Abstract
Postnatal skeletal stem cells are a unique class of progenitors with biological properties that extend well beyond the limits of stemness as commonly defined. Skeletal stem cells sustain skeletal tissue homeostasis, organize and maintain the complex architectural structure of the bone marrow microenvironment and provide a niche for hematopoietic progenitor cells. The identification of stem cells in the human post-natal skeleton has profoundly changed our approach to the physiology and pathology of this system. Skeletal diseases have been long interpreted essentially in terms of defective function of differentiated cells and/or abnormal turnover of the matrix they produce. The notion of a skeletal stem cell has brought forth multiple, novel concepts in skeletal biology that provide potential alternative concepts. At the same time, the recognition of the complex functions played by skeletal progenitors, such as the structural and functional organization of the bone marrow, has provided an innovative, unifying perspective for understanding bone and bone marrow changes simultaneously occurring in many disorders. Finally, the possibility to isolate and highly enrich for skeletal progenitors, enables us to reproduce perfectly normal or pathological organ miniatures. These, in turn, provide suitable models to investigate and manipulate the pathogenetic mechanisms of many genetic and non-genetic skeletal diseases.
Introduction
Post-natal stem cells self-renew and differentiate to replenish the mature cell compartments of the tissues in which they reside. The very fact that stem cells for bone reside in bone marrow may suffice to highlight the fact that bone and bone marrow are functionally and anatomically continuous with one another. The continuity of bone and bone marrow is best reflected in the use of the term bone/bone marrow organ, which Maureen Owen introduced as the existence of a common progenitor for all skeletal tissues in the bone marrow emerged [1]. Bone and bone marrow share their vascularity, which includes vessels traversing the boundaries between bone and marrow space in both directions and often originating from and returning to the bone marrow after looping through bone. In situ, stem cells for bone are perivascular cells [2, 3], and at least some of the defining phenotypic features of perivascular progenitors in the bone marrow are shared by perivascular cells found within bone proper [4]. Bone formation and adipogenesis, which represent the canonical differentiation pathways of bone marrow stromal progenitors, are both perivascular events, as both osteoblasts and adipocytes are themselves perivascular cells. These simple facts would suggest that any attempt to understand the pathophysiology of bone in terms of cell dynamics should not exclude consideration of the bone marrow. However, the dominant paradigm adopted in pursuing an understanding of bone pathophysiology at the cellular level has been centered for years on the dynamics of osteoblasts and osteoclasts. On the other hand, and understandably enough, the dominant view of stem cells in bone has been centered, as in other fields, on the potential use of stem cells as therapeutic tools: replacement bricks for bone tissue engineering, or perhaps vehicles for gene therapy (as successfully pursued in other fields) in what is commonly referred to as “innovative therapies” as part of “regenerative medicine.” However, in all systems, the notion of stem cells is per se coupled to an appreciation that differentiated tissues are part of a lineage, and that diseases of a given system, in turn, can be seen as diseases of differentiated cells, or of the lineage as a whole; and may reflect inherent dysfunction of differentiated cells or of lineages, as well as secondary effects of exogenous signals, regulators or cues. Pathogenic effects of a gene defect can be manifested in mature cells only, as is the case, for example, in sickle cell anemia; or conversely, they can affect the entire lineage, as for example in thalassemia. The following pages are devoted to a brief discussion of how the notion of stem cells in bone can be bent to profit not only for treating, but also for understanding diseases, based on the assumption that proper understanding is key to effective therapy. In doing so, we will adhere to the dual nature and function of skeletal stem cells, which makes them truly unique among all natural objects that we refer to as “stem cells.” Skeletal stem cells act as progenitors, and act as non-progenitors [5]. As progenitors, they generate all different lineages that together comprise the skeleton, and those lineages only. As non-progenitors, they organize the vasculature of bone and bone marrow and also establish the microenvironment for growth and differentiation of hematopoietic cells, as well as the “niche” in which hematopoietic stem cells (HSCs) exist and are retained as such. The manner in which the function of skeletal stem cells is probed (i.e., their heterotopic transplantation to the effect of recapitulating the organogenesis of bone) illustrates these functions and their unique nature most effectively, in sharp contrast with other types of stem cells. Transplantation is the mainstay of stem cell biology. Transplantation of HSCs results in reconstitution of hematopoiesis; transplantation of epithelial stem cells in the reconstitution of epithelial tissues; transplantation of pluripotent embryonic stem cells results in teratomas (i.e., in the chaotic admixture of all differentiated lineages); transplantation of skeletal stem cells results in the generation of different skeletal tissues, yes, but also in the highly coordinated, mutual organization of donor tissues with host tissues in a chimeric organoid [2, 5, 6].
