Abstract
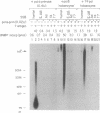
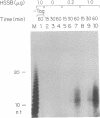
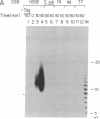
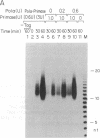
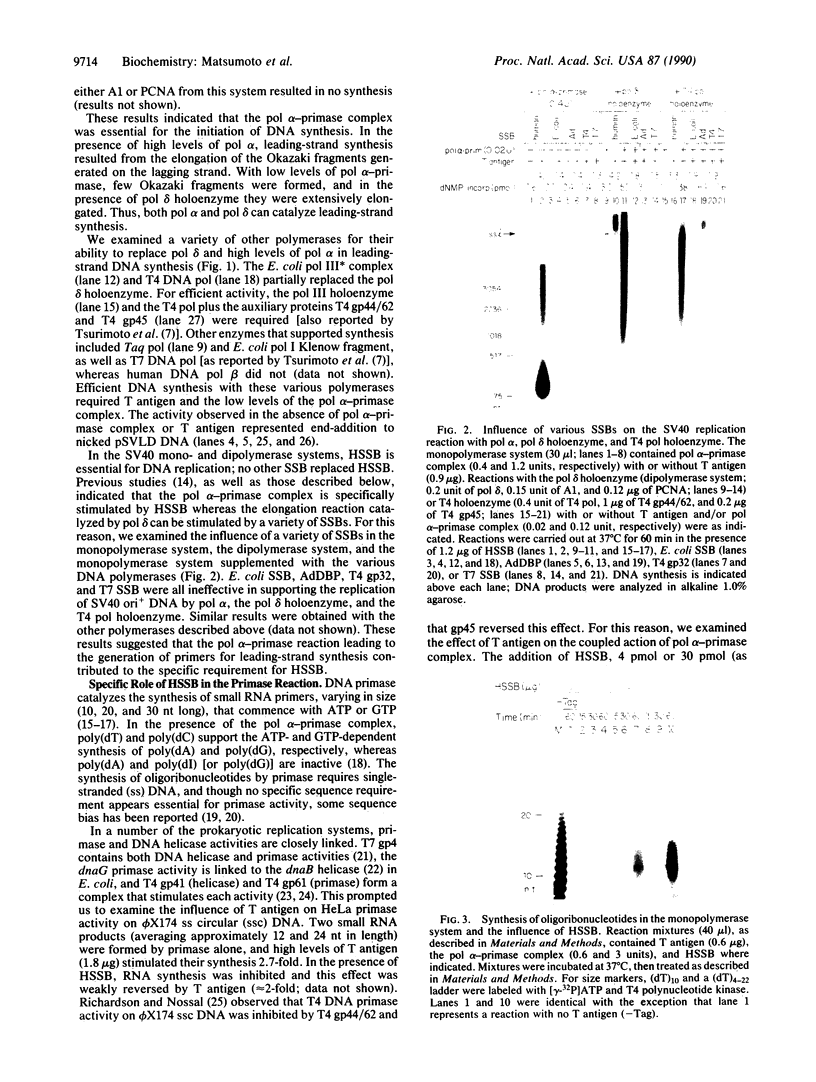
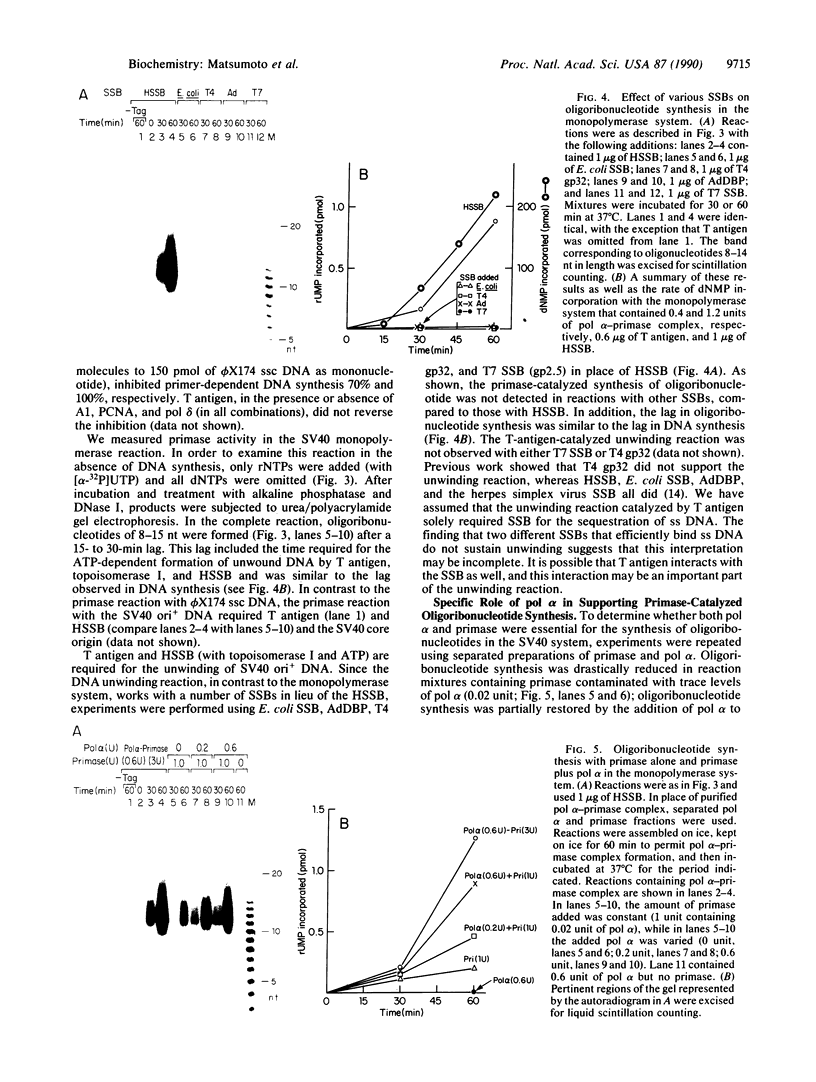
The synthesis of oligoribonucleotides by DNA primase in the presence of duplex DNA containing the simian virus 40 (SV40) origin of replication was examined. Small RNA chains (10-15 nucleotides) were synthesized in the presence of the four common ribonucleoside triphosphates, SV40 large tumor antigen (T antigen), the human DNA polymerase alpha (pol alpha)-DNA primase complex, the human single-stranded DNA-binding protein (HSSB), and topoisomerase I isolated from HeLa cells. The DNA primase-catalyzed reaction showed an absolute requirement for T antigen, HSSB, and pol alpha. The requirement for HSSB was not satisfied by other SSBs that can support the T-antigen-catalyzed unwinding of DNA containing the SV40 origin of replication. Oligoribonucleotide synthesis occurred with a lag that paralleled the lag observed in DNA synthesis. These results indicate that the specificity for the HSSB in the SV40 replication reaction is due to the pol alpha-primase-mediated synthesis of the Okazaki fragments. In contrast to this specificity, the elongation of Okazaki fragments can be catalyzed by a variety of different DNA polymerases, including high levels of pol alpha, the polymerase delta holoenzyme, T4 polymerase holoenzyme, the Escherichia coli polymerase III holoenzyme, and other polymerases. These observations suggest that leading-strand synthesis in the in vitro SV40 replication system can be nonspecific.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Barry J., Bedinger P., Formosa T., Jongeneel C. V., Kreuzer K. N. Studies on DNA replication in the bacteriophage T4 in vitro system. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):655–668. doi: 10.1101/sqb.1983.047.01.077. [DOI] [PubMed] [Google Scholar]
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Cha T. A., Alberts B. M. Studies of the DNA helicase-RNA primase unit from bacteriophage T4. A trinucleotide sequence on the DNA template starts RNA primer synthesis. J Biol Chem. 1986 May 25;261(15):7001–7010. [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey S. K., Faust E. A. Murine DNA polymerase.alpha-primase initiates RNA-primed DNA synthesis preferentially upstream of a 3'-CC(C/A)-5' motif. J Biol Chem. 1990 Mar 5;265(7):3611–3614. [PubMed] [Google Scholar]
