Abstract
Estrogen-mimicking chemicals, such as cadmium, may be associated with increased susceptibility to hormone-dependent cancers, though supporting data are sparse, particularly for endometrial cancer. The Health and Environmental Exposure Research (HEER) study worked with the Arkansas Central Cancer Registry, Iowa Cancer Registry and Missouri Cancer Registry to obtain names of women diagnosed with endometrial cancer who were willing to be contacted for participation in our case control study. Voter registration lists from Iowa and Missouri were used to randomly select similarly aged women as represented in the case population. Participants were interviewed by telephone to obtain information on known or suspected endometrial risk factors. Urine kits were sent to participants for home collection and returned for analysis. Our case-control study consisted of 631 incident cases of endometrial cancer diagnosed from January 2010 to October 2012 and 879 age-matched population-based controls, ages 18–81 years (mean age 65 years). We quantified cadmium amounts in urine and standardized these values through creatinine adjustment. Using data from all survey completers, we developed a multivariable model for endometrial cancer. Creatinine-adjusted cadmium concentration was added to this model. Odds ratio (OR) and 95% confidence intervals (CIs) for endometrial cancer were calculated. After multivariable adjustment, higher creatinine-adjusted cadmium exposure was associated with a statistically significant increase of endometrial cancer risk (OR: 1.22; 95% CI: 1.03–1.44). Our results provide evidence that cadmium may increase the risk of endometrial cancer, possibly through estrogenic effects.
Introduction
Cadmium is a toxic, bioaccumulating, non-essential, and highly persistent metal with a variety of adverse health effects. These include renal dysfunction; breast, lung, pancreatic, and endometrial cancer risk; and disturbances in calcium homeostasis [1–5]. Ingesting food containing cadmium (e.g., kidney, liver, crustaceans, cereals) is the primary non-occupational source [6,7]. Smoking is the second major source of cadmium exposure in the general population [7]. Tobacco plants readily take up agricultural sources of cadmium [8]. A doubling of urinary cadmium levels may be found in heavy smokers (>300 pack years (number of smoking years times usual number of cigarettes per day)) compared to non-smokers [9].
Because the estimated biological half-life of cadmium is from 10 to 30 years [10], only a small fraction of inhaled or ingested cadmium is excreted, and the body burden increases over time. Women generally have higher cadmium levels than men [11], and parous women are more likely to have depleted iron stores and therefore absorb more cadmium when compared to nulliparous women [12].
Endometrial cancer, the fourth most common cancer for women, occurs primarily in postmenopausal women. In 2016, approximately 60,050 women are expected to be diagnosed with cancer of the body of the uterus (endometrial cancers and uterine sarcomas), with 10,470 projected deaths [13]. Endometrial cancer is associated with both endogenous and exogenous estrogen exposure [14]. Cadmium mimics estrogen and may increase estrogen-receptor-mediated proliferation—the classical estrogen signaling, thereby potentially contributing to endometrial cancer risk [15,16]. Two nuclear estrogen receptors, (estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ)) are involved in the classical estrogen signaling. The ERα and ERβ directly interact with specific DNA binding sites that regulate gene expression [17,18]. Alternatively, Ali and colleagues demonstrated that cadmium exposure in mice affected the height of the uterine luminal epithelium in a dose-dependent manner. They suggested that the effects of cadmium exposure was through non-classical estrogen receptor signaling [19]. In 2008, Akesson et al reported a significant association between endometrial cancer and cadmium intake based on assignment of cadmium exposure levels from a food frequency questionnaire [1]. However, more recent studies using food frequency questionnaires to estimate cadmium levels have not reported an association [20–22]. Vacchi-Suzzi [23] reported minimal correlation between urinary cadmium levels and estimates of dietary cadmium intake. This finding and inconsistent observational studies using estimated cadmium exposure from self-reported dietary intake has brought the validity of food frequency questionnaires for cadmium estimation into question [24–26].
The Health and Environmental Exposure Research (HEER) study aimed to investigate the association between endometrial cancer risk from cadmium exposure after adjusting for confounders in a population-based case-control study using cases from the Arkansas, Iowa and Missouri state cancer registries and age-matched controls from voter registration lists.
Materials and methods
Study population
Incident cases of endometrial carcinoma diagnosed from January 2010 to October 2012 were obtained from three cancer registries: Arkansas Central Cancer Registry, Iowa Cancer Registry and Missouri Cancer Registry. In situ and non-carcinomas, such as Müllerian and mesodermal malignant tumors, were excluded. Case ascertainment also included local or regional stages, excluding metastatic endometrial cancer cases. Cases were contacted by each registry to obtain written permission to pass their names to our study. Of those that agreed, contact information was sent to HEER study staff.
Age-matched controls were randomly selected from the Iowa and Missouri voter registration lists only as the Arkansas voter registration list was not available. The Iowa voter registration list provided date of birth, whereas the Missouri voter registration list provided age. For Missouri controls, the age of the participant was at the last voter registration period and was thus approximately one to three years younger than the current year. We therefore used the listed age plus 2 years for matching. For each case, four to five controls were randomly selected from the voter registration lists with replacements on names for which the telephone number or residential address in Missouri or Iowa was missing. Imperfect age matching occurred due to variable responses by potential controls. The age or year of cancer diagnosis for cases was used as the reference age or year for the matched controls, respectively. Most questions asked for information during the year before diagnosis, i.e. reference age/year minus one.
Introductory letters with a study brochure and consent information were sent to all potential participants. For participants who did not contact the study to decline participation and for whom a valid address was obtained, contact information was sent to the University of South Carolina Call Center for telephone interviews.
Assessment of cadmium and covariates
All participants were interviewed by telephone by trained interviewers. The 35-minute interview asked about physical activity, reproductive history, alcohol consumption, height and weight, use of oral contraceptives and hormone replacement therapy, personal and family medical history, demographic factors, a limited set of dietary components, and smoking history.
Upon completion of the telephone interview a kit to collect saliva and urine at home and return to the study center was sent to participants. Urine sample containers and urine hats used to collect the sample were exhaustively cleaned using multistep acid leachings before sending to participants [27]. Analyses of acid leaching solutions from kit materials revealed no detectable cadmium. Urine collection containers were used to prepare method blanks. Detailed photo-essay instructions were carefully designed for the urine collection kits to minimize trace element contamination during specimen collection and handling.