Skeletal stem cells are found in the bone marrow stroma. In situ, the bone marrow stroma is a highly elusive tissue, due to the simple fact that the key cell type, the adventitial reticular cell, escapes detection in conventional histological sections, and can only be visualized using cytochemical (alkaline phosphatase) [7–9] or immunocytochemical markers (e.g., CD146, CD105, CD90) [2]. Changes in number, density, phenotype and function of stromal cells result in gross changes in the organization of the bone marrow stroma, which accompany changes in bone. Osteoporosis, the most common bone disease, is not only a reduction in bone mass, it is also an increase in marrow adiposity and a reduction in Alkaline phosphatase expressing stromal cells[10]. Endosteal fibrosis of secondary hyperparathyroidism is the local accumulation of bone marrow stromal cells at the endosteum [11, 12]. The fibrosis of fibrous dysplasia of bone (FD) is the local accumulation of stromal cells in an abnormal marrow space [13], is coupled to the loss of adipocytes and of the hematopoietic microenvironment, and also to profound subversion of bone architecture, chemical composition, mineralization, internal texture and mechanical competence. Vascularity of the bone marrow is profoundly altered in osteoporosis, Paget’s disease, FD, and many more bone diseases. Many more examples could be given illustrating the point that calling an individual disease a “bone disease” rather than a “bone marrow disease” can be seen as the result of a conventional choice, or simply of a bias.
Skeletal stem cells and genetic diseases
The introduction of the induced pluripotent stem (iPS) cell technology [14] was saluted with enthusiasm as it conveyed both a reliable technological tool for generating pluripotent cells and theoretically any differentiated lineage, and relief from a heated “ethical” controversy, while illustrating the extraordinary notion that less than a handful of genes could reprogram an adult cell into pluripotency. Shortly thereafter, the value of iPS cells as tools for modeling disease became widely appreciated [15], and currently predominates over the still immature use of iPS cells for direct replacement of diseased tissues. The use of iPS cells for disease modeling encompasses investigative as well as directly applicative avenues: the generation of patient-specific diseased and differentiated cell types, in which to seek disease mechanisms, but also a tool for high-throughput drug screening. iPS cells have been used to model rare diseases such as Fibrodysplasia Ossificans Progressiva [16] and metatropic dysplasia [17], revealing altered patterns of cartilaginous differentiation through the use, notably, of assays in fact developed for the study of postnatal stem cells. However, the notion that skeletal diseases could be modeled through stem cells precedes the development of the iPS cell technology. Based on the recognition that obvious changes in the bone marrow stroma occur in FD, Bianco et al [18] hypothesized that heterotopic transplantation of stromal cells from the abnormal marrow of FD patients could recapitulate in vivo the abnormal architecture of FD bone and bone marrow. This provided evidence that a human non-neoplastic disease could be transferred to immunocompromised mice, and also the first use of stem cells for transferring disease into the mouse. A few years before, John Dick and coworkers had shown that human leukemia could be transferred to SCID/bg mice, through the transplantation of leukemic cells [19, 20]; from these studies, the concept that cancer could be transferred to immunocompromised mice by putative cancer stem cells, and the very idea of cancer stem cells, arose later [21]. The same approach contributed decisively to identify and name Gnathodyaphyseal Dysplasia as a separate disease, distinct from both FD and Osteogenesis Imperfecta, and to predict from the cell-autonomous properties of stromal progenitors, its genetic nature [22], which was identified shortly thereafter [23]. Specific dysfunction in skeletal and dental progenitors were recognized in in Cleidocranial Dysplasia [24], while heterotopic transplants of stromal progenitors from patients with Hurler’s disease, conversely, dispel an inherent disruption of stromal cell differentiation [25]. However, use of novel types of heterotopic transplantation assays [6] reveal specific changes in cartilage metabolism in Hurler’s disease (Serafini et al, manuscript in preparation). Heterotopic transplantation of stromal progenitor cells serves also to demonstrate in vivo the functional impact of gene knockout or of transgenes [26, 27].
The adoption of stem cells as a model of disease has been remarkably productive in the specific area in which it was most intensively pursued, Fibrous Dysplasia. Use of cultures of FD-derived bone marrow stromal cells resulted in the development of simple diagnostic tests for the identification of the causative GNAS mutations [18, 28], and for the quantification of the mutational load in a somatic mosaic disease [29]. Correlation of quantitative estimates of mutational load with patient age and clinical and pathological assessment of organ lesions led to the recognition that GNAS-mutated and wild-type stromal progenitors have different lifespans and self-renewal kinetics, explaining the natural occurrence of a spontaneous sterilization over time of the bone marrow progenitor compartment from the disease gene in some patients [30]. Using clonal populations of GNAS-mutated stromal progenitors, it was also possible to determine the imprinting profile of GNAS transcripts in skeletal progenitors. This revealed that while alternative transcripts of GNAS are expressed in osteoprogenitors and imprinted, Gsα is asymmetrically expressed in different clones in a random fashion, independent on imprinting, but potentially contributing to disease heterogeneity [31]. Finally, recognition of FGF23 as a product of the osteogenic lineage, and consequently of the role of bone as an endocrine organ regulating phosphate metabolism in the kidney, came from the use of stromal osteoprogenitors as an in vitro and in vivo model of FD. Overproduction of FGF23 in FD can account for the occurrence of hypophosphatemic rickets/osteomalacia in patients with severe panostotic forms of the disease [32].
