Abstract
Small cell carcinomas (SCC) make up only 1% of malignancies of the prostate. Reports of several case series have described outcomes of surgery and chemotherapy for SCC of the prostate, but few reports address radiotherapy. We treated a case of SCC of the prostate with intensity-modulated radiation therapy (IMRT) consisting of 70 Gy administered in 35 fractions followed by hormonal therapy using only luteinizing hormone-releasing hormone (LH-RH) agonist. The tumor volume decreased remarkably by 4 months after IMRT. The rapid decrease in tumor size of this SCC of the prostate seemed to suggest a similar high radiosensitivity to that of SCC of the lung, but the tumor increased rapidly thereafter within the radiation fields, and pelvic lymph node metastases had developed by 24 months after IMRT. By 28 months after IMRT, multiple lung metastases developed, and the patient died of SCC of the prostate 31 months after initial diagnosis.
Keywords: IMRT, Prostate cancer, Radiotherapy, Small cell carcinoma
1. Introduction
Small cell carcinoma (SCC) is uncommon in the prostate gland and accounts for less than 1% of prostatic malignancies.1 Its prognosis is considered worse than that of common adenocarcinoma despite several aggressive treatments that include surgery and/or chemotherapy and/or hormonal therapy. Recent reviews have reported the dismal outcome of SCC, with 5-year survival of 14.3% and average survival less than one year.2 No standard treatment is established for this rare variant, which seems generally resistant to hormonal therapy. Surgery is sometimes recommended, but many cases are judged unresectable at initial diagnosis because of the tumor's aggressive growth with distant metastases. On the other hand, SCC is often considered to be a systemic disease, and recommended chemotherapy includes drugs such as carboplatin, etoposide, and docetaxel. Though we found several case-series reports that described outcomes of surgery and chemotherapy for SCC of the prostate, we believe few reports describe radiotherapy. We report a case with SCC of the prostate that we treated with IMRT consisting of a total dose of 70 Gy administered in 35 fractions followed by hormonal therapy using only luteinizing hormone-releasing hormone (LH-RH) agonist.
2. Case report
An 81-year old man visited our hospital with symptoms of ischuria; his performance status was graded one based on the guidelines of the Eastern Cooperative Oncology Group, and he was admitted for further evaluation. The patient had many comorbidities, including hypertension, type 2 diabetes mellitus, interstitial pneumonia, and chronic renal failure. He had no family history of prostate cancer. An elastic hard mass in the right lobe of the prostate at rectal examination and initial serum level of prostate-specific antigen (PSA) of 7.42 ng/mL suggested prostate cancer. Magnetic resonance (MR) imaging demonstrated a large mass in the right lobe of the prostate with extracapsular extension and invasion to the right seminal vesicle. The tumor showed iso-signal intensity on T1-weighted image, low signal intensity on T2-weighted image, and high signal intensity on diffusion-weighted image (Fig. 1). A transrectal prostate biopsy confirmed small cell carcinoma (SCC) of the prostate histology, and there was a strong positive staining for chromogranin A, a secretory protein, and MIB1, an antibody against the protein Ki-67, a protein expressed in proliferating cells (Fig. 2).
Fig. 1.
