than that of metastatic lymph nodes due to differences in cellularity.39
Metastases
Skull metastases most commonly arise from breast, prostate, and lung primaries in adults, and neuroblastomas in children and typically affect the bones of the medullary region such as the greater wing of the sphenoid and petrous apex. DWI improves the conspicuity of metastatic lesions over conventional MRI (Figure 15). Although skull metastases show a wide range of ADC values with a mean reported value of 0.99 × 10–3 mm2/s due to histologically diversity and high cellularity, they usually show restricted diffusion. This helps in differentiating them from benign skull lesions such as hemangioma and arachnoid granulations. The ADC values of metastases may overlap with the ADC values of other malignant lesions, such as lymphoma and nasopharyngeal carcinoma, as well as benign entities, such as osteomyelitis.14,40
Figure 15.
Skull metastases: a 52-year-old female with breast carcinoma ((a), (b)). T2 and post-contrast T1-weighted images show multiple T2 isointense (yellow arrows) enhancing lesions (red arrows) in the bony calvarium. The lesions show diffusion restriction (blue arrow) with low ADC (green arrow) ((c), (d)). Similar lesions are also seen in the clivus, body, and greater wing of the sphenoid (green and yellow circle) with diffusion restriction (red circle) with low ADC (purple circle) ((e)–(h)). ADC: apparent diffusion coefficient.
Rhabdomyosarcoma
Rhabdomyosarcomas are the most common soft tissue sarcomas of head and neck region in children, with relatively less incidence in adults. Due to high cellularity and low cytonucleoplasmic ratio, the rhabdomyosarcoma usually shows restricted diffusion with a high signal on DWI and a low signal on the ADC map.41 However, there is paucity of adequate data on DW characteristics of rhabdomyosarcomas in the literature and more studies are needed to accurately define the role of DWI in rhabdomyosarcoma (Figure 16). A study by Razek et al., including four patients of rhabdomyosarcomas showed a lower ADC value of 0.75 × 10–3 mm2/s.9 Another study including four cases orbital rhabdomyosarcomas has shown similar results and distinctly different diffusion characteristics compared with capillary hemangiomas, which can otherwise demonstrate overlapping appearances on conventional MRI sequences.42
Figure 16.
Rhabdomyosarcoma: a 12-year-old boy with a large left neck mass. T1-weighted image shows a large enhancing left infratemporal mass, with invasion of middle cranial fossa and associated right-sided pharyngeal displacement (yellow circles). The mass shows restricted diffusion on DWI (red circles) with an a low ADC value, suggesting malignant etiology. PET-CT shows hypermetabolic activity in the left infratemporal region (blue circle), compatible with a malignant mass. This is a typical appearance of rhabdomyosarcoma in this age group. DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; PET-CT: positron emission tomography computed tomography.