One of the most challenging and at the same time attractive facets of the stem cell notion is the prospect of being able to tackle systemic genetic diseases of the skeleton. Osteogenesis Imperfecta was the first disease for which a stem cell-based type of intervention was envisioned [33], and in which targeting the genetic defect in stem cells ex vivo was attempted [34, 35]. The gene defect causing FD is a dominant, gain-of-function point mutation in a ubiquitously expressed, indispensable gene. Gene correction in FD thus requires silencing of the mutated allele with absolute specificity, which per se is a greater challenge in gene therapy than gene replacement. Nonetheless, the FD-causing mutation can be efficiently and specifically corrected in human stromal progenitor ex vivo using lentivirally expressed shRNAs, resulting in reversion of the fundamental cellular phenotype represented by excess production of cAMP [36]. Of note, as specific genetic defects can be corrected ex vivo in skeletal stem cells, several systemic, often lethal, skeletal diseases such as Osteogenesis Imperfecta and FD could be cured as of today, if systemic infusion of skeletal stem cells was at all feasible in the simplistic way in which it was first envisioned. Unfortunately, we are not there yet. Nonetheless, use of stem cells, including gene-corrected stem cells for treating systemic diseases of the skeleton remains unfeasible until ways to deliver stem cells systemically to the skeleton becomes feasible. Conversely, stable transduction of normal stromal progenitors with disease genes using last generation lentiviral vectors provides an additional tool for investigating the functional effects of a disease gene. In the case of FD, this exercise revealed for example the induction of RANKL as a robust and specific effect of the GNAS mutation directly relevant to the origin of excess osteoclastogenesis and remodeling in FD [36], and made it possible to investigate the transcriptome of newly mutated cells with appropriate controls and statistical robustness, circumventing the unpredictable variability of primary cultures derived from clinical material (manuscript in preparation).
Skeletal stem cells and cancer
Hematopoietic and non-hematopoietic cancer (primary and secondary) is a major determinant of skeletal morbidity, and for this reason, cancer in bone is the source of major clinical, social and healthcare concern. Until very recently, myeloma and metastatic growth of primary epithelial cancers were the specific focus of interest, reflecting both the occurrence of gross bone lesions as a result of their growth, and of the ease with which such lesions could be traced to an unbalance in remodeling. In this context, interest in the interaction of cancer cells with bone essentially excluded consideration of a potential role for skeletal stem cells as partners or players of the cancer-bone interaction, and in most cases even consideration of a role for bone marrow stromal cells at large. The notion of stem cells in bone currently contributes to expanding the range of cancer types seen as connected conceptually to the biology of bone cells, and to changing the visual angle on the interactions of cancer and bone at the cellular and molecular level. In general, a role of skeletal stem cells in cancer can be seen as running in parallel with their dual physiological functions - as progenitors and as the microenvironment.
