Abstract
PURPOSE: To determine whether volumes based on the contours of the mucosal surface instead of the oral cavity can be used to predict grade ≥3 acute oral mucosa toxicity in patients with locally advanced nasopharyngeal carcinoma (LANPC) treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. METHODS AND MATERIALS: A standardized method for the oral cavity (oral cavity contours, OCC) and a novel method for the mucosal surface (mucosal surface contours, MSC) were developed for the oral mucosa and prospectively applied to the radiation treatment plans of 92 patients treated with concurrent IMRT and chemotherapy for LANPC. Dose–volume histogram (DVH) data were extracted and then toxicity was analyzed. Receiver operating characteristic analysis and logistic regression were carried out for both contouring methods. RESULTS: Grade ≥3 acute oral mucosa toxicity occurred to 20.7% (19/92) of patients in the study. A highly significant dose–volume relationship between oral mucosa irradiation and acute oral mucosa toxicity was supported by using both oral cavity and mucosal surface contouring techniques. In logistic regression, body weight loss was an independent factor related to grade ≥3 acute toxicity for OCC and MSC (P = .017 and 0.005, respectively), and the independent factor of dosimetric parameters for OCC and MSC were V30Gy (P = .003) and V50Gy (P = .003) respectively. In the receiver operating characteristics curve, the areas under V30Gy of the OCC curves was 0.753 (P = .001), while the areas under V50Gy of MSC curves was 0.714 (P = .004); the cut-off value was 73.155% (sensitivity, 0.842; specificity, 0.671) and 14.32% (sensitivity, 0.842; specificity, 0.575), respectively. CONCLUSION: DVH analysis of mucosal surface volumes accurately predicts grade ≥3 acute oral mucosa toxicity in patients with LANPC receiving concurrent IMRT and chemotherapy, but in clinical practice the MSC method appears no better than the OCC one.
Introduction
The oral mucosa represents the dose-limiting structure in the treatment of head and neck cancer with therapeutic radiation. The early clinical sign of mucositis appears at cumulative doses of head and neck radiation of about 10Gy (generally after 1 week). The acute mucositis reached by the highest grade at the end of radiotherapy, and it remains its peak for at least 2 weeks following the completion of radiotherapy. After that, symptoms of acute oral mucositis can then persist for up to 8 weeks. Complications associated with mucositis include oral pain, dysphagia [1], [2], [3], weight loss [4], and secondary infections. The acute mucositis unwantedly leads to radiotherapy interruptions [5] especially after the fifth week of initiation, resulting in accelerated repopulation of resistant clones and compromised disease control. The critical weight loss predicts poor prognosis in patients with nasopharyngeal carcinoma (NPC) treated with intensity-modulated radiation therapy (IMRT) [6], [7]. Radiotherapy, being a local treatment, may trigger acute mucositis in the irradiated area, while chemotherapy may cause local mucositis and further affect the entire mucosa. Radiation-induced acute oral mucositis with grade ≥3 toxicity is associated with 23.3% to 36.2% of NPC patients receiving chemoradiotherapy [8], [9], [10], [11].
Before the 3-dimensional (3D) treatment planning era, a dose–volume relationship between radiation of the mucosa and acute toxicity had been assumed by a 2D characteristic. In the era of dose-painting intensity-modulated radiation therapy, it becomes crucial to protect healthy tissues to improve the patient's quality of life. Heterogeneous dose distributions are delivered to the mucosa with IMRT, allowing the oral mucosa to be partially guarded [12]. For acute radiation-induced oral mucositis (ROM), the potential benefit of IMRT has involved attempting to identify key dose or dose-volume thresholds for toxicity. However, the literature available to correlate acute oral mucosa toxicity with doses remains sparse compared with that available for other organs. A literature review for Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) overview in 2010 did not mention any study on quantitative dose-volume information for the oral mucosa [13].
Some results have been published to find the best dosimetric parameters as predictors of acute oral mucositis [14], [15], [16], [17], including two studies on head and neck tumors, one on oral tongue squamous cell carcinoma, and another on oropharyngeal and oral cavity cancer. Patients varied significantly in terms of the type and amount of chemotherapy received, the preoperative versus postoperative status, the radiation techniques, and the irradiated volumes. This heterogeneity in patient, tumor, and treatment factors confounds attempts to quantify a dose–volume relationship for oral mucosa and to provide generalizable dose constraints.
Lacking of a standardized method for contouring and reporting dose to the oral mucosa also accounts for this variability. One method is oral cavity contours (OCC), commonly employed in most clinical studies, depicting the oral cavity as a whole. And in Sun et al.'s report [18] the limits have been defined. Another method is to individually contour different parts of the oral mucosa surface. In Musha et al.'s study [16], by developing an oral mucosal dose surface model of tongue and palate, they concluded that the model was useful for predicting the location and severity of acute radiation mucositis. Jamie et al. [19] exploited a new method named as “mucosa surface contours” (MSC),to obtain anatomically accurate contours of the oral mucosa surfaces with high expectation that it could be used to improve toxicity modeling of acute oral mucositis. However, a consistent definition of the “oral mucosa surface” is still in urgent need, and a dose–volume relationship between this volume and acute oral mucosa toxicity has not been established either.
By means of directly comparing contouring techniques for the oral mucosa in a homogenous group of NPC patients treated with concurrent IMRT and chemotherapy, this study is meant to determine whether dose–volume histogram (DVH) data, based on contours of the oral mucosa surface instead of the oral cavity can be used to predict for grade ≥3 acute oral mucosa toxicity. And the aim coming next is to establish dose limit by using both volumes and the development of reproducible criteria for contouring the oral mucosa surface.
