Abstract
In recent years it has become increasingly clear that astrocytes play a much more active role in neural processes than the traditional view of them as supporting cells suggests. Although not electrically excitable, astrocytes exhibit diverse Ca2+ dynamics across spatial and temporal scales, more or less dependent on the animal's behavioral state. Ca2+ dynamics range from global elevations lasting multiple seconds encompassing the soma up to the finest processes, to short elevations restricted to so-called microdomains within fine processes. Investigations of astrocyte Ca2+ dynamics have particularly benefitted from the development of Genetically-Encoded Calcium Indicators (GECIs). GECI expression can be achieved non-invasively in a cell type-specific manner and it can be genetically targeted to subcellular domains. The GCaMP family, a group of GECIs derived from the green fluorescent protein, has experienced some of the fastest advancements during the past decade. As a consequence we are now facing the challenge of needing to compare published data obtained with different versions of GECIs. With the intention to provide some guidance, here we compared Ca2+ dynamics across scales in awake transgenic mice expressing either the well-established GCaMP3, or the increasingly popular GCaMP6f, specifically in astrocytes. We found that locomotion-induced global Ca2+ elevations in cortical astrocytes displayed only minor kinetic differences and their apparent dynamic ranges for Ca2+ sensing were not different. In contrast, Ca2+ waves in processes and microdomain Ca2+ transients were much more readily detectable with GCaMP6f. Our findings suggest that behavioral state-dependent global astrocyte Ca2+ responses can be studied with either GCaMP3 or GCaMP6f whereas the latter is more appropriate for studies of spatially restricted weak and fast Ca2+ dynamics.
Introduction
Fluorescent Ca2+ indicators are an incredibly valuable tool for measuring neural activity. They enable localizing and monitoring activity levels in cell populations. Traditionally, chemical dyes have been employed. The first widely used dye, Fura-2, has been developed as a fast, ratiometric Ca2+ indicator that enables quantitative Ca2+ measurements [1]. Chemical Ca2+ indicators can be esterified with a lipophilic acetoxymethyl moiety that facilitates permeation of dyes through intact cell membranes and leads to accumulation of the dye following intracellular hydrolysis of the lipophilic moiety [2]. This approach has been extended to the bulk loading of hundreds of brain cells with chemical dyes in intact animals [3], and led to the first observations of Ca2+ dynamics in cortical astrocytes and cerebellar Bergmann glia in anesthetized and awake behaving mice [4,5]. However, bulk loading of chemical dyes has numerous drawbacks: it requires local injection of the loading buffer to the tissue under investigation and therefore is invasive and causes damage, loading is not cell type-specific, and sufficient dye concentration is maintained only for a couple of hours, precluding chronic experiments.
Genetically encoded Ca2+ indicators were sought as a method to overcome most of these drawbacks. Early approaches involved multiple-fluorophore sensors that rely on Förster resonance energy transfer (FRET). Calmodulin (CaM) is used as Ca2+ sensor that associates with the M13 domain from myosin light chain kinase when it has Ca2+ bound and induces a conformational rearrangement facilitating FRET between the fluorophores [6]. In 1999, Baird et al. developed circularly permutated (cp) fluorescent versions of green fluorescent protein (GFP) and the yellow and cyan versions YFP and CFP, in which the N- and C-terminal halves of the fluorophore were swapped and linked [7]. They were able to insert the protein calmodulin (CaM) into YFP with the result that Ca2+ binding increased the fluorescence intensity of YFP. The paper also suggested the same could be done for GFP. In 2001, Nakai and colleagues accomplished this task by combining cpGFP with the CaM/M13 strategy, developing the first version of GCaMP [8]. In this construct, Ca2+ induces conformational changes in CaM/M13, which prevents the solvent from reaching the chromophore, increasing fluorescence. The progress in GECI development made it possible to genetically target the molecule to specific tissues using viruses (lenti, adeno-associated) or transgenic mice [9]. The first generation GCaMP and other contemporary GECIs still had a number of drawbacks compared to synthetic Ca2+ dyes. The baseline fluorescence and sensitivity were much lower. The dynamic range (Fmax/Fmin) was narrow. The ratio of Ca2+ concentration to fluorescence was not linear. They were not reliably functional in vivo at physiological temperatures and pH. The kinetics were much slower, making it impossible to resolve individual action potentials. However, it had already been predicted that altering the amino acid sequence of GCaMP would change its properties significantly with the possibility of alleviating these problems. 2006 saw the creation of GCaMP2, a brighter and more stable version of GCaMP that made in vivo Ca2+ imaging of mouse cardiomyocytes possible [10]. Two years later the structure of GCaMP2 was resolved, which yielded insight into the mechanism of Ca2+ sensing and the associated change in fluorescence, and opened the gate towards rational design for further improvements [11,12]. A major advancement was realized with version GCaMP3, which had increased brightness, dynamic range, and affinity for Ca2+, making it possible to detect Ca2+ elevations caused by individual action potentials in vitro [13]. GCaMP3 was the first GECI version for which transgenic mice were generated that allowed non-invasive, global and chronic expression in a Cre recombinase-dependent manner [14,15]. The availability of these mice greatly propagated the utility of GCaMP3. By screening for beneficial mutations in the primary structure, a family of new versions of GCaMP were developed that provide even greater signal to noise ratios, giving more reliable and sensitive measures of neuronal activity [16]. They are called GCaMP 6s (slow), 6m (medium), and 6f (fast), based on the rise and decay kinetics. With improved kinetics, response amplitude and signal-to-noise ratio GCaMP6f has been the first GECI on par with Oregon Green BAPTA, one of the most popular chemical Ca2+ dyes. GCaMP 6s and 6m realized even larger improvements in response amplitude, at the cost of kinetics. Mice have also been generated for Cre recombinase-dependent expression of the GCaMP6 variants [17,18].