Primary bone tumors
Skeletal stem cells may represent direct progenitors of sarcomas. In spite of the identification of specific molecular pathways underpinning specific types of bone tumors, classical and predominant (and to some extent, partially obsolete) paradigms of histogenesis of bone tumors have largely remained indifferent to the notion that skeletal tissues emanate from a common progenitor. As a result, classification and textbooks of pathology still identify primary bone tumors based on their predominant phenotype and/or clinical behavior. However, recent work has highlighted the significance of skeletal progenitor cells for understanding the biology of bone tumors. Transformation of murine bone marrow stromal cells in culture is a far more common event than currently appreciated (perhaps accounting for some reports of extraordinary numbers of population doublings, mistaken as “self-renewal” in some reports). Screening of multiple murine “MSC” lines by in vivo transplantation assays (conducted to probe their osteogenic capacity) easily reveals their tumorigenic properties (our unpublished results). The latter, in turn, are easily conceived of as the effect of the known chromosomal instability characteristic of murine cell cultures, at variance with humans. Spontaneous immortalization in cultures of human BMSCs, regardless of sporadic reports [37], is admittedly an exceptional event, reflecting uncontrolled growth conditions. More importantly, while forced expression of hTERT in human skeletal stem cells can boost their osteogenic capacity [38] [39], prolonged culturing of hTERT-immortalized human skeletal progenitors results in multiple genetic hits that may culminate with acquisition of full-blown tumorigenesis as assayed by in vivo transplantation [40]. By suggesting that inordinately high rates of proliferation over prolonged time can lead to transformation of skeletal progenitors, these data provide a direct view of sarcomagenesis as related to skeletal stem cells. More specifically, a pathogenetic link between Ewing’s sarcoma (a highly malignant bone tumor, EWS) and skeletal progenitors has been suggested recently. The transformation of murine “mesenchymal” (skeletal) stem cells by EWS-FLI1 (the fusion gene underpinning familial forms of EWS) generated EWS-like tumors [41]; conversely, the silencing of EWS-FLI1 in EWS cell lines rescued neoplastic cells to a “mesenchymal” cell phenotype and function [42]. Suva et al also isolated a CD133 positive subpopulation of stem cells from EWS that was able to initiate the growth of serially transplantable tumors (a putative cancer stem cell) while retaining the ability to differentiate along the adipogenic, osteogenic, and chondrogenic lineages [43]. A direct involvement of skeletal progenitors in tumorigenesis has also been hypothesized for murine and human osteosarcoma. Mohseny et al generated a murine “mesenchymal” stem cell system that formed osteosarcoma in vivo reproducing clinically relevant genetic aberrations [44], and osteosarcoma cell lines have been generated from transformed human “MSCs” [45]. Cells similar to skeletal stem cells, characterized by high invasiveness and drug resistance, have been isolated from human and murine tumors by using STRO1 and CD117 as markers [46]. It must be mentioned, however, that other studies have questioned the pathogenetic relevance of “MSCs” in both EWS and osteosarcoma, suggesting that “MSCs” are the major non-malignant component of the tumoral stroma [47] [48]..
Skeletal stem cells and the cancer microenvironment
While the idea that the bone marrow stroma as a whole provides a microenvironment for hematopoiesis and a niche for HSCs (the HME) goes back to classical hypotheses and experimental work, a revived interest in bone cells as niche-maintaining cells arose in the last ten years, prompting investigation of the “niche” as a determinant of tumor growth in bone. Later, a specific role for stem cells of the skeleton in providing the HME and niche functions became apparent, placing stromal osteoprogenitors at center stage of cancer-bone interactions (reviewed in [4] [49]. In the background, the classical “seed and soil” hypothesis of Stephen Paget [50] taken as a paradigm of the elective tropism of certain types of cancer for bone applies in a similar way to the interaction of blood-borne hematopoietic progenitors with an HME. Direct identification of skeletal stem/progenitor cells as the cells establishing the HME/niche, and of their own residence in a perivascular niche, thus highlights the potential key role of skeletal progenitors in the homing and growth of cancer in bone.
Currently, the terms “niche” and “microenvironment” tend to be used interchangeably. However, even though bone marrow stromal progenitors may exert both functions, the two functions are distinct. The ability of certain types of cancer to home to, and grow in bone selectively can reflect either the ability of the bone/bone marrow organ to provide a “niche” for cancer-initiating cells, or to provide a microenvironment suitable for the growth of their progeny. In the first instance, the existence of a cancer stem cell (CSC) is postulated. However, the concept of a CSC remains controversial for most types of tumors. Indeed, CSCs for non-hematopoietic cancer are probed by heterotopic xenotransplantation of limiting numbers of putative CSCs in immunocompromised mice, outside of a niche. For this reason, demonstration of a CSC in most instances coincides with demonstration of the dispensability of any niche. There is, conversely, little doubt of the fact that most hematopoietic cancers grow primarily in the bone/bone marrow organ, and some types of both hematopoietic (lymphoma) and non-hematopoietic cancer have an exquisite tropism for bone as a secondary site.
Hematopoietic cancer
The specific role for bone marrow stromal progenitors in supporting the growth of hematopoietic cancer can be as diverse as the variety of hematopoietic cancers themselves, and can directly reflect on the pattern of their growth and the type of local organ damage they can produce. In multiple myeloma, for example, the CXCL12/CXCR4 axis, which is thought to operate in the recruitment of a variety of blood borne cells including circulating cells from epithelial cancer and normal HSCs, can account for both the recruitment of myeloma cells to bone marrow and the promotion of their local survival [51] [52]. Myeloma cells are thought to represent post-germinal center B cells with somatic hypermutation and a phenotype consistent with memory B cells [53], which in a way makes myeloma a unique kind of “metastatic-only” cancer that involves selectively the bone marrow but may not arise within it. The unique ability of myeloma to produce lytic lesions in bone, on the other hand, can in turn be traced to different mechanisms, in turn centered on the interaction of myeloma cells with stromal osteoprogenitors. Dickkopf-1 (DKK-1), a Wnt antagonist, is involved in inhibition of the osteogenic potential of stromal osteoprogenitors, while RANKL overexpression and downregulation of osteoprotegerin in stromal cells are intuitively linked to promotion of bone resorption culminating in the production of osteolytic lesions [54]. A number of additional mechanisms can, however, contribute to this effect, including the generation of Th17 cells, immune inhibition of clonal growth in the pre-myelomatous monoclonal gammopathies of undefined significance (MGUS), and modulation of macrophage and dendritic cell function. The role of stromal progenitors in most of these mechanisms is conceivable but remains to be defined [55].