Materials and Methods
Patients
Between November 2016 and January 2017, consecutive patients with locally advanced nasopharyngeal carcinoma (LANPC) receiving IMRT concurrent with chemotherapy at the Zhejiang Cancer Hospital were evaluated. Eligibility included histopathologically confirmed NPC; stage T3-4NxM0 or TxN2-3 M0 according to Union for International Cancer Control (2010); age above 18; performance status of 0 or 1 by Eastern Cooperative Oncology Group; and adequate bone marrow, renal, and hepatic function. Patients with a prior (i.e., within 5 years) or synchronous malignancy were excluded. According to these criteria, 92 patients, whose characteristics are shown in Table 1, were included.
Table 1.
Patients' Characteristics and Their Association with Grade ≥3 Acute Oral Mucosa Toxicity in 92 Cases
| RTOG Radiation Oral Mucositis |
||||||
|---|---|---|---|---|---|---|
| Grade 0–2 |
Grade ≥3 |
|||||
| Characteristic | n | % | n | % | P-Value | |
| Sex | Male | 51 | 76.1% | 16 | 23.9% | 0.210 |
| Female | 22 | 88.0% | 3 | 12.0% | ||
| Age (y) median 52 range (27–70) | <60 | 55 | 80.9% | 13 | 19.1% | 0.541 |
| ≥60 | 18 | 75.0% | 6 | 25.0% | ||
| Diabetes | Yes | 6 | 85.7% | 1 | 14.3% | 0.665 |
| No | 67 | 78.8% | 18 | 21.2% | ||
| Dental disease | Yes | 47 | 78.3% | 13 | 21.7% | 0.742 |
| No | 26 | 81.3% | 6 | 18.7% | ||
| T | 1 | 4 | 66.7% | 2 | 33.3% | 0.394 |
| 2 | 11 | 78.6% | 3 | 21.4% | ||
| 3 | 38 | 86.4% | 6 | 13.6% | ||
| 4 | 20 | 71.4% | 8 | 28.6% | ||
| N | 0 | 1 | 100% | 0 | 0% | 0.952 |
| 1 | 21 | 80.8% | 5 | 19.2% | ||
| 2 | 43 | 78.2% | 12 | 21.8% | ||
| 3 | 8 | 80.0% | 2 | 20.0% | ||
| Stage (UICC 2010) | 3 | 47 | 83.9% | 9 | 16.1% | 0.310 |
| 4a | 18 | 69.2% | 8 | 30.8% | ||
| 4b | 8 | 80% | 2 | 20% | ||
| Ib irradiated | Yes | 25 | 78.1% | 7 | 21.9% | 0.832 |
| No | 48 | 80% | 12 | 20% | ||
| Induction chemotherapy cycles | 0 | 1 | 50.0% | 1 | 50.0% | 0.183 |
| 1 | 0 | 0% | 1 | 100% | ||
| 2 | 24 | 82.1% | 5 | 17.9% | ||
| 3 | 49 | 81.4% | 11 | 18.6% | ||
| 4 | 1 | 50.0% | 1 | 50.0% | ||
| Concurrent chemotherapy | Cisplatin | 35 | 85.4% | 6 | 14.6% | 0.235 |
| Nedaplatin | 35 | 72.9% | 13 | 27.1% | ||
| Carboplatin | 3 | 100% | 0 | 0% | ||
| Concurrent chemotherapy cycles | 1 | 15 | 75.0% | 5 | 25.0% | 0.587 |
| 2 | 58 | 80.6% | 14 | 19.4% | ||
| Nimotuzumab | Yes | 8 | 61.5% | 5 | 38.5% | 0.087 |
| No | 65 | 82.3% | 14 | 17.7% | ||
| Actovegin | Yes | 34 | 73.9% | 12 | 26.1% | 0.198 |
| No | 39 | 84.8% | 7 | 15.2% | ||
| Recombinant human interleukin-11 | Yes | 24 | 72.7% | 9 | 27.3% | 0.241 |
| No | 49 | 83.1% | 10 | 16.9% | ||
| Amifostine | Yes | 51 | 78.5% | 14 | 21.5% | 0.745 |
| No | 22 | 81.5% | 5 | 18.5% | ||
| Body weight loss | <5% | 46 | 88.5% | 6 | 11.5% | 0.014 |
| ≥5% | 27 | 67.5% | 13 | 32.5% | ||
N = regional lymph node stage; RTOG = Radiation Therapy Oncology Group; T = primary tumor stage; UICC = Union for International Cancer Control.
Radiation Therapy
Patients were immobilized in tailored-made thermoplastic mask from head to shoulders, with head in a neutral position. Intravenous contrast-enhanced CT using slice thickness of 3 mm from the skull vertex to 2 cm below the head of clavicles was performed for planning. The CT data were imported to treatment planning system for treatment design.
IMRT plans were made for all patients using Raystation version 3.0 treatment planning system (RaySearch Laboratories AB, Stockholm, Sweden). The primary gross tumor volume (GTVnx) and the involved lymph nodes (GTVnd) can be defined as macroscopic tumor after correlative analysis of CT and MRI scan is done. Clinical target volume 1 (CTV1) refers to GTV extended by a margin of 0.5 cm and nasopharyngeal mucosa included. CTV1 extended by 0.5 cm plus posterior epistaxis and maxillary sinus, pterygopalatine fossa, parapharyngeal space, skull base, part of posterior ethmoid sinus, cervical vertebrae, and clivus, and bilateral uninvolved regional nodes (retro- and parapharyngeal nodes, cervical nodes level II, III, IV, and V) are defined as Clinical target volume 2 (CTV2). Ipsilateral cervical nodes level Ib was included if there was a confirmed involved lymph node or the level II region was full of involved nodes. The prescription dose for PTVnx, PTVnd, PTV1, and PTV2 were 7040, 6880, 6400, and 5440 cGy respectively in 32 fractions.