Urine samples were immediately refrigerated (4o Celsius) upon receipt. The University of Missouri Research Reactor (MURR) has a laboratory that is specially designed for trace element analysis and is subject to high-efficiency particulate air filtration. The trace-analysis laboratory at MURR has been has been conducting elemental analyses of biological monitors for 35 years [28]. Cadmium was quantified by using inductively coupled plasma-dynamic reaction cell-mass spectrometry (ICP-DRC-MS) following an established protocol from the National Health and Nutritional Examination Study (NHANES) [29], with an additional correction for the effect of strontium on the tin interference. Trace metal analyses were performed from November 2012 to July 2014. A comprehensive quality-control program incorporating numerous methods including method blanks, monitoring multiple cadmium isotopes, internal and external controls, stability samples, replicates, spikes, and routine inclusion of National Institute of Standards and Technology (NIST) standard reference materials (SRM) ensured high-quality data. The instrument detection limit (DL) for cadmium was 0.0017 μg/L, the method detection limit (MDL) was 0.0082 μg/L, and the limit of quantification (3.3 x MDL) was 0.027 μg/L. Cadmium levels in every sample exceeded the MDL, and in no case did the method blank (prepared using urine vials from empty specimen collection kits) exhibit [Cd] > DL. Measurements of NIST SRM 2670a “low” yielded (average ± standard. deviation, % relative standard deviation [RSD]) 0.045 ± 0.006 μg/L, 13%RSD (n = 19); 2670a “high” yielded 4.90 ± 0.16 μg/L, 3.3%RSD (n = 18). Three participant samples were aliquoted and measured repeatedly throughout the study, yielding 0.223 ± 0.016 μg/L, 7.2%RSD (n = 17); 0.184 ± 0.013 μg/L, 7.0%RSD (n = 17); 0.650 ± 0.030 μg/L, 4.7%RSD (n = 17). Urine creatinine level was also measured using a colorimetric assay based on the Jaffé reaction to control for kidney function [30].
This study and all HEER consent documents and procedures were approved by the University of Missouri Health Sciences Institutional Review Board as well as the review boards of the respective state cancer registries. Participants provided written consent to have their names passed to the HEER study. Each cancer registry kept these written consents. Invitation letters were sent to registry-consented participants with an explanation of the study as well as information about the next step—to telephone the participant to conduct the survey. Contact information was also included in the invitation letter for those who did not wish to be called to opt out of being telephoned. Upon telephoning the participants, the study was explained with an opportunity to ask questions to clarify any concerns. Those who agreed to be interviewed were considered to have provided verbally informed consent.
Statistical analysis
We initially compared creatinine-adjusted cadmium concentration between cases and controls using a t-test. We performed cross-tabulations of survey variables with case-control status to screen for inclusion in a multivariable model. Missing data were relatively rare however we imputed missing values with multiple imputation using the Markov chain Monte Carlo method to impute 20 datasets to be used in the multivariable conditional logistic models. Prior to multiple imputation, we imputed menopausal status based on participant report, smoking status, and use of hormone replacement therapy. A woman was classified as premenopausal if she reported still having periods and was not using hormone replacement therapy. A participant was classified as postmenopausal if she reported an oophorectomy or natural menopause (no menstrual periods for at least 6 months) before the reference date. Women who were taking postmenopausal hormones and still having periods, and women who reported hysterectomy alone were classified as premenopausal if their reference ages were in the first decile of age at natural menopause among the controls (32 years old for current smokers and 36 years old for nonsmokers), and postmenopausal if their reference ages were in the highest decile for age at natural menopause in the control group (56 years old for both current smokers and nonsmokers). For six women in the intermediate age category (second to ninth deciles), menopausal status was considered unknown. Thus, we defined three categories of menopausal status: premenopausal, postmenopausal, and unknown [31].
We developed a multivariable conditional logistic model, stratifying on age at diagnosis. Variables that were selected a priori (race, marital status, body mass index (BMI)), menopause at age 56 or older, menopausal status at diagnosis, smoking history, second hand smoke exposure, age at menarche, number of live births, weight gain, history of weight loss attempt, family history of endometrial cancer in a first degree relative, exposure to unopposed estrogen, oral contraceptive use, history of breast or ovarian cancer, history of uterine fibroids, history of diabetes, sleep habits, irregular work schedule, alcohol use, protein shake and whole milk consumption) were tested for association with case-control status in a multivariable conditional logistic regression. Those with p<0.1 were retained for further analysis. BMI, weight in kilograms divided by squared height in meters, was capped at a maximum value of 70 kg/m2 to avoid undue influence of extreme outliers. Variables were selected by both forward stepwise inclusion and backwards elimination, using p = 0.1 for both entry and exit. Both stepwise models retained the same set of variables. Interactions between variables in the model were tested; none were retained.
After the “best” case-control model had been achieved, the base-2 logarithm of creatinine-adjusted cadmium was added to the model to test for its association with case-control status after controlling for other risk factors, using data from women who returned a urine sample. Because the women who opted to provide urine samples differed from those who did not, we developed a multivariable logistic model to adjust for a potential non-response bias in the case-control model [32]. The inverse of the predicted probability of returning a sample was used as a weight in the conditional logistic regression model. The final set of independent variables were race; marital status; BMI; history of weight loss attempt; smoking status; pack-years of cigarette smoking for former and current smokers; history of endometriosis, breast cancer, ovarian cancer or uterine fibroids; family history of endometrial cancer; years of oral contraceptive use; years of unopposed estrogen use; menopause at age 56 years or older; menopausal status at diagnosis; protein shake and whole milk consumption; and non-response bias. We also ran the model with only post-menopausal women and a model that excluded smoking status. SAS for Windows v9.4 (SAS Institute Inc., Cary, NC, USA) was used.