The distinct patterns of bone marrow involvement by non-Hodgkin’s lymphomas provide the best visual illustration of the existence of spatially defined microenvironments in the bone-bone marrow organ, sought by distinct populations of cancer cells. Follicular lymphoma grows as paratrabecular nodules, whereas marginal zone lymphomas and other types (hairy cell leukemia, mantle cell lymphoma) characteristically infiltrate sinusoids. Tumor-specific patterns of adhesion molecule expression may underpin such specific tropism for distinct microanatomical sites, the specific stromal composition of which remains to be elucidated. The myelofibrosis and osteosclerosis seen in myeloproliferative neoplasms (MPNs), in turn, represents the best visual demonstration of the involvement of stromal osteoprogenitors in the profound changes occurring in the hematopoietic microenvironment and niche in MPNs. Notably, the appearance of intravascular and extramedullary hematopoiesis in primary myelofibrosis may be linked to a profound subversion of the CXCL12/CXCR4 axis, which normally directs homing of HSCs to the marrow extravascular environment [56]. Human [2] and murine [57] [58] perivascular osteoprogenitors are the prime source of CXCL12 in the perivascular/extravascular environment in bone marrow; stromal osteoprogenitors increase in number in primary myelofibrosis (PMF) [59], but local availability of CXCL12 is decreased due to enhanced clearance and proteolytic degradation, and expression of CXCR4 in HSCs may be decreased [60] [61].
A host of interactions between myeloid cancer cells and stromal progenitors have been described, highlighting a complex bidirectional interplay involving a variety of pathways such as Wnt and adhesion molecule-conveyed signals [62]. Here too, the role of stromal-derived CXCL12 is pivotal in a number of key events including homing, survival and protection of cancer cells from apoptosis or drug effects [63]. Changes in the function of stromal progenitors induced by cancer cells in turn result in tissue changes such as fibrosis and perturbation of niche/microenvironment effects on normal hematopoiesis [64]. No doubt, the most intriguing findings are those suggesting a primary role of osteoprogenitors in directing the leukemogenic process itself. These include the observation of genetic changes in stromal cells in patients with myelodsplasia [65] [66], mouse models of myeloproliferative neoplasia secondary to genetic changes in the stroma [67], and induction of myelodysplasia and leukemia in mice as a result of Dicer-1 knockout in osteoprogenitors proper [68]. These data illustrate at the same time a specific “niche” (as opposed to microenvironment) effect as a function of osteoprogenitors proper.
Bone metastasis
Conceptual models for elucidating the interplay between non-hematopoietic cancer and bone (as a source of major morbidity in cancer patients) have been inscribed in a general paradigm in which remodeling of bone through the regulated action of differentiated bone cells is the key physiological event. No doubt, ultimate disruption of the balance between formation and resorption of bone is convenient to explain the osteolytic or osteosclerotic effects of bone metastasis [reviewed in [69]]. It must be noted, however, that while directly underpinning bone morbidity, these events come late in the natural history of metastatic growth in bone, and exclude from consideration the critical interplay between blood-borne cancer cells and the local microenvironment that lead to homing of cancer cells to bone (and its marrow) in the first place [70] [71]. Downstream of homing, dormancy of cancer cells [72] [73], or their growth into a sizable metastatic deposit, are alternative events. One might argue that the former illustrates a “niche” function, while the latter rather reflects a “microenvironment” effect. The bone marrow is the repository of circulating tumor cells [74] [75] [76] [77] even in the absence of, or prior to, the establishment of metastasis. All bone metastasis result from the seeding of cancer cells in the bone marrow. Redirecting the focus on early steps of the metastatic process may have obvious applicative and clinical implications, and it implies redirecting the attention on the interaction of cancer cells with stromal progenitors. Capturing the early events of the metastatic process in clinical material is difficult. Analysis of bone marrow biopsies taken from patients with known or unknown primary cancer, but free from signs and symptoms of local involvement, is a convenient way to visualize natural early metastasis in bone. This shows that conventional distinctions between “lytic” or “sclerotic” types of metastasis do not apply to early metastasis, in which an excess of medullary bone formation is a regular event, independent on the type and site of primary cancer, and therefore also of the gross “lytic” or “sclerotic” pattern that could be ultimately expected in the single case. Although a number of studies have utilized cultures of bone marrow stromal cells to model their interaction with cancer cells, an in vitro approach does not easily capture the dynamic events of cancer growth in a bone microenvironment. Attempts have recently been made towards the transfer in vivo of stromal/cancer co-cultures established ex vivo [78]. Current models of bone metastasis mostly rely on the intracardiac injection of large numbers of cancer cells [79] [80]. This approach was a major advance over prior practices involving the direct injection of cancer cells into bone, but was essentially utilized to analyze the gross effects of cancer growth in bone, and may not reflect accurately the metastatic process in at least two respects: one, it bypasses the venous phase of cancer cell circulation, and second, it analyzes the ability of specific cancer cell lines to grow in a murine microenvironment. Specific types of cancer may not grow as efficiently in mouse bone as they do in a human microenvironment, hence the need for humanized models [81]. This general approach is reflected into varied attempts to explore the homing of prostate cancer (manuscript in preparation), myeloma cells [82], leukemia [83], and breast cancer cells [84] to humanized microenvironments.