Efforts shall be made to constrain the dosage applied to OAR under the framework of Radiation Therapy Oncology Group (RTOG) 0225 protocol [20]. The dose limits for organs at risk (OAR) were 54 Gy when applied to brainstem, optic chiasm, and optic nerves. The dose limits on temporal lobes, mandible, TMJs, and 1 mL of the cervical spinal cord were 60, 65, 65, and 45 Gy respectively. The dose constrains to 50% of the volume values of the left and right parotid glands were 30 Gy. The mean dose limits on inner/middle ears, eyes, lens, and glottic larynx were 50, 35, 9, and 45 Gy, respectively. IMRT was delivered through seven fixed-gantry angles with step-and-shoot treatment techniques on a linear accelerator (Varian Trilogy).
Oral Mucosa Structure
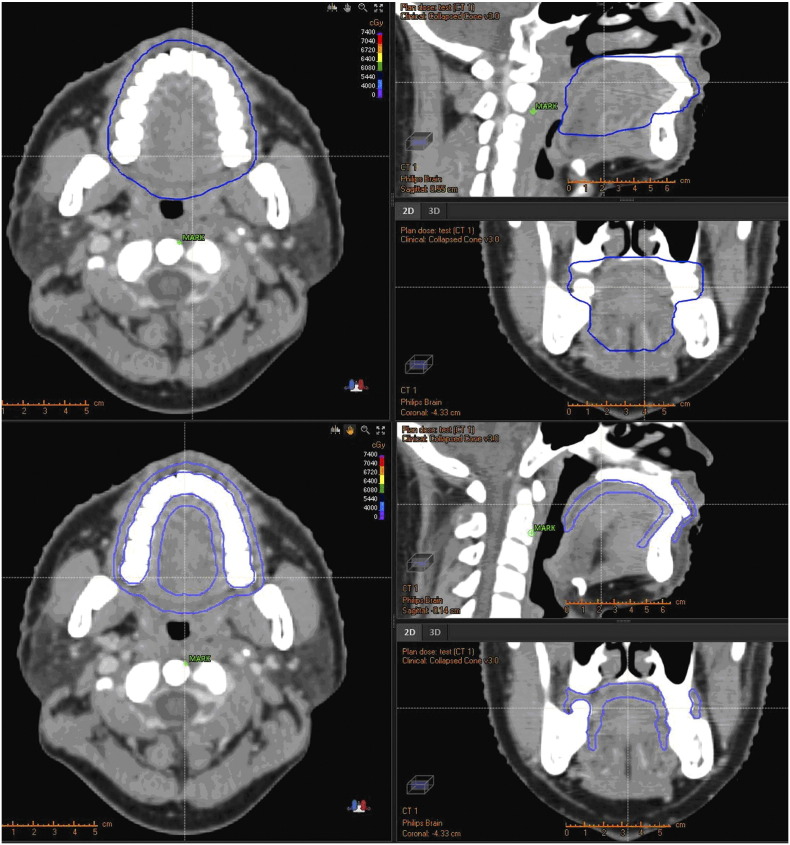
Oral mucosa was prospectively contoured using both the OCC and MSC methods by a single observer, and was subsequently reviewed by another radiation oncologist. Adopting OCC method, the oral mucosa was limited as follows: above to hard palate, underneath to floor of mouth, anterrior to the buccal mucosa around the teeth, and posterior to tongue surface and uvula in reference to Sun Y′s report [18] (Figure 1). While using MSC method, the oral mucosa were defined as a 3-mm thick wall of tissue based on researches done by Ueno et al. [21], and included the following surface: buccal mucosa, buccal gingiva, gingiva proper, lingual gingiva, lingual frenulum, alveolar mucosa, labial mucosa, labial gingiva, labial frenulum, mucosal surface of the floor of the mouth, the mucosal surface of the tongue anterior to the terminal sulcus, the mucosal surface of the hard palate, and the inferior mucosal surface of the soft palate (Figure 1). Absolute cumulative DVH of the structures was extracted from each patient for analysis.
Figure 1.
Computed tomography (CT) scan of a nasopharyngeal carcinoma patient with oral cavity contours (OCC) (up) and mucosal surface contours (MSC) (down) shown with a blue line. The OCC outlines more solid tissue, tongues for instance, whereas what the MSC depicts is the mucosal surface with more accuracy.
Chemotherapy
All patients received 0 to 4 cycles of induction platinum-based chemotherapy. Each patient received 1–3 cycles of concurrent chemotherapy, which was prescribed as: (i) cisplatin 75 mg/m2 on day 1, every 3 weeks; (ii) nedaplatin 75/ mg/m2 on day 1, every 3 weeks; and (iii) carboplatin was dosed to the target area under the concentration-time curve of 5 on day 1, every 3 weeks. The treatment was delayed or halted if the leukocyte counts were lower than 3000/mm3, or the platelet count was less than 75,000/mm3 until recovery was observed. Some patients received concurrent nimotuzumab [22], a kind of targeted therapy, which is a humanized anti-EGFR IgG1 monoclonal antibody.