Astrocytes are not electrically excitable. As a consequence, with current technology, monitoring astrocyte Ca2+ dynamics represents the most accessible way of obtaining insight into their real-time functional state. Astrocytes undergo a wide range of Ca2+ dynamics regarding kinetics as well as spatial distribution within individual astrocytes as well as within the astrocyte population throughout the brain [19]. One extreme within this range of astrocyte Ca2+ dynamics is represented by global Ca2+ elevations that encompass the entire astrocyte, last longer than 5 s, and occur simultaneously in astrocytes in different regions of the brain [15]. This global astrocyte Ca2+ activation can be triggered by a mouse transitioning from a resting state to active locomotion [5,15] or by other forms of arousal, and depends on α1-adrenergic signaling [15,20]. Consistent with a role of noradrenergic signaling in arousal, attention and learning, noradrenergic signaling in hippocampal astrocytes has been reported to be involved in ATP release and subsequent weakening of glutamatergic synaptic transmission through a postsynaptic mechanism [21,22]. Similarly, it has been reported that noradrenergic signaling through cortical astrocytes leads to release of ATP and D-serine and is required for long term potentiation of neuronal responses [23]. Global Ca2+ activation of cortical astrocytes can also be induced with electrical stimulation of the nucleus basalis of Meynert, which leads to cortical acetylcholine release, and plays a role in cortical plasticity [24,25]. Cholinergic signaling through astrocytes in the hippocampus is involved in setting the inhibitory tone [26]. The other extreme within the range of astrocyte Ca2+ dynamics are spatially restricted (< 5 μm in diameter) and short (< 2 s) Ca2+ elevations within microdomains of the finest processes. These microdomain Ca2+ dynamics are only partially dependent on intracellular Ca2+ mobilization from the endoplasmic reticulum [27]. Ca2+ influx through the plasma membrane seems to play an important role for these faster and spatially restricted signals [28–31]. It has been proposed that astrocyte microdomain Ca2+ dynamics can cause vesicular release of glial signaling molecules, regulate plasma membrane neurotransmitter transport, and control the synthesis of membrane-permeable signaling molecules to influence neuronal excitability, synaptic strength and capillary blood flow [28,31–35]. Astrocyte Ca2+ elevations along individual processes may represent to varying degrees combinations of the mechanisms underlying global and microdomain Ca2+ dynamics.
The advantages of GCaMP3 for studying astrocyte Ca2+ dynamics together with the availability of transgenic mice to achieve non-invasive cell type-specific expression [14,15] has made this a very popular tool to study astrocytes [28,29,35–46]. With the advent of the more advanced sensor GCaMP6f, researchers are starting to migrate towards this new GECI [18,27,42]. As a consequence, we are now facing the challenge of needing to compare astrocyte data obtained with GCaMP6f to published GCaMP3 data acquired in different laboratories, using different equipment and protocols. To provide some aid to resolve this challenge, we sought to compare Ca2+ dynamics in cortical astrocytes of awake mice monitored with GCaMP3 or GCaMP6f using the same equipment and protocol. We studied global responses, microdomain Ca2+ dynamics, and Ca2+ waves in processes. We found that GCaMP3 and GCaMP6f were equivalent in many regards when measuring global responses, with GCaMP6f reporting the time course of Ca2+ transients more faithfully; however, GCaMP6f was considerably more sensitive in studies of microdomain Ca2+ dynamics and Ca2+ waves.
Materials and methods
Animals
Cre recombinase-conditional GCaMP3 [15], GCaMP6f [17] and tdTomato (Ai14) [47] mice were crossed to GLAST-CreER [15] mice to enable expression in astrocytes. GCaMP or tdTomato expression was induced by 3 intraperitoneal injections of 100 mg/kg body weight tamoxifen, dissolved in sunflower seed oil, within 5 days during the fourth postnatal week. For some experiments in S1 Fig we crossed GLAST-CreER(+/-);R26-lsl-GCaMP3(+/-) mice with IP3R2 knockout mice [48]. All experiments were conducted in accordance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committees at UT Health San Antonio.
Chronic cranial window surgery
Craniotomies were performed in two steps. In the first surgery, a custom designed stainless steel plate was attached to the skull for immobilization of the head under the objective. In the second surgery, performed at least 3 days later, the bone was removed and replaced with a glass coverslip. Mice were anaesthetized by i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). As soon as animals were unconscious, petroleum jelly was applied to the eyes. Following hair removal with Nair and skin disinfection with 70% ethanol, the scalp was incised and resected. The periosteum was then shaved off and approximately 3 mm of muscle surrounding the exposed skull was covered with a thin layer of cyanoacrylate cement. After drying, an 11 mm wide aluminum head plate with a 2 mm x 4 mm oval opening was centered above primary visual cortex V1 at lambda, 2.5 mm lateral from midline and attached to the skull using dental cement (C&B Metabond; Parkell Bio-Materials Div.). The second surgery was performed at least three days after mounting of the head plate under 1.5–2% vol./vol. isoflurane in O2. A 2.5 mm x 2.5 mm area of skull in the center of the opening was removed using a #12 scalpel blade. Three layers of No.1 cover glass, stacked with Norland Optical Adhesive #81 (Norland Products), replaced the skull and the edges were sealed with dental cement (Ortho-Jet Crystal, Lang Dental Manufacturing). Imaging was initiated at least two weeks after surgery. The time during recovery from the second surgery was used to habituate the mouse to the linear treadmill and the imaging environment.
Two-photon microscopy
Fluorescence images were collected using a Movable Objective Microscope (MOM) (Sutter Instrument) equipped with a resonant scanner and a Nikon 16x, 0.8 NA objective. The microscope was controlled by a personal computer equipped with an Intel Core i7 CPU 4930 @ 3.4 GHz and 16 GB of RAM running ScanImage (v5.0) software (Pologruto et al., 2003). Acquired frames were 400 μm by 400 μm for locomotion-induced global Ca2+ dynamics and 50 μm by 50 μm for microdomain or process wave Ca2+ dynamics, both at 512 by 512 pixel resolution. Image acquisition rate was 30 frames/s. Two photon excitation was achieved using a Titanium:Sapphire laser (Chameleon Ultra II, Coherent) tuned to 920 nm and attenuated, so that an average power of 60 mW or less entered the brain. For signal detection with all experiments we employed H10770PA-40 GaAsP detectors (Hamamatsu) with the control voltage set to 530 mV, and DHPCA-100 high-speed current amplifiers (FEMTO) with the gain set to 10 kV/A. The head of the mouse was immobilized by attaching the head plate to a custom-machined stage mounted on the microscope table. Mice were kept on the stage for a maximum of two hours.
Locomotion paradigm
Head immobilized mice were placed on a custom designed linear treadmill. The treadmill was either freely movable so that animals could move at will, or under motor control. The motion of the belt of the treadmill was monitored with a mechanically coupled optical encoder. The signal of the optical encoder was digitized at 20 kHz simultaneously with the position signal of the slow scan mirror (custom-written LabVIEW routines controlling PXIe-6363, National Instruments) for post hoc determination of movement velocity during corresponding images.
Electromyography
Body surface potential differences were recorded as the voltage between two silver wires placed subcutaneously at the right shoulder and left hip using an EXT-02 B amplifier (npi electronic). Data were digitized at 20 kHz (custom-written LabVIEW routines controlling PXIe-6363, National Instruments) for post hoc analysis. Muscle activity was extracted by applying a fast Fourier transform to the data and determining the power in the range 200 Hz—1 kHz. Fold increase was determined as power / powerBaseline.
Visual stimulation
A UV-LED (UVTOP-355-TO39-FW, Sensor Electronic Technology Inc.) with a Lambertian emission profile was used as a light source at a distance of 40 mm centered between the eyes to achieve uniform light exposure. The light power entering each eye with a pupil diameter of 2 mm was 7 nW. To eliminate optical cross talk between visual stimulation and two-photon fluorescence detection, the objective was shielded from the light source.