Conclusions
Stem cells in bone bring forth a remarkable change of perspective in bone medicine. They allow for consideration of diseases that affect bone as an organ rather than as a tissue. They provide the tool needed to understand diseases of the skeleton other than osteoporosis, while also contributing to the understanding of osteoporosis and bone aging. They provide a novel angle, centered on bone progenitors, in the study of major hematological diseases. They open the prospect of understanding the interaction of bone and cancer using the understanding of the HME/niche as a blueprint. Finally, pursuing these avenues of strict medical relevance can advance our understanding of bone disease, which can feed back on our understanding of bone physiology.
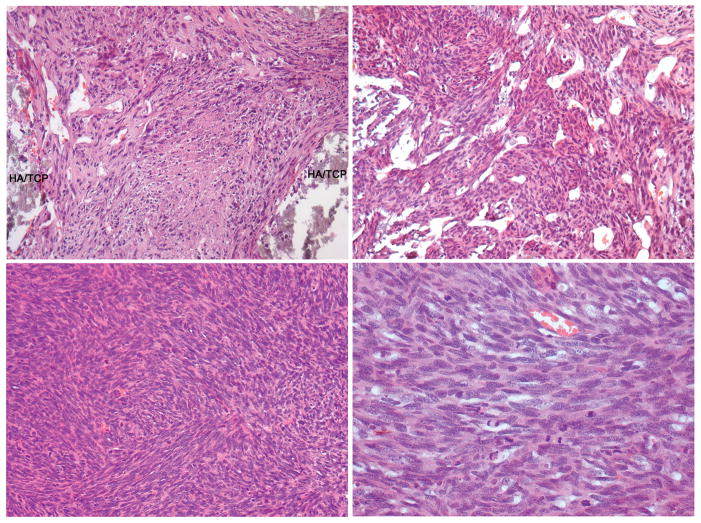
Figure 1.
Heterotopic transplantation of a spontaneously immortalized murine bone marrow stromal cell line, demonstrating sarcomatous growth (HA/TCP, hydroxyapatite/tricalcium phosphate carrier)
Acknowledgments
Personal work mentioned in this article was supported by Telethon (Grant GGP09227), MIUR, Fondazione Roma, Fondazione Cenci Bolognetti, Ministry of Health of Italy, EU (Plurimes consortium) and Sapienza University of Rome
References
- 1.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–8. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serafini M, Sacchetti B, Pievani A, Redaelli D, Remoli C, Biondi A, Riminucci M, Bianco P. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 2014;12:659–672. doi: 10.1016/j.scr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Westen H, Bainton DF. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J Exp Med. 1979;150:919–37. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco P, Costantini M, Dearden LC, Bonucci E. Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br J Haematol. 1988;68:401–3. doi: 10.1111/j.1365-2141.1988.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 9.Bianco P, Boyde A. Confocal images of marrow stromal (Westen-Bainton) cells. Histochemistry. 1993;100:93–9. doi: 10.1007/BF00572894. [DOI] [PubMed] [Google Scholar]
- 10.Bianco P, Bradbeer JN, Riminucci M, Boyde A. Marrow stromal (Western-Bainton) cells: identification, morphometry, confocal imaging and changes in disease. Bone. 1993;14:315–20. doi: 10.1016/8756-3282(93)90158-7. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Bonucci E. Endosteal surfaces in hyperparathyroidism: an enzyme cytochemical study on low-temperature-processed, glycol-methacrylate-embedded bone biopsies. Virchows Arch A Pathol Anat Histopathol. 1991;419:425–31. doi: 10.1007/BF01605077. [DOI] [PubMed] [Google Scholar]
- 12.Lotinun S, Sibonga JD, Turner RT. Evidence that the cells responsible for marrow fibrosis in a rat model for hyperparathyroidism are preosteoblasts. Endocrinology. 2005;146:4074–81. doi: 10.1210/en.2005-0480. [DOI] [PubMed] [Google Scholar]
- 13.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–600. [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89:655–61. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Hayashi Y, Schlieve CR, Ikeya M, Kim H, Nguyen TD, Sami S, Baba S, Barruet E, Nasu A, Asaka I, Otsuka T, Yamanaka S, Conklin BR, Toguchida J, Hsiao EC. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J Rare Dis. 2013;8:190. doi: 10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitta B, Passarini J, Sareen D, Ornelas L, Sahabian A, Argade S, Krakow D, Cohn DH, Svendsen CN, Rimoin DL. Patient-Derived Skeletal Dysplasia iPSCs Display Abnormal Chondrogenic Marker Expression and Regulation by BMP2 and TGFbeta1. Stem Cells Dev. 2014 doi: 10.1089/scd.2014.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101:1737–44. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamel-Reid S, Letarte M, Sirard C, Doedens M, Grunberger T, Fulop G, Freedman MH, Phillips RA, Dick JE. A model of human acute lymphoblastic leukemia in immune-deficient SCID mice. Science. 1989;246:1597–600. doi: 10.1126/science.2595371. [DOI] [PubMed] [Google Scholar]