- Goetz G. S., Hurwitz J. Studies on the role of the phi X174 gene A protein in phi X viral strand synthesis. I. Replication of DNA containing an alteration in position 1 of the 30-nucleotide icosahedral bacteriophage origin. J Biol Chem. 1988 Nov 5;263(31):16421–16432. [PubMed] [Google Scholar]
- Gronostajski R. M., Field J., Hurwitz J. Purification of a primase activity associated with DNA polymerase alpha from HeLa cells. J Biol Chem. 1984 Aug 10;259(15):9479–9486. [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y., Claude A., Bullock P., Hurwitz J. Complete enzymatic synthesis of DNA containing the SV40 origin of replication. J Biol Chem. 1988 Dec 25;263(36):19723–19733. [PubMed] [Google Scholar]
- Johanson K. O., McHenry C. S. The beta subunit of the DNA polymerase III holoenzyme becomes inaccessible to antibody after formation of an initiation complex with primed DNA. J Biol Chem. 1982 Oct 25;257(20):12310–12315. [PubMed] [Google Scholar]
- Kenny M. K., Lee S. H., Hurwitz J. Multiple functions of human single-stranded-DNA binding protein in simian virus 40 DNA replication: single-strand stabilization and stimulation of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9757–9761. doi: 10.1073/pnas.86.24.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J Biol Chem. 1990 May 5;265(13):7693–7700. [PubMed] [Google Scholar]
- Lee S. H., Eki T., Hurwitz J. Synthesis of DNA containing the simian virus 40 origin of replication by the combined action of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7361–7365. doi: 10.1073/pnas.86.19.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Hurwitz J. Mechanism of elongation of primed DNA by DNA polymerase delta, proliferating cell nuclear antigen, and activator 1. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5672–5676. doi: 10.1073/pnas.87.15.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- Miller J., Bullock P., Botchan M. Simian virus 40 T antigen is required for viral excision from chromosomes. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7534–7538. doi: 10.1073/pnas.81.23.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole S. E., Gannon J. V., Ford M. J., Lane D. P. Structure and function of SV40 large-T antigen. Philos Trans R Soc Lond B Biol Sci. 1987 Dec 15;317(1187):455–469. doi: 10.1098/rstb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Hinton D. M. Bacteriophage T4 DNA primase-helicase. Characterization of the DNA synthesis primed by T4 61 protein in the absence of T4 41 protein. J Biol Chem. 1987 Aug 5;262(22):10879–10885. [PubMed] [Google Scholar]