Data analysis—Locomotion-induced global Ca2+ elevations
Data were processed and analyzed in MATLAB using built-in functions integrated into custom routines. Images were first processed with a spatial Gaussian filter (1.52 SD per pixel distance) to reduce stochastic noise of the detector. To compensate for motion artifacts during image series, we acquired weak autofluorescence signals through the red detection path as reference signal to the simultaneously acquired GCaMP signal through the green detection path. Whole-frame normalized 2D cross-correlation (built-in function “normxcorr2”) was determined for reference images, and individual frames were registered to maximize correlation. The same registration parameters were then applied to images of GCaMP fluorescence. Data were temporally averaged by a factor of 6, preserving a frame rate of 5 Hz. ΔF/F fluorescence intensity ("Ca2+ change") traces represent (F—Fmedian) / Fmedian with F representing mean fluorescence value of all pixels within a region of interest (ROI) of one image frame and Fmedian representing median F of all image frames before the locomotion event. ROIs were the thresholded area of an image frame distinguishing GCaMP expressing astrocytes from background. Quantification of individual Ca2+ responses represents mean ΔF/F within 2–12 s following the onset of locomotion. The peak amplitude of calcium response was the point of maximal Ca2+ ΔF/F during a trial. Quantification of the time course of Ca2+ responses was done by calculating the rate of change in fluorescence intensity 10 seconds before and after the peak following locomotion. Area under the curve was determined by integrating the normalized ΔF/F 10 seconds before and after the peak. Trials in which Ca2+ changes resulting from voluntary locomotion or startle responses before or after enforced locomotion exceeded 9% ΔF/F were excluded to avoid distortion of the time course of enforced locomotion-induced fluorescence changes.
Data analysis—Spontaneous microdomain Ca2+ dynamics
Two-photon image series covering 50 μm by 50 μm and lasting 100 s were processed as described for global Ca2+ data up to the temporal averaging step. We then eliminated all episodes of image frames which contained a global astrocyte Ca2+ elevation. Spatial binning of the original 512 by 512 pixel image frames was applied to achieve a final pixel size of 1.56 μm. For each pixel we applied an averaging filter using the mean of that pixel value in four consecutive image frames. We then calculated the differential signal (difference of any pixel value between two consecutive image frames) and filtered that differential signal as described above. A search for the threshold which identified the largest number of solitary or pairs of supra-threshold pixels followed, since microdomain Ca2+ dynamics are spatially restricted to less than 3 μm. Despite our finding of significantly more frequent microdomain Ca2+ events with GCaMP6f than with GCaMP3 (see Results), the thresholds that yielded the highest number of microdomain locations was not different (GCaMP3: 12.6 ± 1.1% ΔF/F; 9 mice—61 astrocytes; GCaMP6f: 10.1 ± 1.7% ΔF/F; 11 mice—82 astrocytes; p = 0.253). Once we had identified the locations (pixels or pixel pairs) of microdomain Ca2+ events, we counted all events that occurred during the observation period and calculated the frequency.
Data analysis—Spontaneous Ca2+ waves along processes
For analysis of Ca2+ waves along processes (non-global Ca2+ dynamics that sequentially encompass more than 3 μm), we used the image series described for microdomain Ca2+ dynamics following the spatial binning. Using MATLAB, we calculated the mean of the Pearson's linear correlation coefficients between all pairs of image frames of an astrocyte. The rationale for this analysis was that the microdomain Ca2+ events, which are short and encompass only 1–2 of the up to 1024 pixels of an image frame in this analysis, will have a minor influence on image variability. On the other hand, following image registration and removal of global Ca2+ elevations, Ca2+ waves along astrocyte processes will dominate variability among fluorescence images. For imaging tdTomato fluorescence in V1 astrocytes, we applied 50–70 mW of 800 nm at the window surface and employed all other imaging and analysis procedures as described above for GCaMP experiments.
Statistical analysis
For all locomotion-induced Ca2+ data sets, measurements from individual astrocytes within one 400 μm by 400 μm field of view per mouse were averaged. For all microdomain Ca2+ dynamics or Ca2+ waves, the analysis results from 3–10 astrocytes per mouse were averaged. In all cases, the statistical power was based on the number of mice. We first conducted a Lilliefors test to determine if statistical tests for normally or non-normally distributed populations were applicable. We used the unpaired Student's t-test for normally distributed data sets, and we used the Kruskal-Wallis test for non-normally distributed data sets, as indicated in the figure captions. For S1 Fig we used one-way ANOVA followed by Bonferroni correction for multiple comparisons. For all tests, p values smaller than 0.05 were considered to indicate significance. All data values have been summarized in S1 File with explanations of the data organization in S2 File.
Results
For studying astrocyte-specific Ca2+ dynamics we used adult (2–6 months old) transgenic mice heterozygous for GLAST-CreERT and R26-lsl-GCaMP3 [15] or R26-lsl-GCaMP6f [17]. Tamoxifen treatment induced astrocyte-specific expression of GCaMP3 (not shown; [15]) or GCaMP6f (Fig 1A) in primary visual cortex.
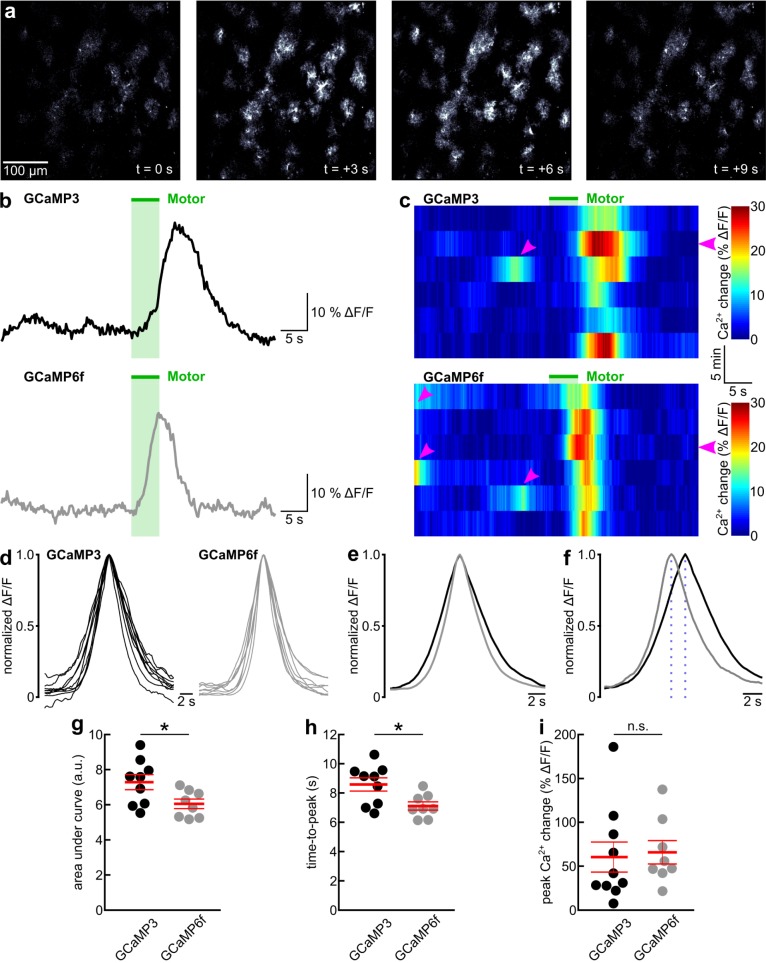
Fig 1. GCaMP6f indicates faster kinetics of astrocyte Ca2+ dynamics than GCaMP3.