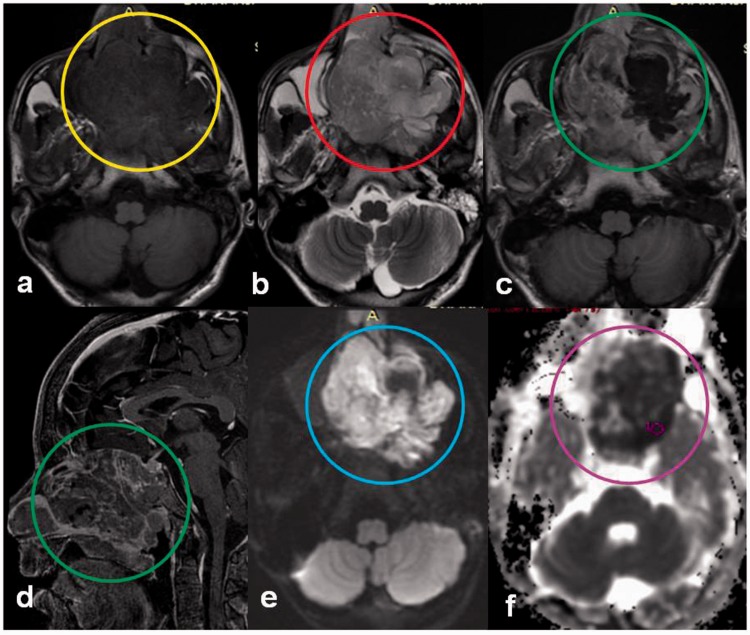
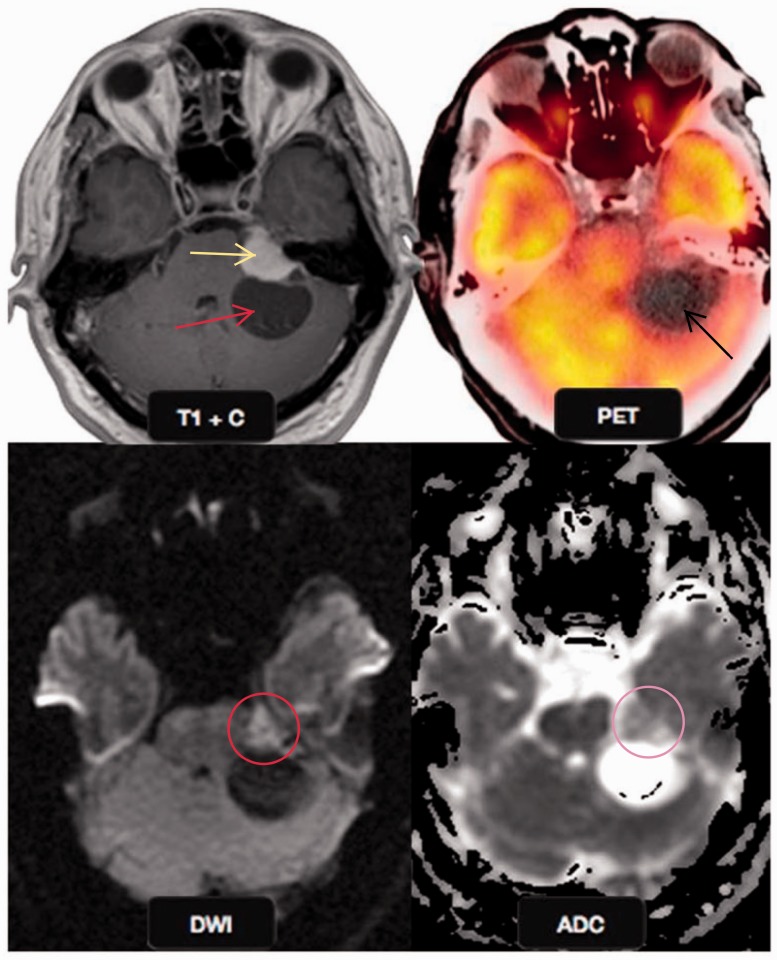
Malignant schwannoma
Vestibular schwannomas are benign tumors arising from the eighth cranial nerve and very rarely undergo a malignant transformation with only a handful cases of de-novo malignancies in the literature, and radiation treatment increases the risk by 10 times in non-neurofibromatosis cases.43 A retrospective study by Pavlisa et al., found no significant differences in ADC values or ADC ratios between typical and atypical meningiomas while schwannomas showed higher mean ADC values than typical and atypical meningiomas (Figure 17). There was no overlap in the ADC values of schwannomas and meningiomas which helps in differentiating the two.44
Figure 17.