Results
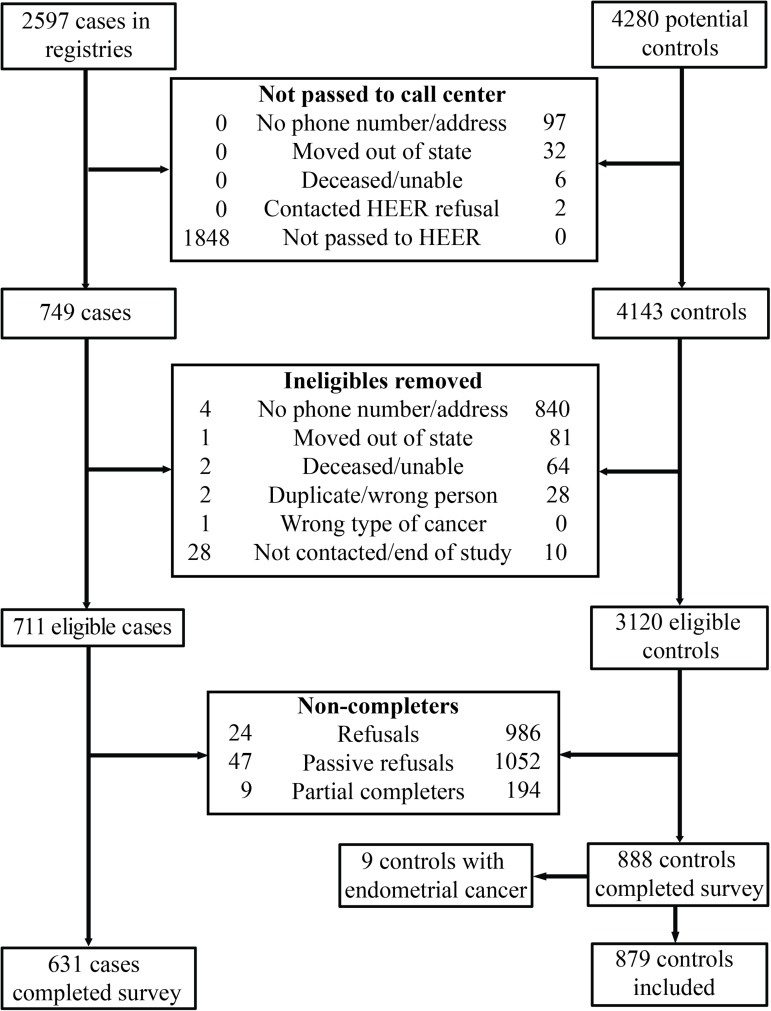
Of those approached by the cancer registries (n = 2597) for permission to send their names to our study for potential enrollment, 29% of the names (m = 749) were sent to the call center for interviewing (approximately 25% of MO and AR and 44% of IA). Of 711 eligible cases 89% (n = 631) completed an interview. In comparison to those who declined to pass their names to our study from the cancer registries, those who passed their names were more likely to be White (94% versus 90%) or married or living with partner (66% versus 55%) and less likely to be diagnosed with endometrioid carcinoma (International Classification of Diseases for Oncology: 8380) (74% versus 78%). The proportion with tumor grade I or II and age at diagnosis were similar between the two groups.
For the controls, 4280 age-matched names were randomly selected from the voter registration lists. Of the 4280 controls, 3120 were eligible and 888 completed the survey (28% participation proportion). Of the 2597 cases, 749 women with endometrial cancer were passed to the HEER study and 631 completed the survey (24% participation proportion for cases). Among these 1519 participants, 498 cases (79%) and 545 controls (61%) also returned urine specimens. For the current study, we excluded 9 controls with a history of endometrial cancer, leaving 1510 participants (631 cases and 879 controls). See Fig 1 for a summary of participant enrollment.
Fig 1. Exclusion and enrollment of participants in the Health and Environmental Exposure Research study.
Controls were slightly older than cases (mean age 63 years versus 60 years, respectively), with less education (high school graduate or less: 43% versus 37%). Both marital status and racial compositions differed between the two groups (Table 1). The cases and controls were similar in income and employment status. Among the cases, 86% had Type I, mostly endometrioid adenocarcinomas, which is driven by hormonal mechanisms, and 14% were Type II, endometrial carcinomas, which often display as serous or clear cell histology [33].
Table 1. Characteristics of endometrial cancer cases and population-based controls*.
| Characteristic (N missing) | Number (Percent) | Cases (N = 631) | Controls (N = 879) | p-value** |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis | < .0001 | |||
| <40 | 32 (2.1) | 20 (3.2) | 12 (1.4) | |
| 40–44 | 38 (2.5) | 24 (3.8) | 14 (1.6) | |
| 45–49 | 54 (3.6) | 30 (4.8) | 24 (2.7) | |
| 50–54 | 144 (9.5) | 82 (13.0) | 62 (7.0) | |
| 55+ | 1242 (82.2) | 475 (75.3) | 767 (87.3) | |
| Mean (SD) | 61.7 (9.1) | 60.1 (9.5) | 62.9 (8.6) | < .0001 |
| Education | ||||
| High school or less | 607 (40.2) | 231 (36.6) | 376 (42.8) | .046 |
| 1–3 years of college | 414 (27.4) | 179 (28.4) | 235 (26.7) | |
| College or more | 489 (32.4) | 221 (35.0) | 268 (30.5) | |
| Employment | .22 | |||
| Not in labor force | 722 (47.8) | 290 (46.0) | 432 (49.2) | |
| Working | 788 (52.2) | 341 (54.0) | 447 (50.8) | |
| Family income, annual (101) | .09 | |||
| ≤ $30,000 | 417 (29.6) | 188 (31.3) | 229 (28.3) | |
| $30,001–$50,000 | 389 (27.6) | 177 (29.4) | 212 (26.2) | |
| $50,001–$100,000 | 424 (30.1) | 160 (26.6) | 264 (32.7) | |
| >$100,000 | 179 (12.7) | 76 (12.6) | 103 (12.8) | |
| Marital status | < .0001 | |||
| Married/living with partner | 1078 (71.4) | 417 (66.1) | 661 (75.2) | |
| Divorced, separated, widowed | 342 (22.6) | 153 (24.2) | 189 (21.5) | |
| Never married | 90 (6.0) | 61 (9.7) | 29 (3.3) | |
| Race/ethnicity (6) | ||||
| Hispanic | 11 (0.7) | 5 (0.8) | 6 (0.7) | .79 |
| Non-Hispanic Black | 37 (2.5) | 26 (4.1) | 11 (1.3) | .0004 |
| Non-Hispanic White | 1437 (95.6) | 595 (94.6) | 842 (96.2) | |
| Other Non-Hispanic | 19 (1.3) | 3 (0.5) | 16 (1.8) | .024 |
| Work schedule (723) | .32 | |||
| Regular daytime shift | 650 (82.6) | 286 (84.1) | 364 (81.4) | |
| Other shift | 137 (17.4) | 54 (15.9) | 83 (18.6) | |
| Dietary habits | ||||
| Drink alcoholic beverages, days/week (18) | ||||
| 0 | 706 (47.3) | 290 (46.5) | 416 (47.9) | .07 |
| <1 | 405 (27.1) | 190 (30.4) | 215 (24.8) | |
| 1–2.9 | 183 (12.3) | 70 (11.2) | 113 (13.0) | |
| 3 or more | 198 (13.3) | 74 (11.9) | 124 (14.3) | |
| Protein shake consumption (5) | .02 | |||
| None | 1328 (88.2) | 542 (86.0) | 786 (89.8) | |
| Less than 2 per week | 73 (4.8) | 31 (4.9) | 42 (4.8) | |
| 2 per week or more | 104 (6.9) | 57 (9.0) | 47 (5.4) | |
| Whole milk consumption | .038 | |||
| 5 or more times per week | 75 (5.0) | 40 (6.3) | 35 (4.0) | |
| Less than 5 times per week | 1435 (95.0) | 591 (94.7) | 844 (96.0) | |
| Physical activity and weight | ||||
| Body mass index, year before diagnosis (20) | ||||