(a) Series of representative fluorescence images of astrocytes in layer 1 of primary visual cortex of an awake GLAST-CreER(+/-);R26-lsl-GCaMP6f(+/-) mouse. Image series covers one 5 s enforced locomotion event. (b) Black and gray traces represent time course of mean GCaMP3 or GCaMP6f fluorescence, respectively, from population of astrocytes as shown in a, in response to enforced locomotion (green bar). (c) Pseudocolored time course of GCaMP3 or GCaMP6f fluorescence in astrocytes in response to 6 consecutive trials of enforced locomotion (green bar). Flat magenta arrowheads indicate trials presented in b. Angled magenta arrowheads highlight prominent fluorescence transients in response to voluntary locomotion events. (d) Overlay of peak-aligned and normalized representative GCaMP3 (9 mice) or GCaMP6f (8 mice) fluorescence traces in response to enforced locomotion. (e) Overlay of peak-aligned and normalized fluorescence traces in response to enforced locomotion from astrocytes expressing GCaMP3 (black trace, 9 mice) or GCaMP6f (gray trace, 8 mice). (f) Same traces as in e, time-shifted to represent mean time from onset of enforced locomotion to peak (highlighted by dotted lines) of GCaMP3 (black trace) or GCaMP6f (gray trace) fluorescence. (g) Population data representing area under enforced locomotion-induced GCaMP fluorescence transient shown in e. a.u. represents seconds multiplied by normalized ΔF/F. Asterisk indicates p = 0.032 (unpaired t-test). Red lines represent mean ± SEM. (h) Population data representing time from onset of enforced locomotion to peak of GCaMP fluorescence shown in f. Asterisk indicates p = 0.017 (unpaired t-test). Red lines represent mean ± SEM. (i) Population data representing peak amplitudes of enforced locomotion-induced astrocyte GCaMP fluorescence transients. GCaMP3, 10 mice; GCaMP6f, 8 mice; n.s. indicates p = 0.813 (unpaired t-test). Red lines represent mean ± SEM.
Locomotion-induced global astrocyte Ca2+ elevations
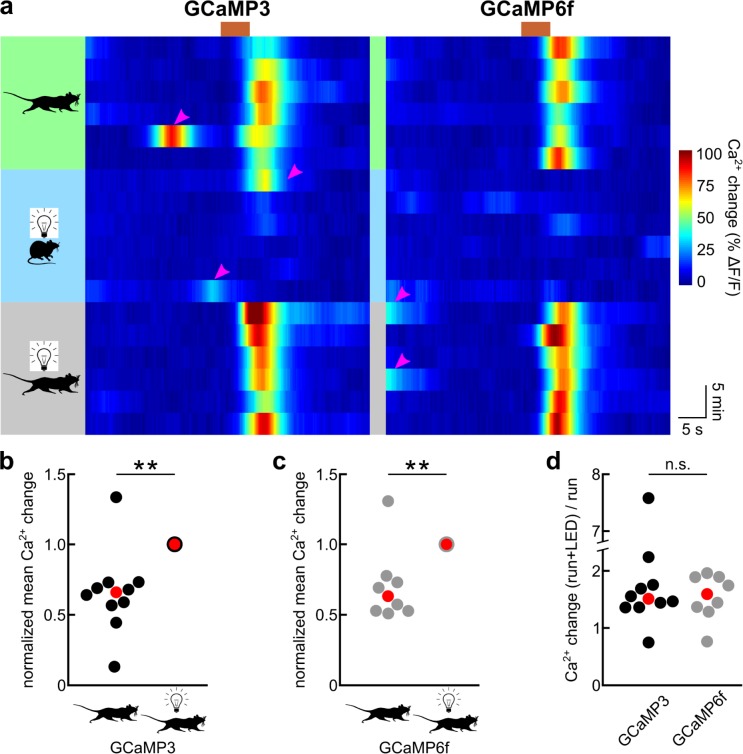
For a quantitative comparison of GCaMP3 and GCaMP6f fluorescence signals in response to astrocyte Ca2+ dynamics in awake behaving mice, we employed the enforced locomotion of head-fixed mice on a linear treadmill, a paradigm that we previously developed [15]. For behavioral state-dependent global Ca2+ dynamics, we acquired image series of 3–29 primary visual cortex astrocytes in one 400 μm by 400 μm field of view per mouse at a rate of 5 images per second (after posthoc averaging). Both GCaMP3 as well as GCaMP6f underwent robust, slowly rising fluorescence increases in astrocyte somata and processes lasting longer than 5 s in response to a 5 s enforced locomotion event (Fig 1A–1C). Facilitated by the well-controlled enforced locomotion stimulus, we could quantify three functional parameters of GCaMP3 and GCaMP6f responses to locomotion-induced global Ca2+ elevation: kinetics, amplitude and dynamic range. For the comparison of GCaMP response kinetics, we normalized locomotion-induced fluorescence increases and temporally aligned them to the response peak (Fig 1D). The area under the normalized fluorescence transient, as a compound parameter controlled by rise and decay, was smaller with GCaMP6f (6.05 ± 0.27 s * normalized ΔF/F; 8 mice) than with GCaMP3 (7.28 ± 0.43 s * normalized ΔF/F; 9 mice, p = 0.032) (Fig 1E and 1G). This finding suggests faster kinetics of GCaMP6f responses to global astrocyte Ca2+ elevations. Indeed, GCaMP6f fluorescent transients had a larger maximum rate of rise (35.2 ± 2.0% of peak / s; 8 mice) than GCaMP3 fluorescent transients (27.9 ± 1.8% of peak / s; 9 mice, p = 0.014), and there was a trend towards a faster maximum rate of fluorescence decay of GCaMP6f signals (-26.2 ± 2.1% of peak / s) compared with GCaMP3 signals (-21.6 ± 1.3% of peak / s; p = 0.080). Consistent with these kinetic parameters, GCaMP6f signals reached the peak response sooner following onset of locomotion (7.11 ± 0.29 s; 8 mice) than GCaMP3 signals (8.58 ± 0.45 s; 9 mice, p = 0.017) (Fig 1F and 1H). In contrast, the peak amplitude of fluorescence change over baseline reached following enforced locomotion was not different (GCaMP3: 60.33 ± 16.19% ΔF/F, 10 mice; GCaMP6f: 65.75 ± 13.29% ΔF/F, 8 mice; p = 0.813) (Fig 1I). Changes in sensor kinetics are often linked to changes in affinity for the ligand, with faster decay kinetics usually being indicative of a lower affinity [49]. Therefore, we sought to compare the dynamic ranges for Ca2+ sensing of GCaMP3 and GCaMP6f expressed in astrocytes. Ideally, we would like to determine the Ca2+ concentration—fluorescence relationship for each of the two GCaMPs. However, given the constraints of awake mouse experiments, we sought to compare the respective ratio of fluorescence responses to two different Ca2+ concentrations within the dynamic range of the sensor. We previously found that locomotion-induced Ca2+ elevations in primary visual cortex astrocytes can be potentiated by simultaneous visual stimulation, and that visual stimulation alone is not sufficient to cause a global astrocyte Ca2+ response [15]. This observation indicates that locomotion-induced astrocyte Ca2+ elevations in primary visual cortex do not saturate GCaMP3. We confirmed this observation here, as locomotion-induced GCaMP3 fluorescence was approximately two thirds the size reached with combined locomotion and visual stimulation (GCaMP3 responselocomotion normalized to GCaMP3 responselocomotion+LED: 0.66 ± 0.09, 10 mice; p = 0.001) (Fig 2A and 2B). Like GCaMP3 (Fig 2A) [15], GCaMP6f did not indicate global primary visual cortex astrocyte Ca2+ elevations in response to visual stimulation when mice were at rest (Fig 