Basic Oral Care and Management
Basic oral care was performed in all patients both during and after IMRT, including nutritional support and a daily oral hygiene routine development (e.g., brushing the teeth and rinsing the mouth). The management to prevent ROM, such as amifostine [23], actovegin [24], and recombinant human interleukin-11 [25], was carried out in some patients from the beginning of IMRT.
Assessment of Acute Radiation-Induced Oral Mucositis
Acute ROM was scored prospectively using the RTOG/European Organization for Research and Treatment of Cancer (EORTC) radiation morbidity score system for each patient. Daily examination was performed through transoral inspection of the oral cavity during IMRT, and weekly examination was implemented for 8 times following the completion of radiotherapy. Only the highest grade of acute ROM in each patient's toxicity assessment was used in the analysis. Grade 3 and above ROM was defined as severe acute ROM.
It is well known that acute ROM will have reached by the highest grade at the end of radiotherapy, and it remains at its peak for at least 2 weeks following the completion of radiotherapy. After that, symptoms of acute oral mucositis can then last for up to 8 weeks [26]. The highest grade of acute ROM in each toxicity assessment was used in the analysis. Grade ≥3 acute toxicity in the oral mucosa was defined as severe acute ROM.
Statistical Analysis
Absolute DVH data, exported from the RayStation version 3.0 treatment planning system, was used to determine the absolute volume of oral mucosa minus target PTVs receiving a given dose, and was tabulated at 5-Gy dose intervals (0–70 Gy). The oral mucosa xGy radiation volumes received were defined as Vx, which were recorded for further study in the overall statistical analysis. The average dose (Dmean) and maximum dose (Dmax) received in the structure of oral mucosa minus target PTVs were also considered for the analysis. The ratio between oral mucosa minus target PTVs/total oral mucosa was also included for analysis.
The relationship between clinical categoric variables and severe ROM was tested by using Pearson's χ2 test, whereas independent samples T-test was used to identify the differences between dosimetric parameters from patients with and without acute severe ROM. Parameters that were statistically significant on the χ2 test and T-test analysis were considered for a binary logistic regression analysis to identify the determined factor for acute severe ROM. Receiver operating characteristic (ROC) curves were generated to predict the absolute volume of acute severe ROM.
Statistical analysis was carried out with SPSS version 13.0. A p-value inferior to 0.05 was considered significant.
Results
As this study analysis suggests, acute grade ≥3 oral mucosa toxicity occurred to 20.7% (19/92) of patients. 5 patients (5.4%) was graded 0 with acute oral mucosa toxicity, while 27 patients (29.3%) graded 1, 41 patients (44.6%) graded 2; and no grade 4 case was registered.
The median volumes of total oral mucosa and oral mucosa minus target PTVs with the OCC method were 150.40 cc (range: 115.11–210.17 cc) and 146.02 cc (range: 112.39–205.86 cc) respectively. With the MSC method, the median volumes become 43.64 cc (range: 31.81–68.33 cc) and 41.5 cc (range: 30.45–63.71 cc) respectively. The average dose of oral mucosa minus target PTVs for patients experiencing grade ≥3 acute toxicity was 3697 cGy, compared with 3534 cGy for patients with grade 0–2 acute toxicity (P = .005) with the OCC method. Doses appear to be 3758 cGy and 3588 cGy, respectively, (P = .004) with the MSC method. The ratio between oral mucosa minus target PTVs/total oral mucosa was also significantly associated with ROM, but only with the OCC method (P = .026).
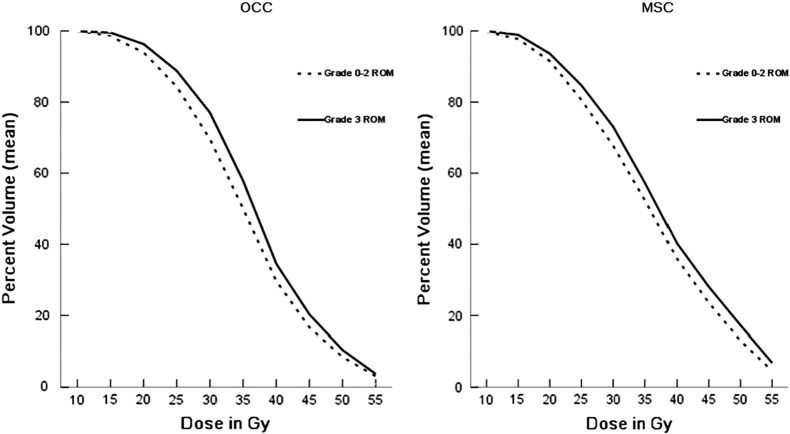
For the oral mucosa minus target PTVs with the OCC method, the association maintained at each 5-Gy dose interval was from 10 to 50 Gy; with the MSC method, it was from 10 to 55 Gy (Table 2). Figure 2 depicts the association of mean oral mucosa volume with each method at 5-Gy dose intervals from 10 to 55 Gy.
Table 2.