| Mean (SD) | 32.0 (8.9) | 35.7 (9.9) | 29.3 (7.0) | < .0001 |
| MET-hours/week, total (8) | ||||
| Mean (SD) | 38.2 (58.9) | 32.5 (51.5) | 42.3 (63.4) | .001 |
| Weight gain since age 25 (23) | ||||
| Mean (SD) | 20.5 (18.3) | 25.0 (20.4) | 17.2 (15.9) | < .0001 |
| Smoking history | ||||
| Smoke cigarettes (3) | .003 | |||
| Never | 946 (62.8) | 413 (65.7) | 533 (60.7) | |
| Former | 418 (27.7) | 175 (27.8) | 243 (27.7) | |
| Current | 143 (9.5) | 41 (6.5) | 102 (11.6) | |
| Smoking, total pack-years (6) | ||||
| Mean for ever-smokers (SD) | 9.0 (18.8) | 7.6 (13.5) | 10.9 (16.7) | .0002 |
| Medical history | ||||
| Breast cancer (3) | .0002 | |||
| Yes | 75 (5.0) | 16 (2.5) | 59 (6.7) | |
| No | 1432 (95.0) | 614 (97.5) | 818 (93.3) | |
| Diabetes (4) | ||||
| Yes | 291 (19.3) | 155 (24.7) | 136 (15.5) | < .0001 |
| No | 1215 (80.7) | 476 (75.3) | 742 (84.5) | |
| Endometrial cancer in first degree relative (67) | .0001 | |||
| Yes | 48 (3.3) | 33 (5.5) | 15 (1.8) | |
| No | 1395 (96.7) | 570 (94.5) | 825 (98.2) | |
| Endometriosis (31) | .07 | |||
| Yes | 250 (16.9) | 116 (19.0) | 134 (15.4) | |
| No | 1229 (83.1) | 494 (81.0) | 735 (84.6) | |
| Hypertension (8) | .008 | |||
| Yes | 799 (53.2) | 359 (57.3) | 440 (50.3) | |
| No | 703 (46.8) | 268 (42.7) | 435 (49.7) | |
| Ovarian cancer (10) | < .0001 | |||
| Yes | 46 (3.1) | 35 (5.6) | 11 (1.3) | |
| No | 1454 (96.9) | 590 (94.4) | 864 (98.7) | |
| Medications and treatments | ||||
| Birth control, years used (7)*** | ||||
| Mean (SD) | 5.8 (7.2) | 4.8 (6.0) | 6.5 (7.9) | < .0001 |
| Hormone replacement therapy, years used (22) | ||||
| Mean (SD) | 3.2 (7.1) | 2.0 (5.6) | 4.1 (7.9) | < .0001 |
| Reproductive history | ||||
| Age of menarche 11 or earlier | .004 | |||
| Yes | 330 (21.8) | 161 (25.5) | 169 (19.2) | |
| No | 1180 (78.2) | 470 (74.5) | 710 (80.8) | |
| Menopause status, year before diagnosis (6) | < .0001 | |||
| Premenopausal | 220 (14.6) | 149 (23.6) | 71 (8.1) | |
| Postmenopausal | 1284 (85.0) | 481 (76.2) | 803 (91.4) |
*9 controls who reported a diagnosis of endometrial cancer were excluded. Variables refer to the year prior to diagnosis of endometrial cancer unless otherwise specified.
**P-value for chi-square analysis except for means, which were compared with a t-test. Fisher’s Exact test was used for race/ethnicity where there were low numbers of participants in one category.
***Birth control refers to methods that release hormones, such as birth control pills, injections, patches, progestogen implants (Norplant), progestin-releasing intrauterine devices, or vaginal rings.
Characteristics of refusal at enrollment
Of those who actively declined to participate, 17% (9 cases and 194 controls) agreed to answer a few demographic questions. Compared to participants who completed the interview, these helpful refusals were more likely to be high school graduate or less (40% versus 54%) and be married or living with partner (71% versus 82%). The educational attainment of spouse or partner, percent of Hispanic ethnicity, race, income and sexual orientation were similar between these two groups.
Reliability substudy
Of the 165 respondents contacted for a second interview, 2 individuals refused to be interviewed, 29 could not be reached, and 134 were re-interviewed (81%; 69 cases and 65 controls). Mean time between interviews was 13.9 months (range 3.1–20.8 months). Time between interviews was not different between cases and controls.
Participation in moderate and vigorous physical activity showed good concordance between interviews (93% and 82% respectively); the kappa for moderate physical activity was 0.54 (lower confidence limit (LCL) 0.27) and vigorous physical activity was 0.64 (LCL 0.51). History of ever using mineral supplements showed good concordance (84.2%) with a low kappa (0.27, LCL 0.05). The kappa for BMI category was .84 (LCL .75) with 89.6% concordance. There was high concordance for ever being diagnosed with polycystic ovarian syndrome (97%); the kappa was 0.48 (LCL 0.05). Ever being diagnosed with diabetes showed high concordance (95.5%); the kappa was 0.85 (LCL .74). Having a biological family member that was ever diagnosed with endometrial cancer had high concordance between interviews (88.1). Correlation between the repeated measures of weight, height, BMI, and age of diabetes diagnosis was high (0.98, 0.97, 0.95, and 0.92, respectively).
Cadmium analysis
Creatinine-adjusted cadmium levels ranged from to 0.005 to 0.417 (mean 0.037) μg/g in case participants and from 0.006 to 0.649 (mean 0.041) μg/g in control subjects. A t-test of the creatinine-adjusted cadmium concentration between cases and controls was not statistically significant (p = .101).