- O'Donnell M., Studwell P. S. Total reconstitution of DNA polymerase III holoenzyme reveals dual accessory protein clamps. J Biol Chem. 1990 Jan 15;265(2):1179–1187. [PubMed] [Google Scholar]
- Richardson R. W., Nossal N. G. Trypsin cleavage in the COOH terminus of the bacteriophage T4 gene 41 DNA helicase alters the primase-helicase activities of the T4 replication complex in vitro. J Biol Chem. 1989 Mar 15;264(8):4732–4739. [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Enomoto T., Masutani C., Hanaoka F., Yamada M., Ui M. DNA primase-DNA polymerase alpha assembly from mouse FM3A cells. Purification of constituting enzymes, reconstitution, and analysis of RNA priming as coupled to DNA synthesis. J Biol Chem. 1989 Jun 15;264(17):10065–10071. [PubMed] [Google Scholar]
- Syvaoja J., Linn S. Characterization of a large form of DNA polymerase delta from HeLa cells that is insensitive to proliferating cell nuclear antigen. J Biol Chem. 1989 Feb 15;264(5):2489–2497. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Jan;78(1):205–209. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Prussak C. E. Sequence and structural requirements for primase initiation in the SV40 origin of replication. Nucleic Acids Res. 1989 Mar 11;17(5):1953–1963. doi: 10.1093/nar/17.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, alpha and delta. EMBO J. 1989 Dec 1;8(12):3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. Mechanism of DNA elongation catalyzed by Escherichia coli DNA polymerase III, dnaZ protein, and DNA elongation factors I and III. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3511–3515. doi: 10.1073/pnas.73.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. Studies on deoxyribonucleic acid polymerases from yeast. 1. Parial purification and properties of two DNA polymerases from mitochondria-free cell extracts. Eur J Biochem. 1970 Mar 1;13(1):11–19. doi: 10.1111/j.1432-1033.1970.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA replicase. DNA polymerase associated with a novel RNA polymerase activity to synthesize initiator RNA of strict size. J Biol Chem. 1982 Sep 25;257(18):11121–11127. [PubMed] [Google Scholar]