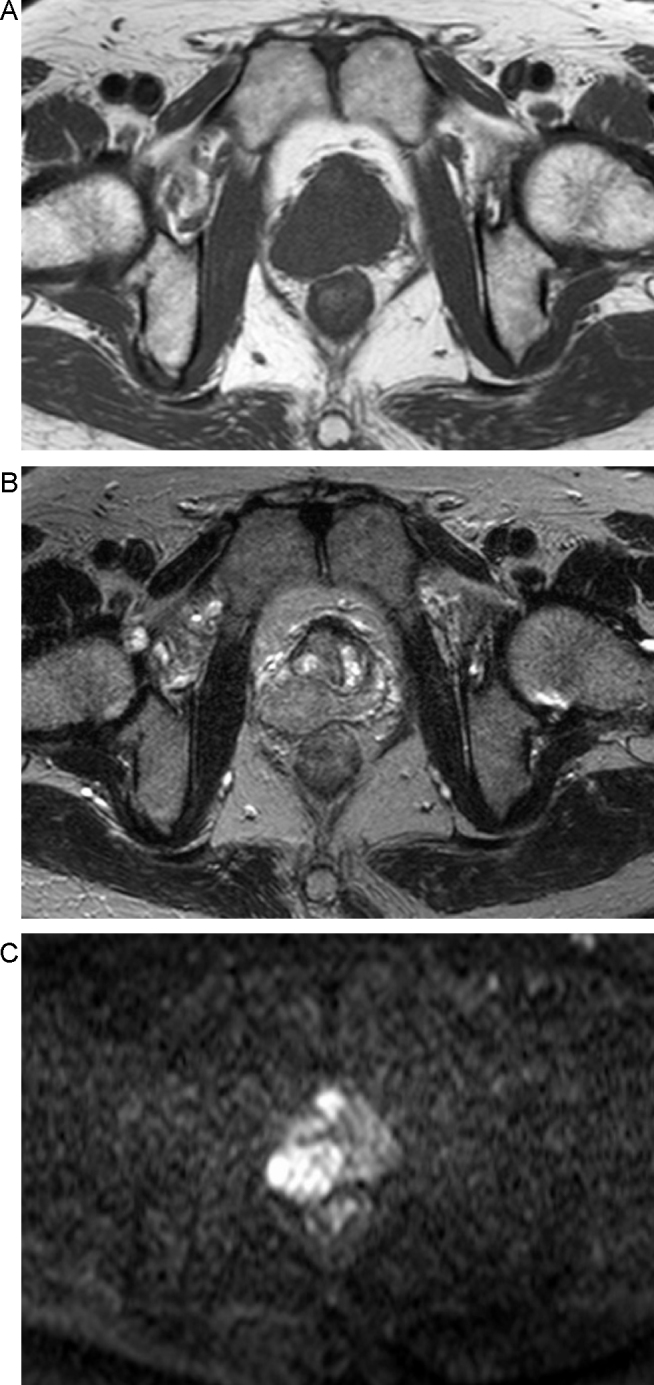
Magnetic resonance imaging demonstrates a large mass in the right lobe of the prostate that invades beyond the surgical capsula of the prostate and to the right seminal vesicle. (A) Iso-signal intensity on T1-weighted image; (B) low signal intensity on T2-weighted image; (C) high signal intensity on diffusion-weighted image.
Fig. 2.

Pathological findings of the biopsy specimen. (A) Hematoxylin and eosin (H&E) staining, 400×. Tumor cells are small and indicate solid alveolar growth and central necrosis. No adenocarcinoma component is seen. (B) Strong positive staining for chromogranin A, 400×; (C) strong positive staining for MIB1 index, 400×.
Four of 5 cores were positive in the right lobe of the prostate, and none of the 5 cores in the left lobe was positive. Other examinations, including chest X-ray and contrast-enhanced computed tomography (CT), revealed no abnormal enlargement of pelvic lymph nodes and no distant metastases. We finally diagnosed the tumor as Stage III (T3bN0M0) SCC of the prostate according to the TNM classification system4 and categorized it as high risk according to the D’Amico classification.5
The tumor was judged unresectable because of its aggressive growth, which included its invasion of the right seminal vesicle, and the patient's many comorbidities precluded chemotherapy. We, therefore, chose IMRT as an initial treatment, utilizing a protocol similar to that for standard treatment of common adenocarcinoma of the prostate. IMRT was delivered by a 10-MV X-ray using 7 portals with a daily dose of 2 Gy administered 5 times a week. We created 3 radiation volumes – the gross tumor volume (GTV), the clinical target volume (CTV), and the planning target volume (PTV). For the first course of radiation, we referred to pretreatment CT and MR imaging to create the GTV and CTV on the treatment-planning CT. The CTV included the GTV, entire prostate gland, and bilateral entire seminal vesicles. The prescription dose was a minimum dose of at least 95% of the CTV, and inverse optimization was performed. The PTV included the CTV with a margin of 0.9–1.0 cm, except for the posterior of the prostate, where a margin of 0.6 cm was used. Finally, a radiation oncologist rearranged the PTV according to the dose limit of the CTV and organs at risk, including the bladder and rectum. Fig. 3 shows the dose distribution of IMRT. IMRT was performed and continued to a total dose of 70 Gy in 35 fractions in the same radiation fields with no change. Serum PSA was 5.04 ng/mL at completion of the IMRT.