After multivariable adjustment, a doubling of urine cadmium increased the endometrial cancer risk by 22% (Table 2; odds ratio (OR) = 1.22, 95% confidence interval (CI): 1.03–1.44; p-value = .02) No substantive changes were observed in any parameter estimates when only post-menopausal women were included (S1 Table). A similarly elevated endometrial risk for cadmium exposure was also observed among those diagnosed with Type I endometrial carcinoma (n = 550) compared to controls (OR: 1.21 95% CI 1.03–1.47). Women diagnosed with endometrial carcinoma who were also 50 pounds over ideal weight had an increased endometrial cancer risk for cadmium exposure (S2 Table). When we re-ran the model and did not include current smoking, cadmium concentration remained statistically significant (OR: 1.19, 95% CI: 1.01–1.41) (S3 Table). A similarly elevated endometrial risk for cadmium exposure was also observed when we re-ran the model and include two additional established risk factors, age of menarche and number of live births, (S4 Table). We also re-ran the model and included creatinine concentration as a separate covariant and base-2 logarithm of unadjusted cadmium concentration. Cadmium concentration remained statistically significant (S5 Table).
Table 2. Multivariable conditional logistic regression of risk factors for endometrial cancer.
| Base model with all participants | Model with adjusted Cd* and inverse probability weights | |||
|---|---|---|---|---|
| Characteristic | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value |
| Non-Hispanic African-American race | 3.07 (1.33, 7.10) | .0085 | 4.91 (1.88, 12.82) | .0012 |
| Marital status (reference never married) | ||||
| Married, living with partner | 0.47 (0.27, 0.82) | .0077 | 0.36 (0.17, 0.77) | .0088 |
| Divorced, separated, widowed | 0.58 (0.32, 1.06) | .0791 | 0.44 (0.20, 1.00) | .0497 |
| Body Mass Index at diagnosis† | 1.08 (1.06, 1.10) | < .0001 | 1.09 (1.06, 1.11) | < .0001 |
| History of trying to lose weight | 1.75 (1.18, 2.60) | .0058 | 1.59 (0.99, 2.57) | .0573 |
| Current smoker | 0.52 (0.32, 0.85) | .0086 | 0.52 (0.28, 0.96) | .0381 |
| Cigarette smoking (10 pack-years) | 0.94 (0.87, 1.01) | .0864 | 0.88 (0.80, 0.97) | .0086 |
| History of endometriosis | 1.64 (1.17, 2.29) | .0037 | 1.66 (1.10, 2.50) | .0151 |
| History of breast cancer | 0.47 (0.25, 0.88) | .0182 | 0.39 (0.16, 0.93) | .0337 |
| History of ovarian cancer | 4.27 (1.89, 9.69) | .0005 | 9.99 (2.67, 37.38) | .0006 |
| History of uterine fibroids | 0.77 (0.57, 1.03) | .0797 | 0.71 (0.50, 1.00) | .0508 |
| Endometrial cancer in first degree relative | 2.70 (1.32, 5.52) | .0065 | 3.31 (1.37, 8.01) | .0079 |
| Oral contraceptive use (5 years) | 0.86 (0.79, 0.95) | .0014 | 0.88 (0.79, 0.97) | .0138 |
| Unopposed estrogen use (5 years) | 0.64 (0.53, 0.77) | < .0001 | 0.67 (0.53, 0.84) | .0006 |
| Menopause at age 56 or later | 1.90 (1.33, 2.70) | .0004 | 1.70 (1.13, 2.56) | .0113 |
| Post-menopausal at diagnosis | 0.43 (0.26, 0.70) | .0009 | 0.33 (0.21, 0.52) | < .0001 |
| Protein shake consumption, days/week | 1.12 (1.01, 1.24) | .0276 | 1.20 (1.04, 1.39) | .0133 |
| Whole milk consumption, ≥ 5 days/week | 1.96 (1.13, 3.42) | .0173 | 2.57 (1.28, 5.15) | .0078 |
| Base-2 logarithm of adjusted cadmium concentration | 1.22 (1.03, 1.44) | .0212 | ||
CI = confidence interval
*Cadmium concentration adjusted by urine concentration of creatinine
†Body mass index is weight in kilograms divided by (height in meters)2
Discussion
In this population-based case-control study of Midwestern U.S. women, we found a statistically significant positive association between urine cadmium levels and endometrial cancer risk. Specifically, a 22% increased risk of endometrial cancer was associated with doubling cadmium exposure. Our confidence in the findings is strengthened by our large number of cases, evaluation of those who declined to participate at various points in our study, use of population-based controls that were age-matched to cases, inclusion of a reliability study, the use of urine as the biomarker to ascertain lifetime cadmium exposure, additional analyses to account for potential bias, and using an established protocol for urine analysis with an additional correction factor.
To our knowledge, this is the first published report on cadmium exposure and endometrial cancer risk using urine as a biomarker for cadmium measurement. Four other cohort studies conducted in Japan, Denmark, Sweden, and the United States have reported on this association; all have estimated cadmium exposure using food frequency questionnaires with mixed results. Eriksen et al reported a null finding for cadmium exposure and risk of endometrial cancer (192 endometrial cancer cases over 13 year period) using a Danish population-based prospective study as did Sawada in the Japan Public Health Center-based Prospective Study (75 endometrial cancer cases over 9 year period) [20,34]. The Women’s Health Initiative has also reported a null finding on cadmium exposure and endometrial cancer (1198 endometrial cancer cases over 10 year period) [21]. In contrast, Akesson et al reported an increased endometrial cancer risk (relative risk [RR]: 1.39; 95% CI, 1.04–1.86) among the Swedish Mammography Cohort comprised of postmenopausal women (378 endometrial cancer cases over 16 year period) [1]. In a meta-analysis of dietary cadmium intake and cancer risk, Cho reported an increase cancer risk among studies with Western populations (RR: 1.15; 95% CI 1.08–1.23) particularly for hormone-related cancers (prostate, breast and endometrial) though only two of the aforementioned four cohort studies were included in Cho’s meta-analysis [22]. One recent cancer mortality study using NHANES III data (1988–1994) and creatinine adjusted urinary cadmium found a suggestion of an increased risk for uterine cancer among those with highest level of urinary Cd (n = 7 deaths, mean follow-up 14 years; adjusted hazard ratio (aHR): 1.03; 95% CI 0.23–4.62 and aHR per 2-fold urinary Cd: 1.63; 95% CI 1.06–2.51) [35].