2A). Similarly, GCaMP6f-mediated fluorescence increases to locomotion were smaller than responses to combined locomotion and visual stimulation (GCaMP6f responselocomotion normalized to GCaMP6f responselocomotion+LED: 0.71 ± 0.09, 8 mice; p = 0.007) (Fig 2A). If GCaMP6f had a similar apparent Ca2+ affinity in astrocytes as GCaMP3, we would predict that they would have a similar ratio of responses to simultaneous locomotion and visual stimulation over responses to locomotion alone. Indeed, simultaneous visual stimulation potentiated locomotion-induced GCaMP6f responses similarly strongly as with GCaMP3 (GCaMP3: median fold potentiation: 1.51x (range: 0.75x - 7.58x), 10 mice; GCaMP6f: median fold potentiation: 1.59x (range: 0.76x - 1.96x), 8 mice; p = 0.859) (Fig 2D). Together, these data indicate that GCaMP6f detects astrocyte Ca2+ dynamics with moderately faster kinetics while preserving the useful dynamic range of GCaMP3 for sensing behavioral state-dependent global astrocyte Ca2+ elevations. For these slow global Ca2+ transients the kinetic improvements with GCaMP6f did not translate into increased signal amplitudes.
Fig 2. GCaMP3 and GCaMP6f operate with a similar dynamic range in astrocytes.
(a) Pseudocolored time course of primary visual cortex astrocyte GCaMP fluorescence in 6 consecutive trials of enforced locomotion (green field), visual stimulation (blue field) or enforced locomotion combined with visual stimulation (gray field) during the time window indicated by the brown bar. Magenta arrowheads highlight prominent fluorescence transients in response to voluntary locomotion events. (b) Population data representing mean GCaMP3 fluorescence increases in response to enforced locomotion normalized to responses to enforced locomotion with simultaneous visual stimulation in the same experimental session (10 mice; asterisks indicate p = 0.001 (Kruskal-Wallis test)). Red symbol indicates median. (c) Population data representing mean GCaMP6f fluorescence increases in response to enforced locomotion normalized to responses to enforced locomotion with simultaneous visual stimulation in the same experimental session (8 mice; asterisks indicate p = 0.007 (Kruskal-Wallis test)). Red symbol indicates median. (d) Population data representing fold increase of locomotion-induced mean GCaMP fluorescence increases by simultaneous visual stimulation (n.s. indicates p = 0.859 (Kruskal-Wallis test)). Red symbol indicates median.
Spontaneous astrocyte microdomain Ca2+ dynamics and Ca2+ waves
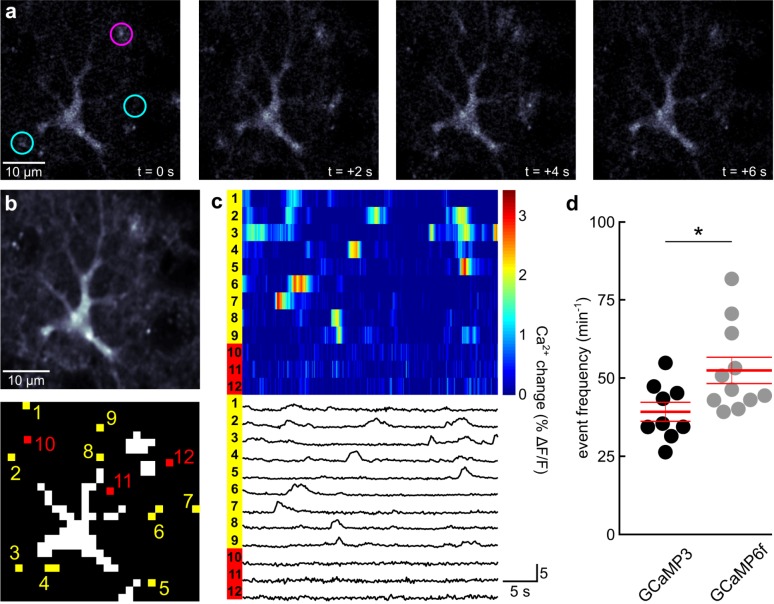
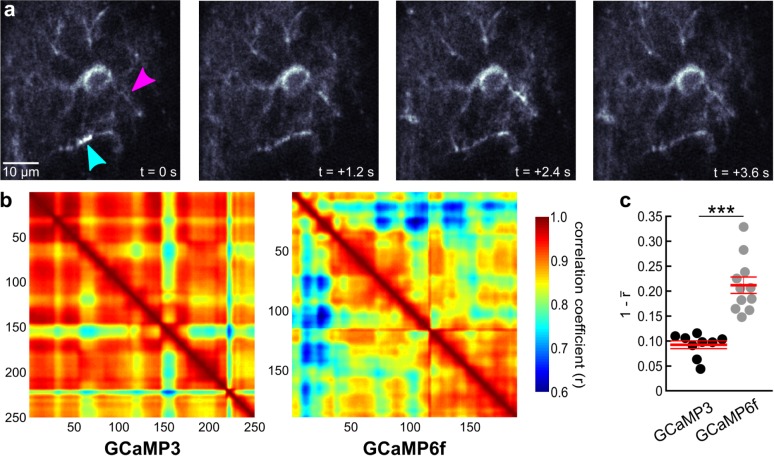
The faster kinetics of GCaMP6f signaling suggested benefits for the detection of shorter Ca2+ transients, such as in spatially restricted microdomains of fine astrocyte processes (Fig 3A). We acquired image series at 5 image frames per second (after posthoc averaging) for 100 s per astrocyte. Following removal of images with a global astrocyte Ca2+ elevation, we applied a fully automatic analysis algorithm for unbiased quantification of microdomain Ca2+ dynamics. The analysis algorithm (see Materials and Methods for details) searched for the threshold that yielded the largest number of individual size-restricted supra-threshold domains and determined the number of fluorescence transients that occurred at the detected domains during the time interval of observance (Fig 3B). As expected for microdomain Ca2+ dynamics, fluorescence transients were short (less than 5 s) and occurred in an asynchronous, uncoordinated manner (Fig 3C). We found that GCaMP6f made it possible to detect microdomain Ca2+ events in individual astrocytes of resting, awake mice more frequently (52.43 ± 4.20 events / s; 11 mice—82 astrocytes) than when imaged using GCaMP3 (39.21 ± 3.02 events / s; 9 mice—61 astrocytes, p = 0.025). Astrocytes also exhibit intermediate, wave-like Ca2+ dynamics along individual processes. Since Ca2+ waves are constituted by a spatial and temporal sequence of short Ca2+ transients at any location along a process (Fig 4A), we hypothesized that GCaMP6f might also prove to be more sensitive for the detection of astrocyte subcellular Ca2+ waves. For quantifying astrocyte Ca2+ waves in a completely unbiased way, we reasoned that the highly variable pattern of Ca2+ waves that can encompass considerable fractions of the astrocyte processes would reduce the average cross-correlation among frames of an image series (Fig 4B). We first tested whether this analysis preferentially reflects Ca2+ wave activity in contrast to microdomain Ca2+ events. Ca2+ waves, like global astrocyte Ca2+ elevations, are strongly reduced in inositol trisphosphate receptor 2 (IP3R2) knockout mice, whereas microdomain Ca2+ events are smaller but not less frequent [27]. Consistent with the notion that image cross-correlation analysis is not sensitive to microdomain Ca2+ dynamics, the distance to a perfect correlation of 1 was not significantly different when a Ca2+ unresponsive tdTomato signal was analyzed (0.04 ± 0.01; 6 mice) compared to the GCaMP3 signal in IP3R2 (-/-) mice (0.05 ± 0.01; 8 mice; S1 Fig). In contrast, image series acquired with GCaMP3 in wildtype mice revealed a considerably larger distance to a perfect correlation of 1 (0.09 ± 0.01; 9 mice; Fig 4C and S1 Fig) compared to tdTomato mice (p < 0.05—ANOVA). Importantly, image series acquired in wildtype mice using GCaMP6f revealed a much larger distance to a perfect correlation of 1 (0.21 ± 0.02; 11 mice, p < 0.001—unpaired t-test) than with GCaMP3 (Fig 4C and S1 Fig) Together, these data indicate that, compared with GCaMP3, GCaMP6f is more sensitive to the detection of fast astrocyte Ca2+ dynamics at small process terminals, or of Ca2+ waves along processes.