Association of Dose Volume Parameters of Oral Mucosa Minus Target PTVs with Toxicity with OCC and MSC Methods
| OCC | Toxicity | Mean | SD | P-value | MSC | Toxicity | Mean | SD | P-value |
|---|---|---|---|---|---|---|---|---|---|
| V5 (%) | Grade 0–2 | 99.91 | 0.33 | 0.925 | V5 (%) | Grade 0–2 | 99.97 | 0.09 | 0.543 |
| Grade ≥3 | 99.92 | 0.14 | Grade ≥3 | 99.98 | 0.04 | ||||
| V10 (%) | Grade 0–2 | 99.78 | 0.44 | 0.049 | V10 (%) | Grade 0–2 | 99.73 | 0.54 | 0.002 |
| Grade ≥3 | 99.92 | 0.16 | Grade ≥3 | 99.95 | 0.10 | ||||
| V15 (%) | Grade 0–2 | 98.48 | 2.02 | 0.000 | V15 (%) | Grade 0–2 | 97.73 | 2.52 | 0.003 |
| Grade ≥3 | 99.52 | 0.50 | Grade ≥3 | 98.91 | 1.06 | ||||
| V20 (%) | Grade 0–2 | 93.95 | 4.14 | 0.001 | V20 (%) | Grade 0–2 | 91.51 | 4.83 | 0.014 |
| Grade ≥3 | 96.31 | 2.20 | Grade ≥3 | 93.62 | 2.64 | ||||
| V25 (%) | Grade 0–2 | 84.31 | 6.84 | 0.010 | V25 (%) | Grade 0–2 | 80.57 | 6.96 | 0.020 |
| Grade ≥3 | 88.77 | 5.16 | Grade ≥3 | 84.67 | 5.61 | ||||
| V30 (%) | Grade 0–2 | 69.58 | 9.35 | 0.002 | V30 (%) | Grade 0–2 | 67.70 | 8.10 | 0.008 |
| Grade ≥3 | 77.02 | 7.19 | Grade ≥3 | 73.09 | 6.22 | ||||
| V35 (%) | Grade 0–2 | 50.00 | 10.96 | 0.005 | V35 (%) | Grade 0–2 | 52.40 | 8.61 | 0.027 |
| Grade ≥3 | 57.86 | 9.64 | Grade ≥3 | 57.31 | 8.07 | ||||
| V40 (%) | Grade 0–2 | 29.69 | 9.43 | 0.045 | V40 (%) | Grade 0–2 | 35.89 | 7.46 | 0.030 |
| Grade ≥3 | 34.54 | 8.54 | Grade ≥3 | 40.22 | 8.30 | ||||
| V45 (%) | Grade 0–2 | 16.66 | 6.41 | 0.027 | V45 (%) | Grade 0–2 | 23.57 | 6.60 | 0.009 |
| Grade ≥3 | 20.29 | 5.52 | Grade ≥3 | 28.04 | 6.27 | ||||
| V50 (%) | Grade 0–2 | 8.47 | 3.92 | 0.046 | V50 (%) | Grade 0–2 | 13.02 | 5.87 | 0.005 |
| Grade ≥3 | 10.43 | 3.06 | Grade ≥3 | 17.28 | 5.44 | ||||
| V55 (%) | Grade 0–2 | 3.11 | 2.23 | 0.260 | V55 (%) | Grade 0–2 | 4.58 | 3.54 | 0.020 |
| Grade ≥3 | 3.74 | 1.93 | Grade ≥3 | 6.81 | 4.02 | ||||
| V60 (%) | Grade 0–2 | 0.72 | 0.98 | 0.370 | V60 (%) | Grade 0–2 | 0.83 | 1.22 | 0.087 |
| Grade ≥3 | 0.95 | 1.01 | Grade ≥3 | 1.63 | 1.83 | ||||
| V65 (%) | Grade 0–2 | 0.09 | 0.25 | 0.528 | V65 (%) | Grade 0–2 | 0.07 | 0.21 | 0.399 |
| Grade ≥3 | 0.14 | 0.27 | Grade ≥3 | 0.11 | 0.18 | ||||
| V70 (%) | Grade 0–2 | 0.004 | 0.02 | 0.737 | V70 (%) | Grade 0–2 | 0.0001 | 0.000 | 0.613 |
| Grade ≥3 | 0.002 | 0.01 | Grade ≥3 | 0.0000 | 0.000 | ||||
| Dmean (cGy) | Grade 0–2 | 3524 | 246.5 | 0.005 | Dmean (cGy) | Grade 0–2 | 3588 | 231.7 | 0.004 |
| Grade ≥3 | 3697 | 184.5 | Grade ≥3 | 3758 | 188.8 | ||||
| Dmax (cGy) | Grade 0–2 | 6618 | 293.0 | 0.957 | Dmax (cGy) | Grade 0–2 | 6388 | 314.1 | 0.131 |
| Grade ≥3 | 6613 | 349.8 | Grade ≥3 | 6508 | 274.3 | ||||
| Ratio | Grade 0–2 | 0.97 | 0.026 | 0.026 | Ratio | Grade 0–2 | 0.97 | 0.042 | 0.072 |
| Grade ≥3 | 0.95 | 0.030 | Grade ≥3 | 0.94 | 0.073 |
Ratio: the ratio between oral mucosa minus target PTVs/total oral mucosa.
SD = standard deviation.
Figure 2.
Mean oral mucosa minus target PTVs volumes versus dose for patients experiencing grade ≥3 acute toxicity compared with patients experiencing grade 0–2 acute toxicity. Grade ≥3 acute toxicity was associated with a greater volume irradiated for each 5-Gy dose increment from 10 to 55 Gy. CI = confidence interval; MSC = mucosal surface contours; OCC = oral cavity contours; ROM = radiation-induced oral mucositis.