After humans ingest or inhale cadmium, the body excretes only a very small fraction and efficiently retains the rest [7]. Among the potential matrices used to measure cadmium (blood, nail, hair, urine), urine specimens more closely reflect lifetime cadmium exposure than the other matrices [11]. Although not without criticism [36], using creatinine-adjusted values in the field of toxicology for spot urine samples, such as in this study, is common and several papers support this analytic technique [37]. For example, the Jaffé reaction may cause overestimation of creatinine, as mentioned in National Health and Nutrition Examination material [38]. Barr et al suggest using urinary creatinine as an independent variable which allows for urinary dilution and demographic difference adjustment [39]. When we included the base-2 logarithm of unadjusted cadmium concentration and creatinine concentration in our model, results were essentially unchanged from those reported in Table 2. Our unadjusted geometric mean of cadmium was slightly higher (0.32, 95% CI: 0.31–0.34 μg/l) than those reported from 1999–2010 NHANES for women age 20–85 years (0.25, 95% CI: 0.24–0.26 μg/l), though this may reflect a different age structure between these two samples. As noted by Adams and Newcomb, urinary cadmium values for those aged 60–69 years was 2.7 fold greater compared to those 20–29 years old [40].
Cadmium levels are related to level of smoking. Heavy smokers may have twice as much cadmium, and moderate smokers’ cadmium burden may increase by approximately sixty percent compared to non-smokers [9]. Former smokers have a cadmium body burden that is intermediate [24,41]. Smoking has been shown to decrease the risk of endometrial cancer, likely through endometrial atrophy [42]. However, Brinton and others suggest that smoking in conjunction with use of exogenous estrogens significantly multiplies the risk of developing endometrial cancer, especially in thin women [43]. Unfortunately we did not have a sufficient number of thin smokers to confirm this finding and only 6.5% of cases were smokers.
With our extensive survey we were able to consider numerous variables that are known or suspected to increase the risk of endometrial cancer in the logistic regression models. Well documented endometrial cancer risk factors include late menopause, early menarche, nulliparity, and obesity [14]. Among these risk factors, obesity is strongly associated with an increased endometrial cancer risk [43]. Davies et al suggests this is especially true for those who are 50 pounds or heavier than their ideal weight [44]. Additional risk factors that may be associated with endometrial cancer risk include hypertension [45], family history of cancers (breast, endometrial, ovarian, and/or Lynch’s syndrome) [46], history of endometriosis [47], sleep [48], irregular work schedule [49], uterine fibroids [50], alcohol consumption [51], and dietary choices [52], such as milk consumption [53]. We explored the risk of endometrial cancer and consumption of protein shakes. Besides the typical consumption of milk as part of the protein shake, undeclared anabolic androgenic steroids are found in up to 15% of commercially available non-hormonal supplements (i.e., protein drinks) [54]. Though no data are available on this consumption and endometrial cancer, one risk factor of PCOS relates to dysfunction of androgen receptors leading to hyperandrogenism and an increased risk of endometrial cancer [55,56]. Protective factors may be physical activity [57] and oral contraceptive use [58]. Among these risk factors, we found obesity, late age of menopause, selected dietary choices (milk and protein shakes), history of ovarian cancer or endometriosis, and family history of endometrial cancer as characteristics that increased the risk of endometrial cancer. In our analysis, years of oral contraceptive use, years of unopposed estrogen use, history of breast cancer, smoking and being married were protective factors.
Several limitations should be considered in evaluating our results. One limitation was the low participation proportion of women diagnosed with endometrial cancer. Obtaining consent was a two-stage process. Each cancer registry approached eligible cancer cases and required written consent to pass their names to the HEER study. The second stage involved verbal consent upon contact by telephone by the HEER study team. Only 25%-44% of the women agreed to let their respective cancer registry pass their names onto HEER for enrollment. The second stage participation proportion was 89% of the eligible cases; another 1% who declined to participate answered a few questions to obtain a few details. Those not consenting at stage one were more likely to be married or living with partner while those opting to not enroll at stage two were less likely to be married or living with partner. Differences were also observed in racial composition. Those not consenting to pass their names were more likely to be Black, but no difference was observed in declining to enrollment by race. Proportion of tumor grade I and II and age of diagnoses was similar at both stage one and two among the two groups—those consenting and those declining to participate. Among the controls, our participation proportion was 28%. Although we assessed some characteristics between the two groups at the time of interview (n = 203) in our refusal sub-study, we cannot rule out the possibility of selection bias.
As with any case-control study, the risk of recall bias is also a limitation. All data except information about urinary cadmium measurement and tumor characteristics for cases ere self-reported. However, of the few questions we re-asked of participants, the concordance between the two time periods was quite good. Nevertheless, we cannot eliminate the possibility of recall bias.
In conclusion, our results provide evidence that cadmium may increase the risk of endometrial cancer, possibly through estrogenic effects. Further studies that employ urinary cadmium as the biomarker are necessary given the weak association with estimated cadmium from dietary sources. A comprehensive list of suspected and known risk factors should also be collected to fully adjust the regression models.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors acknowledge that the case data used in this report were provided by three cancer registries: the Arkansas Department of Health, Arkansas Central Cancer Registry (ACCR), Little Rock, AR; State Health Registry of Iowa, Iowa Cancer Registry (ICR), Iowa City Iowa; and the Missouri Cancer Registry and Research Center (MCR-ARC), Columbia, MO. Michele West and Jason Brubaker were key Iowa personnel involved in data collection for this project. Telephone interviews were conducted by the University of South Carolina’s Survey Research Laboratory. Analytical instrumentation used in this study was acquired with support of the National Science Foundation, grant # BCS-0922374.
Data Availability
Rules Pertaining to the Arkansas Cancer Registry are duly adopted and promulgated by the Arkansas State Board of Health pursuant to the authority expressly conferred by the laws of the State of Arkansas, specifically Ark. Code Ann. §§ 20-15-201-205. As part of this rule, Arkansas Department of Health and Arkansas State Board of Health are the only entities that can approve the release of data. Due to the protocol and IRB that was approved by the Arkansas State Board of Health, data from the HEER study to other parties cannot be released. To obtain data, please contact Abby Holt at the Arkansas Central Cancer Registry: (Email: Abby.Holt@arkansas.gov, Phone: 501-280-4830); Charles Lynch at the Iowa Cancer Registry: (charles-lynch@uiowa.edu, Phone: 319-384-5006); and Sumei Yun at Missouri Department of Health and Senior Services (oversees the Missouri Cancer Registry): Shumei.Yun@health.mo.gov, Phone: 573-522-2809.