Fig 3. Astrocyte microdomain Ca2+ fluctuations can be detected more readily with GCaMP6f than with GCaMP3.
(a) Series of representative fluorescence images of an astrocyte in layer 1 of primary visual cortex of an awake GLAST-CreER(+/-);R26-lsl-GCaMP6f(+/-) mouse. Magenta circle highlights area of persistently enhanced fluorescence. In contrast, cyan circles highlight areas of transient, spatially restricted fluorescence increases, typical for microdomain Ca2+ fluctuations. (b) upper, Average of 250 consecutive astrocyte images, as shown in a. lower, Clusters with not more than two suprathreshold pixels during a 100 s time window are highlighted in yellow (1–9) and are considered locations of microdomain Ca2+ fluctuations. Example pixels representing areas of the astrocyte process tree without suprathreshold events during the observation time window are highlighted in red (10–12). (c) upper, Pseudocolored time course of GCaMP6f fluorescence at 12 labeled locations highlighted in b. lower, corresponding fluorescence traces. (d) Population data of frequency of detected microdomain Ca2+ fluctuations in mice expressing GCaMP3 (9 mice—61 astrocytes) or GCaMP6f (11 mice—82 astrocytes). Asterisk indicates p = 0.025 (unpaired t-test). Red lines represent mean ± SEM.
Fig 4. Calcium waves along astrocyte processes can be detected more readily with GCaMP6f than with GCaMP3.
(a) Series of representative fluorescence images of an astrocyte in layer 1 of primary visual cortex of an awake GLAST-CreER(+/-);R26-lsl-GCaMP6f(+/-) mouse. Magenta arrowhead highlights region where a full wave cycle was captured, cyan arrowhead highlights a region where the decaying phase of a wave was covered. (b) Representative pairwise Pearson correlation coefficient plots of series of indicated number of fluorescence images. Cooler colors indicate lower correlation and more dynamic GCaMP indication of Ca2+ fluctuations. (c) Population data of 1—mean correlation coefficient (r). GCaMP3, n = 9 mice (61 cells) and GCaMP6f, n = 11 mice (82 cells); asterisks indicate p < 0.001 (unpaired t-test). Red lines represent mean ± SEM.
Discussion
Our investigations revealed that GCaMP6f was more sensitive than GCaMP3 in the detection of small and fast microdomain Ca2+ dynamics in cortical astrocytes of awake mice, as well as in the detection of Ca2+ waves along astrocyte processes. In contrast, there was no difference in the amplitude of GCaMP6f or GCaMP3 fluorescence changes in response to locomotion-induced slow, global Ca2+ elevations. While these findings appear contradictory at first glance, they are more conclusive when we consider the properties of these GECIs in more detail. GCaMP6f has been developed from GCaMP5G, which in turn was derived from GCaMP3 [16,37]. GCaMP5G carries three point mutations within the GCaMP3 substrate. Two amino acid substitutions (T302L, R303P) in the linker 2 region, which connects cpEGFP with CaM, lead to an increased dynamic range of Ca2+-induced fluorescence increase at the cost of Ca2+ affinity. A third mutation (D380Y) within the interlobe linker of CaM restitutes Ca2+ affinity and favors low fluorescence signals of GCaMP5G in the absence of Ca2+ at physiological pH. The latter property is critical for the extended dynamic range of fluorescence change of this indicator during in vivo experiments [37]. The GCaMP6 family of GECIs carries a common set of three additional single amino acid substitutions. Two (T381R, S383T) are located in the interlobe linker of CaM and may facilitate anchoring the Ca2+ bound CaM-M13 complex to the cpEGFP, thereby controlling solvent access to the chromophore and controlling its protonation state, accounting for the increased Ca2+-dependent fluorescence increase [50]. The third mutation (R392G) was adapted from the K version of GCaMP5; it is located close to one of the CaM Ca2+ binding sites [50] and makes GCaMP5K the GCaMP5 with the highest Ca2+ affinity [51]. GCaMP6f is the fastest GCaMP due to an additional mutation (A317E) in a region of CaM that is involved in the interaction with the M13 peptide. The substitution of the short hydrophobic alanine with the long, charged glutamate is thought to destabilize the Ca2+-induced CaM-M13 complex formation [50]. Remarkably, the considerably enhanced Ca2+ sensitivity of GCaMP6f in cultured hippocampal neurons compared to GCaMP3 is predominantly noticeable with small and short Ca2+ elevations. For example, GCaMP6f fluorescence increases in response to 1–2 action potentials are up to 10 times larger than when imaged with GCaMP3 [16]. However, with increasing stimulus strength this benefit fades away, and with a burst of 100 action potentials GCaMP6f fluorescence increases are some twofold as large as GCaMP3 responses. Locomotion and startle-induced astrocyte Ca2+ elevations depend on α1-adrenergic signaling [15,20], Ca2+ transients encompass the entire cell and display a time course (>10 s) consistent with Gq-coupled receptor mediated intracellular Ca2+ release. Therefore, our finding that GCaMP6f and GCaMP3 indicate locomotion-induced Ca2+ elevations equally well is not surprising (Fig 1). Our comparison of locomotion-induced fluorescence increases with responses to combined locomotion and visual stimulation even suggests that these indicators operate in a similar portion of their dynamic Ca2+ sensing range (Fig 2). Microdomain astrocyte Ca2+ dynamics and the kinetically related Ca2+ waves along processes are at any location short and relatively weak events, more similar to a neuronal response to one or a few action potentials. It is then comprehensible that GCaMP6f was more sensitive at detecting these faint signals than GCaMP3 (Figs 3 and 4) [16].