Fig. 3.
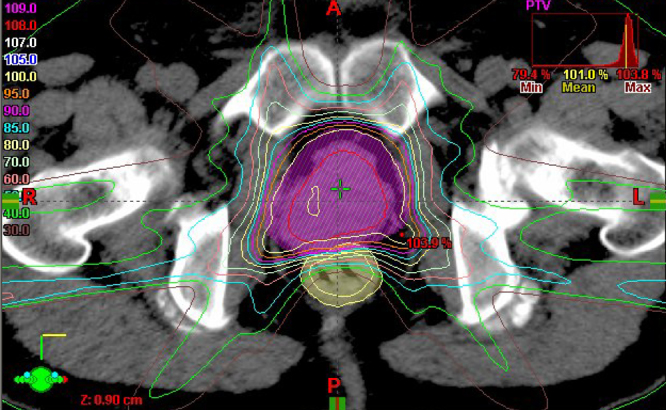
Dose distribution of intensity-modulated radiation therapy. Planning target volume, pink color wash. Number at left indicates the percentage of the prescribed radiation dose.
We evaluated outcomes and toxicities weekly during IMRT and at subsequent patient visits every 1–2 months thereafter. Follow-up examinations included physical examination, serum PSA testing, and imaging studies when necessary. Toxicities were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) (Version 4.0; U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute).
Because the patient's symptoms, including ischuria, had not changed by one month after IMRT, we evaluated grade 2 acute genitourinary toxicity including ischuria and performed hormonal therapy using LH-RH agonist to relieve his symptoms.
CT images obtained 4 months after IMRT showed remarkable shrinkage of the tumor volume (Fig. 4). One year after IMRT, his serum PSA had decreased to 0.01 ng/mL and remained below 0.01 ng/mL thereafter. However, the tumor recurred rapidly within the radiation fields, pelvic lymph node metastases developed 24 months after IMRT (Fig. 5), multiple lung metastases developed 28 months after IMRT, and the patient died from SCC of the prostate 31 months after the initial diagnosis.
Fig. 4.
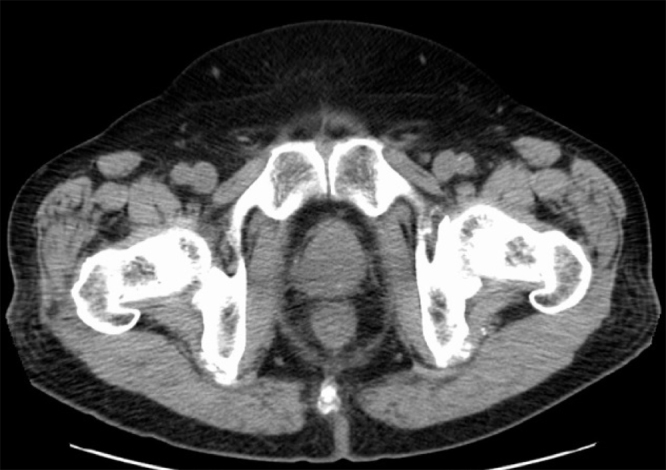
Contrast-enhanced computed tomography 4 months after intensity-modulated radiation therapy shows remarkable shrinkage of the tumor volume.
Fig. 5.