Funding Statement
This work was supported by the American Cancer Society (RSG-10-197-01-CNE; www.acs.org) and the National Science Foundation (BCS-0922374). The Arkansas Central Cancer Registry is fully funded by a grant from the National Program of Cancer Registries, Centers for Disease Control and Prevention (CDC). Missouri Cancer Registry and Research Center is supported in part by a cooperative agreement between the CDC and the Missouri Department of Health and Senior Services (DHSS) (5NU58DP003924-04) and a Surveillance Contract between DHSS and the University of Missouri. State Health Registry of Iowa, Iowa Cancer Registry is funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN261201000032C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res. 2008;68: 6435–6441. doi: 10.1158/0008-5472.CAN-08-0329 [DOI] [PubMed] [Google Scholar]
- 2.McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98: 869–873. doi: 10.1093/jnci/djj233 [DOI] [PubMed] [Google Scholar]
- 3.Friberg L, Elinder CG, Kjellstrom T, Nordberg GF. Cadmium and health: a toxicological and epidemiological appraisal, volume II, effects and response Boca Raton, FL: CRC Press; 1985. [Google Scholar]
- 4.Garcia-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E et al. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect. 2014;122: 363–370. doi: 10.1289/ehp.1306587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ Health Perspect. 2002;110: 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera C, Ortega E, Lorenzo ML, Lopez MC. Cadmium contamination of vegetable crops, farmlands, and irrigation waters. Rev Environ Contam Toxicol. 1998;154: 55–81. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JA, Pahren HR, Lucas JB. Controlling cadmium in the human food chain: a review and rationale based on health effects. Environ Res. 1982;28: 251–302. [DOI] [PubMed] [Google Scholar]
- 8.Chaney RL, Ryan JA, Li YM, Brown SL Soil cadmium as a threat to human health In: Singh BR, McLaughlin MJ, editors. Developments in plant and soil sciences: cadmium in soils and plants. Dordrecht Boston: Kluwer Academic Publishers; 1999. pp. 219–256. [Google Scholar]
- 9.Haswell-Elkins M, McGrath V, Moore M, Satarug S, Walmby M, Ng J. Exploring potential dietary contributions including traditional seafood and other determinants of urinary cadmium levels among indigenous women of a Torres Strait Island (Australia). J Expo Sci Environ Epidemiol. 2007;17: 298–306. doi: 10.1038/sj.jes.7500547 [DOI] [PubMed] [Google Scholar]
- 10.Klaassen CD. Pharmacokinetics in metal toxicity. Fundam Appl Toxicol. 1981;1: 353–357. [PubMed] [Google Scholar]
- 11.Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137: 65–83. [DOI] [PubMed] [Google Scholar]
- 12.Akesson A, Berglund M, Schutz A, Bjellerup P, Bremme K, Vahter M. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Key statistics for endometrial cancer? Atlanta, GA: American Cancer Society; 5 January 2017. Available: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html. Accessed 17 April 2017. [Google Scholar]
- 14.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11: 1531–1543. [PubMed] [Google Scholar]
- 15.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9: 1081–1084. doi: 10.1038/nm902 [DOI] [PubMed] [Google Scholar]
- 16.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104: 85–95. doi: 10.1016/j.envres.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24: 6–19. doi: 10.1021/tx100231n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67: 335–376. doi: 10.1146/annurev.physiol.67.040403.120151 [DOI] [PubMed] [Google Scholar]
- 19.Ali I, Penttinen-Damdimopoulou PE, Makela SI, Berglund M, Stenius U, Akesson A et al. Estrogen-like effects of cadmium in vivo do not appear to be mediated via the classical estrogen receptor transcriptional pathway. Environ Health Perspect. 2010;118: 1389–1394. doi: 10.1289/ehp.1001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksen KT, Halkjaer J, Sorensen M, Meliker JR, McElroy JA, Tjonneland A et al. Dietary cadmium intake and risk of breast, endometrial and ovarian cancer in Danish postmenopausal women: a prospective cohort study. PLoS ONE. 2014;9: e100815 doi: 10.1371/journal.pone.0100815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams SV, Quraishi SM, Shafer MM, Passarelli MN, Freney EP, Chlebowski RT et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women's Health Initiative. Environ Health Perspect. 2014;122: 594–600. doi: 10.1289/ehp.1307054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YA, Kim J, Woo HD, Kang M. Dietary cadmium intake and the risk of cancer: a meta-analysis. PLoS ONE. 2013;8: e75087 doi: 10.1371/journal.pone.0075087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Penn Medicine Program for LGBT Health. Philadelphia, PA: Penn Medicine; 2015. Available: http://www.pennmedicine.org/lgbt/about-us/. Accessed 13 January 2015. doi: 10.1089/lgbt.2014.0054 [Google Scholar]
- 24.McElroy JA, Shafer MM, Hampton JM, Newcomb PA. Predictors of urinary cadmium levels in adult females. Sci Total Environ. 2007;382: 214–223. doi: 10.1016/j.scitotenv.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Quraishi SM, Adams SV, Shafer M, Meliker JR, Li W, Luo J et al. Urinary cadmium and estimated dietary cadmium in the Women's Health Initiative. J Expo Sci Environ Epidemiol. 2016;26: 303–308. doi: 10.1038/jes.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vacchi-Suzzi C, Eriksen KT, Levine K, McElroy J, Tjonneland A, Raaschou-Nielsen O et al. Dietary intake estimates and urinary cadmium levels in Danish postmenopausal women. PLoS ONE. 2015;10: e0138784 doi: 10.1371/journal.pone.0138784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Care and use of Nalgene Labware. Thermo Fisher Scientific Inc. 2017. Available: https://www.thermofisher.com/us/en/home/life-science/lab-plasticware-supplies/lab-plasticware-supplies-learning-center/lab-plasticware-supplies-resource-library/care-use-nalgene-labware.html. Accessed 17 April 2017. [Google Scholar]
- 28.Life sciences: trace element epidemiology Columbia, MO: University of Missouri Research Reactor Center; 2017. Available: http://www.murr.missouri.edu/rd_life_sciences_trace_element.php. Accessed 17 April 2017. [Google Scholar]