Locomotion-induced astrocyte Ca2+ elevations measured with GCaMP6f reached the peak earlier than GCaMP3 signals (Fig 1F and 1H). Had the earlier rise in the GCaMP6f signal occurred due to increased Ca2+ sensitivity or affinity, we would have also expected a longer lasting GCaMP6f fluorescence signal during the Ca2+ decay phase, and as a consequence an increased area under the normalized fluorescence trace. However, in our experiments we found that the area under the normalized GCaMP6f fluorescence trace was smaller than for GCaMP3. This happened through a combination of a faster rise and a trend towards a faster decay (Fig 1E and 1G). This observation was consistent with intrinsically faster kinetics of transition between the fluorescent and non-fluorescent states of GCaMP6f compared to all other GCaMPs [16], and it suggests that GCaMP6f represents the true astrocyte Ca2+ transient more accurately. Fast kinetics of a Ca2+ indicator are particularly important for studies that aim at understanding cause or consequence in the relationship of astrocyte Ca2+ dynamics with local neural processes, such as neuronal activity or blood flow, especially when Ca2+ imaging is combined with fast measurements of electrical or morphological dynamics [19,52].
Conclusions
Our investigations indicate that for relating new studies of behavioral state-dependent global astrocyte Ca2+ dynamics using GCaMP6f to published work where GCaMP3 has been the most popular GECI, as long as detailed kinetic analyses are not involved, GCaMP6f and GCaMP3 can be considered equivalent. However, for kinetic studies of Ca2+ dynamics as well as studies of localization and frequency of small, fast Ca2+ events, we can expect novel insight into the complexity of astrocyte function using GCaMP6f. We have focused our project on the basic comparison of the cytosolic versions of GCaMP3 and GCaMP6f. It should be mentioned that GCaMPs have been targeted to the inner leaflet of the plasma membrane and to intracellular organelles to maximize their exposure to locally confined astrocyte Ca2+ events [38,53–55]. Our findings further support the promise that the recently developed transgenic mouse line, which enables Cre recombinase-dependent expression of membrane-tethered GCaMP6f [18], will offer a significant step forward towards understanding astrocyte Ca2+ dynamics in awake behaving mice.
Supporting information
Population data of 1—mean correlation coefficient (r). astrocyte tdTomato (GLAST-CreER(+/-);R26-lsl-tdTomato(+/-), n = 6 mice (43 cells); GCaMP3;IP3R2(-/-), n = 8 mice (52 cells); GCaMP3, n = 9 mice (61 cells); GCaMP6f, n = 11 mice (82 cells). Comparisons, which were not significantly different from one-way ANOVA followed by Bonferroni correction (n.s.), are highlighted. Red lines represent mean ± SEM.
(EPS)
Excel file containing values of all data points presented in this manuscript and underlying statistical analysis. For explanation of organization of the data file, see S2 File.
(XLSX)
(PDF)
Acknowledgments
We would like to thank Priscilla A. Barba-Escobedo for expert technical assistance with animal husbandry. IP3R2 knockout mice were kindly provided by Dwight Bergles.
Data Availability
All relevant data are in S1 File (Excel document), and S2 File (pdf file) serves as explanation of data organization.
Funding Statement
This work was supported by grants from the NIMH (MH113780), NIAAA (AA022239), the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation as well as by pilot grants from the Center for Biomedical Neuroscience at UT Health San Antonio and UTS BRAIN # 364828 to M.P. M.A.H was supported by the NIH Jointly Sponsored NIH Predoctoral Training Program in the Neurosciences training grant T32 NS082145. A.S. was supported by the NIGMS Initiative for Maximizing Student Development R25 GM095480-04.