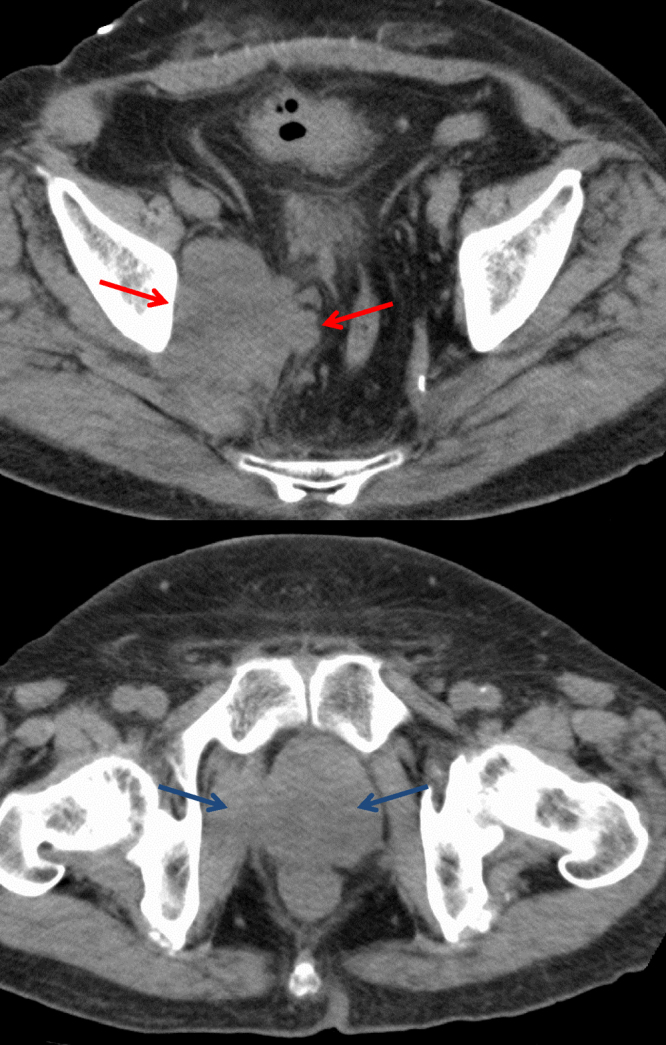
Contrast-enhanced computed tomography reveals rapid recurrence of the tumor within the radiation fields and development of pelvic lymph node metastases 24 months after intensity-modulated radiation therapy.
3. Discussion
SCC of the prostate is very rare and demonstrates an especially poor outcome compared to that of common adenocarcinoma of the prostate.1 Manifestations include a low level of PSA, large tumor volume, lytic bone metastases, and no response to hormonal therapy.1, 3 In general, Gleason score is not evaluated in case of SCC of the prostate because of its immature biology. SCC of the prostate is a neuroendocrine carcinoma, one of a heterogeneous group that also includes common adenocarcinomas of the prostate with focal differentiation, tumors with Paneth-cell-like neuroendocrine differentiation, carcinoid tumors, and large-cell neuroendocrine carcinomas.6 It is important to distinguish SCC of the prostate from non-small cell neuroendocrine-differentiated prostatic adenocarcinoma because the treatment strategy is different. SCC may occur in a pure form (50–60% of cases) or adjacent to or concomitantly with common adenocarcinoma; it is estimated that 40–50% of patients with SCC have a history of prostatic adenocarcinoma.7 SCC of the prostate is often believed to be a systemic disease, and chemotherapy is recommended that includes carboplatin, etoposide, docetaxel, and other drugs. Reports of several clinical trials of chemotherapy for SCC of the prostate have described median overall survival of 9.6–16 months and progression-free survival of 2.1–8 months.8, 9, 10 However, few reports have examined radiotherapy for SCC of the prostate.
Our patient's tumor was diagnosed histologically as a pure SCC of the prostate and showed strong positive staining for chromogranin A and MIB1. Chromogranin A is a specific neuroendocrine marker that is a common constituent of neuroendocrine secretory granules. A high level of the antibody, MIB1, a reliable marker of cell proliferation, indicates aggressive tumor growth. He underwent IMRT with 70 Gy in 35 fractions followed by hormonal therapy with LH-RH agonist alone and survived 31 months after initial diagnosis. The tumor volume decreased remarkably by 4 months after IMRT (8 months after initial diagnosis), but recurred rapidly within the radiation fields, and pelvic lymph node metastases developed 24 months after IMRT and multiple lung metastases, 28 months after IMRT. Despite the initial good response of the patient's SCC of the prostate to radiation therapy, resembling that of SCC of the lung, the radiotherapy failed to control the tumor. Nevertheless, our patient survived longer after initial diagnosis than patients described in previous reports. PSA was not useful for evaluating tumor activity, and immunohistopathological findings resembled those of pulmonary small cell cancer.