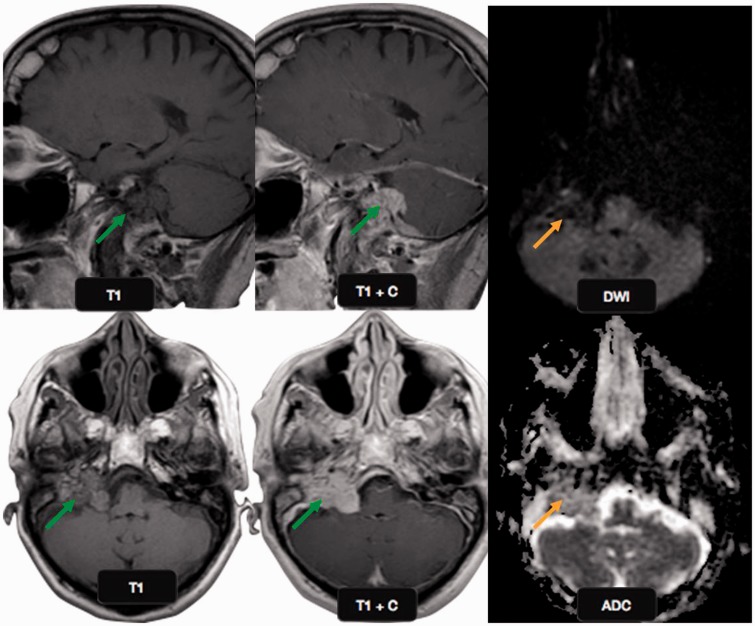
Malignant schwannoma: a 51-year-old male presented with dizziness. Post gadolinium T1W image shows an enhancing left cerebellopontine angle mass (yellow arrow) with a cystic component (red arrow). The solid mass shows restricted diffusion (red circle) on DWI with a low ADC value (yellow circle), suggestive of a malignant mass, however the cystic component did not show any diffusion restriction. PET-CT showed the cystic component to be hypometabolic (black arrow) confirming the findings of DWI. This is a typical location and imaging behavior of malignant schwannoma. DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; PET-CT; positron emission tomography computed tomography.
Conclusions
Although CT and routine MR pulse sequences are very good in defining the extent of skull base lesions and associated bone destruction, they are not reliable in the differentiation of benign from malignant pathologies. DWI is a promising, noninvasive approach that can be used in the characterization of skull base lesions, differentiate malignant tumors from benign lesions and evaluate the pathological grading of malignant tumors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Parmar H, Gujar S, Shah G, et al. Imaging of the anterior skull base. Neuroimaging Clin N Am 2009; 19: 427–439. [DOI] [PubMed] [Google Scholar]
- 2.Borges A. Imaging of the central skull base. Neuroimaging Clin N Am 2009; 19: 441–468. [DOI] [PubMed] [Google Scholar]
- 3.Raut AA, Naphade PS, Chawla A. Imaging of skull base: Pictorial essay. Indian J Radiol Imaging 2012; 22: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll CL, Lane JI. Advances in skull base imaging. Otolaryngol Clin North Am 2007; 40: 439–454. [DOI] [PubMed] [Google Scholar]
- 5.Mathur A, Jain N, Kesavadas C, et al. Imaging of skull base pathologies: Role of advanced magnetic resonance imaging techniques. Neuroradiol J 2015; 28: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malayeri AA, El Khouli RH, Zaheer A, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011; 31: 1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar Y, Khaleel M, Boothe E, et al. Role of diffusion weighted imaging in musculoskeletal infections: Current perspectives. Eur Radiol 2017; 27: 414–423. [DOI] [PubMed] [Google Scholar]
- 8.Kumar Y, Wadhwa V, Phillips L, et al. MR imaging of skeletal muscle signal alterations: Systematic approach to evaluation. Eur J Radiol 2016; 85: 922–935. [DOI] [PubMed] [Google Scholar]
- 9.Razek AA, Mossad A, Ghonim M. Role of diffusion-weighted MR imaging in assessing malignant versus benign skull-base lesions. Radiol Med 2011; 116: 125–132. [DOI] [PubMed] [Google Scholar]
- 10.Ginat DT, Mangla R, Yeaney G, et al. Diffusion-weighted imaging for differentiating benign from malignant skull lesions and correlation with cell density. AJR Am J Roentgenol 2012; 198: W597–W601. [DOI] [PubMed] [Google Scholar]
- 11.Wei XE, Li WB, Li MH, et al. Detection of brain lesions at the skull base using diffusion-weighted imaging with readout-segmented echo-planar imaging and generalized auto calibrating partially parallel acquisitions. Neurol India 2011; 59: 839–843. [DOI] [PubMed] [Google Scholar]
- 12.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 2009; 62: 468–475. [DOI] [PubMed] [Google Scholar]