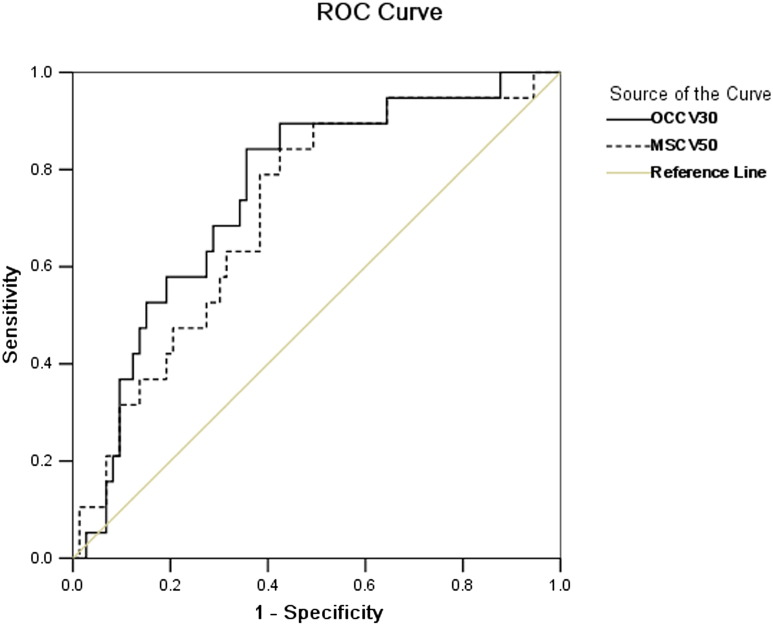
Logistic regression analysis was carried out for all the parameters with statistical significance with the OCC and MSC methods. Body weight loss was an independent risk factor with both the OCC and MSC methods. The risk of grade ≥3 acute toxicity increased around 4 and 6 times (P = .017 and 0.005), respectively, with both the OCC and MSC methods (Table 3). V30 was the determining factor for grade ≥3 acute toxicity with the OCC method (P = .003), while V50 for grade ≥3 acute toxicity with the MSC method (P = .003) (Table 3). The ROC curve was generated for V30 with the OCC method and for V50 with the MSC method (Figure 3). The area under the curve was 0.753 (P = .001) and 0.714 (P = .004), respectively. The cut-off value of V30 with the OCC method was 71.80% for grade ≥3 acute toxicity (sensitivity, 0.842; specificity, 0.644), while it of V50 with the MSC method was 14.32% for grade ≥3 acute toxicity (sensitivity, 0.842; specificity, 0.575).
Table 3.
Multivariate Logistic Regression Analysis of Predictors of Grade ≥3 Acute Oral Mucosa Toxicity with Oral Cavity Contours (OCC) and Mucosal Surface Contours (MSC) Methods
| Method | Predictors | B | SE | Wald | P-Value | R (95% CI) |
|---|---|---|---|---|---|---|
| OCC | V30 | 0.105 | 0.036 | 8.530 | 0.003 | 1.111 (1.035–1.192) |
| Body weight loss | 1.420 | 0.593 | 5.730 | 0.017 | 4.138 (1.294–13.238) | |
| MSC | V50 | 0.173 | 0.059 | 8.659 | 0.003 | 1.189 (1.059–1.334) |
| Body weight loss | 1.820 | 0.642 | 8.024 | 0.005 | 6.169 (1.752–21.726) |
Wald refers to Wald Statistics.
B = regression coefficient CI = confidence interval; R = correlation coefficient: SE = standard error.
Figure 3.
ROC curve of V30 with OCC method and V50 with MSC method for grade ≥3 acute toxicity of oral mucosa. MSC = mucosal surface contours; OCC = oral cavity contours; ROC = receiver operating characteristics.
Discussion
To our current acquaintance, this can be counted as the first study to correlate oral cavity and mucosa surface contouring techniques with acute toxicity in a homogenous population of NPC patients. The data further confirm the relationship between oral mucosa dose–volume and grade ≥3 acute oral mucosa toxicity—one that is maintained whether the contoured volume is composed of the oral cavity space or the oral mucosa surface. OCC and MSC indicate similar correlation with grade ≥3 acute toxicity at each 5-Gy dose increment ranging from 10 to 50 Gy, but differ in terms of the maximum toxicity volumes, with that number being 50 Gy with MSC method while 30 Gy with OCC method respectively.
Conceptually, MSC method outweighs the OCC method in several aspects. It incorporates all the mucosa of irradiated oral structure and excludes tongue and the floor of the mouth. MSC method defines oral mucosa as an anatomically realistic volume instead of an anatomically unrealistic solid organ, helping establish an accurate relationship between mucosa dose–volume and acute toxicity. The MSC method, reducing overlap between the primary PTV and oral mucosa volume, is supposed to be a more sensitive predictor of acute toxicity at every dose level, compared with the OCC method. This shows the disadvantage of the oral cavity space volume with respect to predicting acute toxicity. Compared with contour of individual mucosa surface, the oral cavity space volumes appear to be much larger and more variable among patients, which could have reduced the ability to predict acute oral mucosa toxicity, and may make defining future toxicity cut points more challenging. However, the oral cavity space volumes still display a strong association with toxicity and much convenience in contouring and identifying, compared with contouring an individual mucosa surface.