- 20.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 21.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Riminucci M, Collins MT, Corsi A, Boyde A, Murphey MD, Wientroub S, Kuznetsov SA, Cherman N, Robey PG, Bianco P. Gnathodiaphyseal dysplasia: a syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J Bone Miner Res. 2001;16:1710–8. doi: 10.1359/jbmr.2001.16.9.1710. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi S, Kamata N, Vokes TJ, Maruoka Y, Nakakuki K, Enomoto S, Omura K, Amagasa T, Nagayama M, Saito-Ohara F, Inazawa J, Moritani M, Yamaoka T, Inoue H, Itakura M. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am J Hum Genet. 2004;74:1255–61. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding B, Li C, Xuan K, Liu N, Tang L, Liu Y, Guo W, Liu W, Jin Y. The effect of the cleidocranial dysplasia-related novel 1116_1119insC mutation in the RUNX2 gene on the biological function of mesenchymal cells. Eur J Med Genet. 2013;56:180–7. doi: 10.1016/j.ejmg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gatto F, Redaelli D, Salvade A, Marzorati S, Sacchetti B, Ferina C, Roobrouck VD, Bertola F, Romano M, Villani G, Antolini L, Rovelli A, Verfaillie CM, Biondi A, Riminucci M, Bianco P, Serafini M. Hurler disease bone marrow stromal cells exhibit altered ability to support osteoclast formation. Stem Cells Dev. 2012;21:1466–77. doi: 10.1089/scd.2011.0555. [DOI] [PubMed] [Google Scholar]
- 26.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi L, Kronenberg HM, Schipani E, Robey PG, Bianco P. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167:1113–22. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, Corsi A, Bone HG, Wientroub S, Spiegel AM, Fisher LW, Robey PG. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15:120–8. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- 29.Karadag A, Riminucci M, Bianco P, Cherman N, Kuznetsov SA, Nguyen N, Collins MT, Robey PG, Fisher LW. A novel technique based on a PNA hybridization probe and FRET principle for quantification of mutant genotype in fibrous dysplasia/McCune-Albright syndrome. Nucleic Acids Res. 2004;32:e63. doi: 10.1093/nar/gnh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23:1731–40. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michienzi S, Cherman N, Holmbeck K, Funari A, Collins MT, Bianco P, Robey PG, Riminucci M. GNAS transcripts in skeletal progenitors: evidence for random asymmetric allelic expression of Gs alpha. Hum Mol Genet. 2007;16:1921–30. doi: 10.1093/hmg/ddm139. [DOI] [PubMed] [Google Scholar]
- 32.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain JR, Deyle DR, Schwarze U, Wang P, Hirata RK, Li Y, Byers PH, Russell DW. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–93. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, Underwood RA, Song KM, Sussman M, Byers PH, Russell DW. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 36.Piersanti S, Remoli C, Saggio I, Funari A, Michienzi S, Sacchetti B, Robey PG, Riminucci M, Bianco P. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25:1103–16. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan Q, Fouraschen SM, de Ruiter PE, Dinjens WN, Kwekkeboom J, Tilanus HW, van der Laan LJ. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood) 2014;239:105–15. doi: 10.1177/1535370213506802. [DOI] [PubMed] [Google Scholar]
- 38.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–91. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 39.Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–6. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 40.Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–8. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 41.Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C, Hoffmann F, Trumpp A, Stamenkovic I. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–68. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 42.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–9. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suva D, Clement V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–81. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 44.Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn I, de Jong D, van Pel M, Cleton-Jansen AM, Hogendoorn PC. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Yang R, Zhang W, Dorfman H, Rao P, Gorlick R. Genetically transforming human mesenchymal stem cells to sarcomas: changes in cellular phenotype and multilineage differentiation potential. Cancer. 2009;115:4795–806. doi: 10.1002/cncr.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–12. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brune JC, Tormin A, Johansson MC, Rissler P, Brosjo O, Lofvenberg R, von Steyern FV, Mertens F, Rydholm A, Scheding S. Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int J Cancer. 2011;129:319–30. doi: 10.1002/ijc.25697. [DOI] [PubMed] [Google Scholar]