- 13.Clark MP, Pretorius PM, Byren I, et al. Central or atypical skull base osteomyelitis: Diagnosis and treatment. Skull Base 2009; 19: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozgen B, Oguz KK, Cila A. Diffusion MR imaging features of skull base osteomyelitis compared with skull base malignancy. AJNR Am J Neuroradiol 2011; 32: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dremmen MH, Hofman PA, Hof JR, et al. The diagnostic accuracy of non-echo-planar diffusion-weighted imaging in the detection of residual and/or recurrent cholesteatoma of the temporal bone. AJNR Am J Neuroradiol 2012; 33: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz KM, Lane JI, Bolster BD, Jr, et al. The utility of diffusion-weighted imaging for cholesteatoma evaluation. Am J Neuroradiol 2011; 32: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann P, Saliou G, Brochart C, et al. 3T MR imaging of postoperative recurrent middle ear cholesteatomas: Value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion weighted MR imaging. Am J Neuroradiol 2009; 30: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashida Y, Hirai T, Yakushiji T, et al. Evaluation of diffusion weighted imaging for the differential diagnosis of poorly contrast enhanced and T2-prolonged bone masses: Initial experience. J Magn Reson Imaging 2006; 23: 377–382. [DOI] [PubMed] [Google Scholar]
- 19.Szymanska A, Szymanski M, Czekajska-Chehab E, et al. Invasive growth patterns of juvenile nasopharyngeal angiofibroma: Radiological imaging and clinical implications. Acta Radiol 2014; 55: 725–731. [DOI] [PubMed] [Google Scholar]
- 20.Marfani MS, Jawaid MA, Shaikh SM, et al. Allergic fungal rhinosinusitis with skull base and orbital erosion. J Laryngol Otol 2010; 124: 161–165. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AA, Bashir SH, Ali Shah A, et al. Diagnostic MR imaging features of craniocerebral aspergillosis of sino-nasal origin in immunocompetent patients. Acta Neurochir (Wien) 2006; 148: 155–166. [DOI] [PubMed] [Google Scholar]
- 22.Gaviani P, Schwartz RB, Hedley-Whyte ET, et al. Diffusion weighted imaging of fungal cerebral infection. AJNR Am J Neuroradiol 2005; 26: 1115–1121. [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller-Mang C, Castillo M, Mang TG, et al. Fungal versus bacterial brain abscesses: Is diffusion-weighted MR imaging a useful tool in the differential diagnosis? Neuroradiology 2007; 49: 651–657. [DOI] [PubMed] [Google Scholar]
- 24.Sartoretti-Schefer S, Wichmann W, Aguzzi A, et al. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol 1997; 18: 77–87. [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita Y, Yamasaki F, Tominaga A, et al. Diffusion-weighted imaging and the apparent diffusion coefficient on 3T MR imaging in the differentiation of craniopharyngiomas and germ cell tumors. Neurosurg Rev 2016; 39: 207–213. [DOI] [PubMed] [Google Scholar]
- 26.Mehnert F, Beschorner R, Küker W, et al. Retroclival ecchordosis physalispora: MR imaging and review of the literature. AJNR Am J Neuroradiol 2004; 25: 1851–1855. [PMC free article] [PubMed] [Google Scholar]
- 27.Park HH, Lee KS, Ahn SJ, et al. Ecchordosis physaliphora: Typical and atypical radiologic features. Neurosurg Rev 2017; 40: 87–94. [DOI] [PubMed] [Google Scholar]
- 28.Bonneville F, Sarrazin JL, Marsot-Dupuch K, et al. Unusual lesions of the cerebellopontine angle: A segmental approach. Radiographics 2001; 21: 419–438. [DOI] [PubMed] [Google Scholar]