In a study of 12 patients with head and neck cancer by Narayan et al. [14], it was shown that a cumulative point dose of 39.1 Gy with duration of 3 weeks or longer resulted in acute mucositis, while mild severity (Grade </=1) with short duration (</=1 week) of acute mucositis showed up at cumulative point doses less than 32 Gy. On the basis of this result, Wang et al. [15] protected the oral mucosa outside of the PTV and prescribed a dose <32 Gy to 24 patients with oral tongue squamous cell carcinoma treated postoperatively with IMRT. They found that the incidence of grade 2 and 3 acute mucositis was 0% and 25%, respectively, and the Dmean of oral mucosa was 41.8 ± 7.4 Gy. While Mazzola et al. [17] reported that in 50 oropharyngeal and oral cavity cancer patients treated with volumetric modulated radiation treatment, acute mucositis ≥ grade 2 was statistically related to total oral mucosa Dmean ≥50 Gy and Dmax ≥65 Gy, V45 Gy >40%, V50 Gy >30%, and V55 Gy >20% of the oral mucosa minus target PTVs, and a ratio between total oral mucosa and oral mucosa minus target PTVs >2.5 was related to grade 3 acute mucositis. These parameters were based on patients with a primary tumor in or close to the oral cavity, in which the volume analyzed was constructed by contouring the oral cavity space with [17] or without [14], [15] oropharyngeal. Using this definition of the oral cavity space or simply OCC method, the resultant volume would invariably be much larger than the one obtained through MSC method used in other studies, which covered more parts of the high-dose region. These results were significantly affected by the prescribed dose of the target volume. Thus, the parameters given by these studies must be kept in the context of the single study from which it was derived but unlikely to be generalized.
The only clinical study to investigate oral mucosa acute toxicity using a similar MSC method examined 39 patients with head and neck cancer receiving carbon ion radiotherapy [16]. The authors defined an “oral mucosal dose surface model” (OMDS-model), which seems similar to the mucosa surface volume defined by Jamie et al. [19], but only included the palate and tongue surface. They found that the threshold doses for grade 2–3 acute radiation mucositis in the palate and tongue were 43.0 Gy and 54.3 Gy (relative biological effectiveness), respectively.
In the retrospective study of 11 head and neck radiotherapy patients by Jamie et al. [19], the dose comparison showed that the mean mucosal dose, reduced by 28.7% when the MSC method was used, while the location of the maximum doses, as obtained using the OCC method, were either in the musculature of the tongue or the floor of the mouth within the PTV, which are not included in the MSC volume. In the current study, the inferior mucosal surface of the soft palate was added to our MSC volume, which is different from Jamie et al.'s [19] definition. Since in this study the OCC volume included the soft palate (Figure 1) and also the mucositis on the inferior surface of the soft palate was visible, our MSC volume is more appropriate to be compared with the OCC volume.
There is discordance in the literature regarding whether oral mucosa toxicity is more dependent on the volume of oral mucosa receiving lower doses or higher doses. Whereas this study indicates that the dose most strongly associated with acute toxicity using OCC method was V30, while that one using MSC method was V50. The body weight loss was a consistent risk factor of grade ≥3 acute toxicity in both the OCC and MSC methods. In the ROC curve, the area under the curve of V50 with the MSC method was similar to that of V30 with the OCC method. To diagnose grade ≥3 toxicity, the cut-off value of V30 with the OCC method was 71.80% (sensitivity, 0.842; specificity, 0.644), while that of V50 with the MSC method was 14.32% (sensitivity, 0.842; specificity, 0.575). The V30 of the OCC method seemed more valuable than the V50 of the MSC method on the diagnosis of severe acute oral mucositis. This result is different from the assumption given previously. One possible explanation for this observation is that our study was exclusively composed of NPC patients, in which cases the overlapping parts between the oral mucosa and PTVs were too small with both the OCC and MSC methods to show the advantages of the MSC method over the OCC method. However, the DVH analysis of mucosal surface volumes could still accurately predict grade ≥3 acute oral mucosa toxicity in patients with LANPC receiving concurrent IMRT and chemotherapy in this study.
The increasing use of IMRT will further confound the complex task of defining dose–volume constraints for the oral mucosa. IMRT optimization based on contours of the oral cavity space can realize greater mucosa protection than plans using mucosal surface contours. Though IMRT makes high dose regions more conformal, radiation volumes receiving lower doses may turn out to be larger. As for oral mucosa, the lower dose regions are of prime importance in predicting acute toxicity.
Conclusion
Our data for the first time verify a dose-volume relationship between mucosal surface contours and acute oral mucosa toxicity, suggesting that contouring the mucosal surface can by some means replace contouring oral cavity space. However, in terms of predicting acute toxicity, the oral cavity contouring method appears to be more diagnostically valuable. For locally advanced nasopharyngeal cancer patients receiving concurrent IMRT with chemotherapy, V30 ≥ 71.80% using oral cavity contouring and V50 ≥ 14.32% using mucosal surface contouring have a strong connection with grade ≥3 acute toxicity. While future prospective studies with more patients enrolled are in urgent need to verify those dose limits.
Conflict of Interest
This paper is supported by National Natural Science Foundation of China, No: 81672971;;National Natural Science Foundation of Zhejiang, No: LY16H160037; Medical and Health Science and Technology Program of Zhejiang Province, No: 2016KYA041.
Contributor Information
Ming Chen, Email: chenming@zjcc.org.cn.
Yuanyuan Chen, Email: chenyy@zjcc.org.cn.