It is important to consider the radiotherapy administered for limited SCC of the lung. Standard thoracic irradiation for SCC of the lung is a total dose of 45 Gy administered in fractions of 1.5 Gy twice daily for 5 days per week over 3 weeks, keeping the overall treatment time as short as possible. In addition, a dose-escalation study (60–70 Gy with 2 Gy once daily over 6–7 weeks) is underway to improve local control. Therefore, accelerated fractionated radiotherapy and/or dose escalation might be employed to sterilize SCC of the prostate. Though our patient's tumor shrank remarkably after total irradiation with 70 Gy administered in 35 fractions, the dose seemed insufficient, and recent meta-analysis of randomized controlled trials reported that radiation doses exceeding 80 Gy would be expected to improve biochemical control in all risk groups of prostate carcinoma.11 With regard to toxicity for surrounding normal tissues, including the bladder and rectum, a total dose escalation of up to 74–78 Gy administered in 37–39 fractions might be possible. In addition, compared to conventional radiotherapy, IMRT allows better distribution of the radiation dose to conform to the targeted lesion. We performed adjuvant hormonal therapy using only LH–RH, but this might be insufficient to sterilize tumor cells completely. Intensive concurrent chemotherapy should be considered for patients with a good performance status.
Though irradiation of the entire pelvis might prevent intra-pelvic lymph node metastases, this radiation method remains controversial in routine practice in patients considered at high risk of harboring microscopic disease in pelvic nodes. Reported findings of Trial 94-13 of the Radiation Therapy Oncology Group (RTOG) showed no difference between pelvic versus prostate-only irradiation,12 but the higher dose necessary to eradicate prostate tumors carries a high risk of late intestinal complications. Therefore, care should be taken in the prophylactic radiotherapy of pelvic lymph nodes because the acceptable radiation dose to eliminate micrometastases to these nodes remains unclear. Unlike most cases in the RTOG trial, our patient experienced better progression-free survival in response to pelvic radiotherapy with adjuvant hormonal treatment than those who underwent irradiation of the prostate alone.
We can conclude from this case report that in patients with SCC of the prostate and contraindication for chemotherapy, we should try radiotherapy of sufficient dose and possible hormonal treatment. More data is required to determine the appropriate indication of radiotherapy for SCC of the prostate.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Têtu B., Ro J.Y., Ayala A.G. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Parimi V., Goyal R., Poropatich K. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 3.Deorah S., Rao M.B., Raman R. Survival of patients with small cell carcinoma of the prostate during 1973–2003: a population-based study. BJU Int. 2012;109:824–830. doi: 10.1111/j.1464-410X.2011.10523.x. [DOI] [PubMed] [Google Scholar]
- 4.Sobin L.H., Gospodarowicz M.K., Wittekind Ch., editors. TNM classification of malignant tumours. 7th ed. Wiley-Blackwell; New York: 2009. pp. 243–248. [Google Scholar]
- 5.D’Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 6.di Sant’Agnese P.A., Cockett A.T. Neuroendocrine differentiation in prostatic malignancy. Cancer. 1996;78:357–361. doi: 10.1002/(SICI)1097-0142(19960715)78:2<357::AID-CNCR27>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Nadal R., Schweizer M., Kryvenko O.N. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11:213–219. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fléchon A., Pouessel D., Ferlay C. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumour Group (GETUG) P01 trial. Ann Oncol. 2011;22:2476–2481. doi: 10.1093/annonc/mdr004. [DOI] [PubMed] [Google Scholar]
- 9.Culine S., El Demery M., Lamy P.J. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J Urol. 2007;178(3 Pt 1):844–848. doi: 10.1016/j.juro.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Papandreou C.N., Daliani D.D., Thall P.F. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–3080. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 11.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 12.Lawton C.A., DeSilvio M., Roach M., 3rd An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