- 29.Hakyemez B, Aksoy U, Yildiz H, et al. Intracranial epidermoid cysts: Diffusion-weighted, FLAIR and conventional MR findings. Eur J Radiol 2015; 54: 214–220. [DOI] [PubMed] [Google Scholar]
- 30.Ilica AT, Mossa-Basha M, Zan E, et al. Cranial intraosseous meningioma: Spectrum of neuroimaging findings with respect to histopathological grades in 65 patients. Clin Imaging 2014; 38: 599–604. [DOI] [PubMed] [Google Scholar]
- 31.Nagar VA, Ye JR, Ng WH, et al. Diffusion-weighted MR imaging: Diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 2008; 29: 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyers SP, Hirsch WL, Jr, Curtin HD, et al. Chondrosarcomas of the skull base: MR imaging features. Radiology 1992; 184: 103–108. [DOI] [PubMed] [Google Scholar]
- 33.Yeom KW, Lober RM, Mobley BC, et al. Diffusion-weighted MRI: Distinction of skull base chordoma from chondrosarcoma. AJNR Am J Neuroradiol 2013; 34: 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jesus-Garcia R, Osawa A, Filippi RZ, et al. Is PET-CT an accurate method for the differential diagnosis between chondroma and chondrosarcoma? Springerplus 2016; 5: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgouin PM, Tampieri D, Robitaille Y, et al. Low-grade myxoid chondrosarcoma of the base of the skull: CT, MR, and histopathology. J Comput Assist Tomogr 1992; 16: 268–273. [DOI] [PubMed] [Google Scholar]
- 36.King AD, Lam WW, Leung SF, et al. MRI of local disease in nasopharyngeal carcinoma: Tumour extent vs tumour stage. Br J Radiol 1999; 72: 734–741. [DOI] [PubMed] [Google Scholar]
- 37.Yu XP, Hou J, Li FP, et al. Intravoxel incoherent motion diffusion weighted magnetic resonance imaging for differentiation between nasopharyngeal carcinoma and lymphoma at the primary site. J Comput Assist Tomogr 2016; 40: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang GY, Wang YJ, Liu JP, et al. Pretreatment diffusion weighted MRI can predict the response to neoadjuvant chemotherapy in patients with nasopharyngeal carcinoma. Biomed Res Int 2015, pp. 307943, doi: 10.1155/2015/307943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumi M, Nakamura T. Diagnostic importance of focal defects in the apparent diffusion coefficient based differentiation between lymphoma and squamous cell carcinoma nodes in the neck. Eur Radiol 2009; 19: 975–981. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth AJ, Henson JW, Mullins ME, et al. Improved detection of skull metastasis with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2007; 28: 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassold N, Warmuth-Metz M, Winkler B, et al. Hit the mark with diffusion-weighted imaging: Metastases of rhabdomyosarcoma to the extraocular eye muscles. BMC Pediatr 2014; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lope LA, Hutcheson KA, Khademian ZP. Magnetic resonance imaging in the analysis of pediatric orbital tumors: Utility of diffusion-weighted imaging. J AAPOS 2010; 14: 257–262. [DOI] [PubMed] [Google Scholar]
- 43.Husseini ST, Piccirillo E, Sanna M. On “malignant transformation of acoustic neuroma/vestibular schwannoma 10 years after gamma knife stereotactic radiosurgery” (skull base 2010; 20: 381–388). Skull Base 2011; 21: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlisa G, Rados M, Pazanin L, et al. Characteristics of typical and atypical meningiomas on ADC maps with respect to schwannomas. Clin Imaging 2008; 32: 22–27. [DOI] [PubMed] [Google Scholar]