References
- 1.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, Ang KK, Morrison WH, Rosenthal DI, Garden AS. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngealintensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanguineti G, Gunn GB, Parker BC, Endres EJ, Zeng J, Fiorino C. Weekly dose volume parameters of mucosa and constrictor muscles predict the use of percutaneous endoscopic gastrostomy during exclusive intensity-modulated radiotherapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:52–59. doi: 10.1016/j.ijrobp.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Sanguineti G, Rao N, Gunn B, Ricchetti F, Fiorino C. Predictors of PEG dependence after IMRT chemotherapy for oropharyngeal cancer. Radiother Oncol. 2013;107:300–304. doi: 10.1016/j.radonc.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 5.Russo G, Haddad R, Posner M, Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 6.Lin YH, Chang KP, Lin YS, Chang TS. Evaluation of effect of body mass index and weight loss on survival of patients with nasopharyngeal carcinoma treated with intensity-modulated radiationtherapy. Radiat Oncol. 2015;10:136. doi: 10.1186/s13014-015-0443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Q, Shen LJ, Guo X, Guo XM, Qian CN, Wu PH. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer. 2016;16:169. doi: 10.1186/s12885-016-2214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Wang R, Lu H, Wei B, Feng G, Li G, Liu M, Yan H, Zhu J, Zhang Y. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: Treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112:106–111. doi: 10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, Ng QS, Tan D, Ong WS, Tan SH. Concurrent Chemo-Radiation With or Without Induction Gemcitabine, Carboplatin, and Paclitaxel: A Randomized, Phase 2/3 Trial in Locally Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2015;91:952–960. doi: 10.1016/j.ijrobp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Guan X, Zhu G, Wang X. Induction Chemotherapy With Docetaxel and Nedaplatin Followed by Concurrent IMRT and Nedaplatin for Locally Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2014;90(Suppl. 1):S525–S526. [Google Scholar]
- 11.Ma F, Jin F. Efficacy and Factor Affecting Outcome of Induction Chemotherapy Combined Concurrent Chemoradiation Therapy in 263 Patients With Locally Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2014;90(Suppl. 1):S524. [Google Scholar]
- 12.Sanguineti G, Endres EJ, Gunn BG, Parker B. Is there a mucosa-sparing benefit of IMRT for head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2006;66:931–938. doi: 10.1016/j.ijrobp.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 13.Marks LB, Yorke ED, Jackson A, Haken RKT, Constine LS, Eisbrusch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probility models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan S, Lehmann J, Coleman MA, Vaughan A, Yang CC, Enepekides D, Farwell G, Purdy JA, Laredo G, Nolan K. Prospective evaluation to establish a dose response for clinical oral muscositis in patients undergoing head-and-neck conformal radiotherpy. Int J Radiat Oncol Biol Phys. 2008;73:756–762. doi: 10.1016/j.ijrobp.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZH, Zhang SZ, Zhang ZY, Zhang CP, Hu HS, Tu WY, Kirwan J, Mendenhall WM. Protecting the oral mucosa in patients with oral tongue squamous cell carcinoma treated postoperatively with intensity-modulated radiotherapy: A randomized study. Laryngoscope. 2012;122:291–298. doi: 10.1002/lary.22434. [DOI] [PubMed] [Google Scholar]
- 16.Musha A, Shimada H, Shirai K, Saitoh J, Yokoo S, Chikamatsu K, Ohno T, Nakano T. Prediction of Acute Radiation Mucositis using an Oral Mucosal Dose Surface Model in Carbon Ion Radiotherapy for Head and Neck Tumors. PLoS One. 2015;10(10):e0141734. doi: 10.1371/journal.pone.0141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzola R, Ricchetti F, Fersino S, Fiorentino A, Levra NG, Paola GD, Ruggieri R, Alongi F. Predictors of mucositis in oropharyngeal and oral cavity cancer in patients treated with volumetric modulated radiation treatment: A dose-volume analysis. Head Neck. 2016;38(Issue S1):E815–E819. doi: 10.1002/hed.24106. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Yu XL, Luo W, Lee AW, Wee JT, Lee N, Lee N, Zhou GQ, Tang LL, Tao CJ. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol. 2014;110:390–397. doi: 10.1016/j.radonc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Dean JA, Welsh LC, Gulliford SL, Harrington KJ, Nutting CM. A novel method for delineation of oral mucosa for radiotherapy dose-response studie. Radiother Oncol. 2015;115:63–66. doi: 10.1016/j.radonc.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyneal carcinoma: radiation therapy oncology group phase II trail 0225. J Clin Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno D, Sato J, Igarashi C, Ikeda S, Morita M, Shimoda S, Shiozaki K, Kobayashi M, Kobayashi K. Accuracy of oral mucosal thickness measurements using spiral computed tomography. J Periodontol. 2011;82:829–836. doi: 10.1902/jop.2010.100160. [DOI] [PubMed] [Google Scholar]
- 22.Liu ZG, Zhao Y, Tang J, Zhou YJ, Yang WJ, Qiu YF, Wang H. Nimotuzumab combined with concurrent chemoradiotherapy in locally advancednasopharyngeal carcinoma: a retrospective analysis. Oncotarget. 2016;7(17):24429–24435. doi: 10.18632/oncotarget.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CJ, Wang SZ, Wang SY, Zhang YP. Assessment of the effect of local application of amifostine on acute radiation-induced oral mucositis in guinea pigs. J Radiat Res. 2014;55:847–854. doi: 10.1093/jrr/rru024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SX, Cui TT, Zhao C, Pan JJ, Xu BY, Tian Y, Cui NJ. A prospective, randomized, multi-center trial to investigate Actovegin in prevention and treatment of acute oral mucositis caused by chemoradiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2010;97:113–118. doi: 10.1016/j.radonc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Antin JH, Lee SJ, Neuberg D, Alyea E, Soiffer RJ, Sonis S, Ferrara JL. A phase I/II double-blind, placebo-controlled study of recombinant human interleukin-11 formucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:373–377. doi: 10.1038/sj.bmt.1703394. [DOI] [PubMed] [Google Scholar]
- 26.Moslemi D, Nokhandani AM, Otaghsaraei MT, Moghadamnia Y, Kazemi S, Moghadaminia AA. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother Oncol. 2016;120:13–20. doi: 10.1016/j.radonc.2016.04.001. [DOI] [PubMed] [Google Scholar]