- 29.Jarrett JM, Xiao G, Caldwell KL, Henahan D, Shakirova G, Jones RL. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. J Anal At Spectrom. 2008;23: 962–967. [Google Scholar]
- 30.Elinder CG Normal values for cadmium in human tissues, blood, and urine in different countries In: Friberg L, Elinder CG, Kjellstrom T, Nordberg GF, editors. Cadmium and health: a toxicological and epidemiological appraisal, volume I, exposure, dose, and metabolism. Boca Raton, FL: CRC Press; 1985. pp. 81–102. [Google Scholar]
- 31.Lobo RA, Kelsey J, Marcus R. Menopause: biology and pathobiologoy San Diego, CA: Academic Press; 2000. [Google Scholar]
- 32.Little RJ, Vartivarian S. On weighting the rates in non-response weights. Stat Med. 2003;22: 1589–1599. doi: 10.1002/sim.1513 [DOI] [PubMed] [Google Scholar]
- 33.Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177: 142–151. doi: 10.1093/aje/kws200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T et al. Long-term dietary cadmium intake and cancer incidence. Epidemiology. 2012;23: 368–376. doi: 10.1097/EDE.0b013e31824d063c [DOI] [PubMed] [Google Scholar]
- 35.Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup Environ Med. 2012;69: 153–156. doi: 10.1136/oemed-2011-100111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10: 117–126. doi: 10.1080/13547500500159001 [DOI] [PubMed] [Google Scholar]
- 37.Mason HJ, Williams NR, Morgan MG, Stevenson AJ, Armitage S. Influence of biological and analytical variation on urine measurements for monitoring exposure to cadmium. Occup Environ Med. 1998;55: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NHANES environmental chemical data tutorial: using blood lipid or urine creatinine adjustments in the analysis of environmental chemical data Hyattsville, MD: Centers for Disease Control and Prevention, National Center for Health Statistics; 3 May 2013. Available: https://www.cdc.gov/nchs/tutorials/environmental/critical_issues/adjustments/. Accessed 17 April 2017. [Google Scholar]
- 39.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113: 192–200. doi: 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Expo Sci Environ Epidemiol. 2014;24: 163–170. doi: 10.1038/jes.2013.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jawaid M, Lind B, Elinder CG. Determination of cadmium in urine by extraction and flameless atomic-absorption spectrophotometry. Talanta. 1983;30: 509–513. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med. 2008;121: 501–508. doi: 10.1016/j.amjmed.2008.01.044 [DOI] [PubMed] [Google Scholar]
- 43.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19: 3119–3130. doi: 10.1158/1055-9965.EPI-10-0832 [DOI] [PubMed] [Google Scholar]
- 44.Davies JL, Rosenshein NB, Antunes CM, Stolley PD. A review of the risk factors for endometrial carcinoma. Obstet Gynecol Surv. 1981;36: 107–116. [DOI] [PubMed] [Google Scholar]
- 45.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003;104: 669–676. doi: 10.1002/ijc.10974 [DOI] [PubMed] [Google Scholar]
- 46.Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol. 2015;125: 89–98. doi: 10.1097/AOG.0000000000000563 [DOI] [PubMed] [Google Scholar]
- 47.Olson JE, Cerhan JR, Janney CA, Anderson KE, Vachon CM, Sellers TA. Postmenopausal cancer risk after self-reported endometriosis diagnosis in the Iowa Women's Health Study. Cancer. 2002;94: 1612–1618. doi: 10.1002/cncr.10370 [DOI] [PubMed] [Google Scholar]
- 48.Sturgeon SR, Luisi N, Balasubramanian R, Reeves KW. Sleep duration and endometrial cancer risk. Cancer Causes Control. 2012;23: 547–553. doi: 10.1007/s10552-012-9912-2 [DOI] [PubMed] [Google Scholar]
- 49.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67: 10618–10622. doi: 10.1158/0008-5472.CAN-07-2485 [DOI] [PubMed] [Google Scholar]
- 50.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer. The Iowa Women's Health Study. Am J Epidemiol. 1996;143: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 51.Setiawan VW, Monroe KR, Goodman MT, Kolonel LN, Pike MC, Henderson BE. Alcohol consumption and endometrial cancer risk: the multiethnic cohort. Int J Cancer. 2008;122: 634–638. doi: 10.1002/ijc.23072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360: 861–868. doi: 10.1016/S0140-6736(02)09958-0 [DOI] [PubMed] [Google Scholar]
- 53.Ganmaa D, Cui X, Feskanich D, Hankinson SE, Willett WC. Milk, dairy intake and risk of endometrial cancer: a 26-year follow-up. Int J Cancer. 2012;130: 2664–2671. doi: 10.1002/ijc.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green GA, Catlin DH, Starcevic B. Analysis of over-the-counter dietary supplements. Clin J Sport Med. 2001;11: 254–259. [DOI] [PubMed] [Google Scholar]
- 55.Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37: 66–79. doi: 10.1016/j.bpobgyn.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 56.Tokmak A, Kokanali MK, Guzel AI, Kara A, Topcu HO, Cavkaytar S. Polycystic ovary syndrome and risk of endometrial cancer: a mini-review. Asian Pac J Cancer Prev. 2014;15: 7011–7014. [DOI] [PubMed] [Google Scholar]
- 57.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103: 933–938. doi: 10.1038/sj.bjc.6605902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden). Cancer Causes Control. 1999;10: 277–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Rules Pertaining to the Arkansas Cancer Registry are duly adopted and promulgated by the Arkansas State Board of Health pursuant to the authority expressly conferred by the laws of the State of Arkansas, specifically Ark. Code Ann. §§ 20-15-201-205. As part of this rule, Arkansas Department of Health and Arkansas State Board of Health are the only entities that can approve the release of data. Due to the protocol and IRB that was approved by the Arkansas State Board of Health, data from the HEER study to other parties cannot be released. To obtain data, please contact Abby Holt at the Arkansas Central Cancer Registry: (Email: Abby.Holt@arkansas.gov, Phone: 501-280-4830); Charles Lynch at the Iowa Cancer Registry: (charles-lynch@uiowa.edu, Phone: 319-384-5006); and Sumei Yun at Missouri Department of Health and Senior Services (oversees the Missouri Cancer Registry): Shumei.Yun@health.mo.gov, Phone: 573-522-2809.