References
- 1.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260:3440–50. [PubMed] [Google Scholar]
- 2.Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 1981;290:527–8. [DOI] [PubMed] [Google Scholar]
- 3.Garaschuk O, Milos R-I, Konnerth A. Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nat Protoc 2006;1:380–6. doi: 10.1038/nprot.2006.58 [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Lou N, Xu Q, Tian G-F, Peng WG, Han X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 2006;9:816–23. doi: 10.1038/nn1703 [DOI] [PubMed] [Google Scholar]
- 5.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron 2009;62:400–12. doi: 10.1016/j.neuron.2009.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997;388:882–7. doi: 10.1038/42264 [DOI] [PubMed] [Google Scholar]
- 7.Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci U S A 1999;96:11241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol 2001;19:137–41. doi: 10.1038/84397 [DOI] [PubMed] [Google Scholar]
- 9.Ji G, Feldman ME, Deng K-Y, Greene KS, Wilson J, Lee JC, et al. Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle. J Biol Chem 2004;279:21461–8. doi: 10.1074/jbc.M401084200 [DOI] [PubMed] [Google Scholar]
- 10.Tallini YN, Ohkura M, Choi B-R, Ji G, Imoto K, Doran R, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A 2006;103:4753–8. doi: 10.1073/pnas.0509378103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Shui B, Kotlikoff MI, Sondermann H. Structural basis for calcium sensing by GCaMP2. Structure 2008;16:1817–27. doi: 10.1016/j.str.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akerboom J, Rivera JDV, Guilbe MMR, Malavé ECA, Hernandez HH, Tian L, et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem 2009;284:6455–64. doi: 10.1074/jbc.M807657200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 2009;6:875–81. doi: 10.1038/nmeth.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, et al. A Cre-Dependent GCaMP3 Reporter Mouse for Neuronal Imaging In Vivo. J Neurosci 2012;32:3131–41. doi: 10.1523/JNEUROSCI.4469-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014;82:1263–70. doi: 10.1016/j.neuron.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013;499:295–300. doi: 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 2015;85:942–58. doi: 10.1016/j.neuron.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan R, Lu T-Y, Chai H, Xu J, Huang BS, Golshani P, et al. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron 2016;92:1181–95. doi: 10.1016/j.neuron.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 2016;19:182–9. doi: 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- 20.Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, et al. α1-Adrenergic receptors mediate coordinated Ca(2+) signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013. doi: 10.1016/j.ceca.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pougnet J-T, Toulme E, Martinez A, Choquet D, Hosy E, Boué-Grabot E. ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron 2014;83:417–30. doi: 10.1016/j.neuron.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 22.Pougnet J-T, Compans B, Martinez A, Choquet D, Hosy E, Boué-Grabot E. P2X-mediated AMPA receptor internalization and synaptic depression is controlled by two CaMKII phosphorylation sites on GluA1 in hippocampal neurons. Sci Rep 2016;6:31836 doi: 10.1038/srep31836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankratov Y, Lalo U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front Cell Neurosci 2015;9:230 doi: 10.3389/fncel.2015.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 2011;31:18155–65. doi: 10.1523/JNEUROSCI.5289-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A 2012;109:E2832–41. doi: 10.1073/pnas.1206557109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, et al. Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron 2016;90:853–65. doi: 10.1016/j.neuron.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 2015;18:708–17. doi: 10.1038/nn.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 2011;15:70–80. doi: 10.1038/nn.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rungta RL, Bernier L-P, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, et al. Ca 2+ transients in astrocyte fine processes occur via Ca 2+ influx in the adult mouse hippocampus. Glia 2016;64:2093–103. doi: 10.1002/glia.23042 [DOI] [PubMed] [Google Scholar]
- 30.Boddum K, Jensen TP, Magloire V, Kristiansen U, Rusakov DA, Pavlov I, et al. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat Commun 2016;7:13572 doi: 10.1038/ncomms13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra A, Reynolds JP, Chen Y, Gourine A V, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 2016;19:1619–27. doi: 10.1038/nn.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 1994;369:744–7. doi: 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- 33.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 2007;10:331–9. doi: 10.1038/nn1849 [DOI] [PubMed] [Google Scholar]
- 34.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 2011;14:1276–84. doi: 10.1038/nn.2929 [DOI] [PubMed] [Google Scholar]
- 35.Biesecker KR, Srienc AI, Shimoda AM, Agarwal A, Bergles DE, Kofuji P, et al. Glial Cell Calcium Signaling Mediates Capillary Regulation of Blood Flow in the Retina. J Neurosci 2016;36:9435–45. doi: 10.1523/JNEUROSCI.1782-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shigetomi E, Kracun S, Khakh BS. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biol 2010;6:183–91. doi: 10.1017/S1740925X10000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J Neurosci 2012;32:13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically Encoded Calcium Indicators and Astrocyte Calcium Microdomains. Neurosci 2013;19:274–91. doi: 10.1177/1073858412468794 [DOI] [PubMed] [Google Scholar]
- 39.Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 2013;141:633–47. doi: 10.1085/jgp.201210949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 2014;34:13139–50. doi: 10.1523/JNEUROSCI.2591-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet J-M, et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 2014;18:210–8. doi: 10.1038/nn.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gee JM, Gibbons MB, Taheri M, Palumbos S, Morris SC, Smeal RM, et al. Imaging activity in astrocytes and neurons with genetically encoded calcium indicators following in utero electroporation. Front Mol Neurosci 2015;8:10 doi: 10.3389/fnmol.2015.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosa JM, Bos R, Sack GS, Fortuny C, Agarwal A, Bergles DE, et al. Neuron-glia signaling in developing retina mediated by neurotransmitter spillover. Elife 2015;4 doi: 10.7554/eLife.09590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnell C, Negm M, Driehaus J, Scheller A, Hülsmann S. Norepinephrine-induced calcium signaling in astrocytes in the respiratory network of the ventrolateral medulla. Respir Physiol Neurobiol 2016;226:18–23. doi: 10.1016/j.resp.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 45.Jung Kim K, Ramiro Diaz J, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci 2016;36:1300–16. doi: 10.1523/JNEUROSCI.1300-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfes AC, Ahmed S, Awasthi A, Stahlberg MA, Rajput A, Magruder DS, et al. A novel method for culturing stellate astrocytes reveals spatially distinct Ca 2+ signaling and vesicle recycling in astrocytic processes. J Gen Physiol 2016:jgp.201611607. doi: 10.1085/jgp.201611607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010;13:133–40. doi: 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Zima A V, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res 2005;96:1274–81. doi: 10.1161/01.RES.0000172556.05576.4c [DOI] [PubMed] [Google Scholar]
- 49.Paukert M, Bergles DE. Reduction of motion artifacts during in vivo two-photon imaging of brain through heartbeat triggered scanning. J Physiol 2012;590:2955–63. doi: 10.1113/jphysiol.2012.228114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding J, Luo AF, Hu L, Wang D, Shao F. Structural basis of the ultrasensitive calcium indicator GCaMP6. Sci China Life Sci 2014;57:269–74. doi: 10.1007/s11427-013-4599-5 [DOI] [PubMed] [Google Scholar]
- 51.Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 2012;32:13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci 2014;15:327–35. doi: 10.1038/nrn3725 [DOI] [PubMed] [Google Scholar]
- 53.Shigetomi E, Kracun S, Sofroniew M V, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 2010;13:759–66. doi: 10.1038/nn.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Wang X, Zhang N, Gottipati MK, Parpura V, Ding S. Imaging of mitochondrial Ca2+ dynamics in astrocytes using cell-specific mitochondria-targeted GCaMP5G/6s: Mitochondrial Ca2+ uptake and cytosolic Ca2+ availability via the endoplasmic reticulum store. Cell Calcium 2014;56:457–66. doi: 10.1016/j.ceca.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal A, Wu P-H, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, et al. Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron 2017;93:587–605.e7. doi: 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Population data of 1—mean correlation coefficient (r). astrocyte tdTomato (GLAST-CreER(+/-);R26-lsl-tdTomato(+/-), n = 6 mice (43 cells); GCaMP3;IP3R2(-/-), n = 8 mice (52 cells); GCaMP3, n = 9 mice (61 cells); GCaMP6f, n = 11 mice (82 cells). Comparisons, which were not significantly different from one-way ANOVA followed by Bonferroni correction (n.s.), are highlighted. Red lines represent mean ± SEM.
(EPS)
Excel file containing values of all data points presented in this manuscript and underlying statistical analysis. For explanation of organization of the data file, see S2 File.
(XLSX)
(PDF)
Data Availability Statement
All relevant data are in S1 File (Excel document), and S2 File (pdf file) serves as explanation of data organization.