- 48.Amaral AT, Manara MC, Berghuis D, Ordonez JL, Biscuola M, Lopez-Garcia MA, Osuna D, Lucarelli E, Alviano F, Lankester A, Scotlandi K, de Alava E. Characterization of human mesenchymal stem cells from ewing sarcoma patients. Pathogenetic implications. PLoS One. 2014;9:e85814. doi: 10.1371/journal.pone.0085814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32:2535–47. doi: 10.1038/emboj.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 51.Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM, Jia X, Lu G, Timm M, Kumar A, Cote D, Veilleux I, Hedin KE, Roodman GD, Witzig TE, Kung AL, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–17. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen T, Jensen L, Johnsen HE. The clonal hierachy in multiple myeloma. Acta Oncol. 2000;39:765–70. doi: 10.1080/028418600750063479. [DOI] [PubMed] [Google Scholar]
- 54.Seidl S, Kaufmann H, Drach J. New insights into the pathophysiology of multiple myeloma. Lancet Oncol. 2003;4:557–64. doi: 10.1016/s1470-2045(03)01195-1. [DOI] [PubMed] [Google Scholar]
- 55.Tripodo C, Sangaletti S, Piccaluga PP, Prakash S, Franco G, Borrello I, Orazi A, Colombo MP, Pileri SA. The bone marrow stroma in hematological neoplasms--a guilty bystander. Nat Rev Clin Oncol. 2011;8:456–66. doi: 10.1038/nrclinonc.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migliaccio AR, Martelli F, Verrucci M, Migliaccio G, Vannucchi AM, Ni H, Xu M, Jiang Y, Nakamoto B, Papayannopoulou T, Hoffman R. Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease. Exp Hematol. 2008;36:158–71. doi: 10.1016/j.exphem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Tripodo C, Di Bernardo A, Ternullo MP, Guarnotta C, Porcasi R, Ingrao S, Gianelli U, Boveri E, Iannitto E, Franco G, Florena AM. CD146(+) bone marrow osteoprogenitors increase in the advanced stages of primary myelofibrosis. Haematologica. 2009;94:127–30. doi: 10.3324/haematol.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–10. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 61.Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A, Viarengo G, Meli V, Marchetti M, Guglielmelli P, Bruno E, Xu M, Hoffman R, Barosi G Italian Registry of Myelofibrosis with Myeloid M, Myeloproliferative Disorders Research C. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007;38:280–6. doi: 10.1016/j.bcmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–38. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger JA, Burkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Br J Haematol. 2007;137:288–96. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhao ZG, Liang Y, Li K, Li WM, Li QB, Chen ZC, Zou P. Phenotypic and functional comparison of mesenchymal stem cells derived from the bone marrow of normal adults and patients with hematologic malignant diseases. Stem Cells Dev. 2007;16:637–48. doi: 10.1089/scd.2007.0008. [DOI] [PubMed] [Google Scholar]
- 65.Flores-Figueroa E, Arana-Trejo RM, Gutierrez-Espindola G, Perez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29:215–24. doi: 10.1016/j.leukres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernandez-Campo P, Lopez-Holgado N, Diez-Campelo M, Barbado MV, Perez-Simon JA, Hernandez-Rivas JM, San-Miguel JF, del Canizo MC. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23:664–72. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 67.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 70.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olechnowicz SW, Edwards CM. Contributions of the Host Microenvironment to Cancer-Induced Bone Disease. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Berry JE, Havens AM, Joseph J, Kim JK, Patel L, Carmeliet P, Daignault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia. 2012;14:429–39. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 75.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 77.Shiozawa Y, Taichman RS, Keller ET. Detection and isolation of human disseminated tumor cells in the murine bone marrow stem cell niche. Methods Mol Biol. 2013;1035:207–15. doi: 10.1007/978-1-62703-508-8_18. [DOI] [PubMed] [Google Scholar]
- 78.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, Mariotti V, Buchupalli B, Foster K, Bonnet D, Grigoriadis A, Rameshwar P, Purushotham A, Tutt A, Dontu G. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013;73:6886–99. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 79.Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994;32:73–84. doi: 10.1007/BF00666208. [DOI] [PubMed] [Google Scholar]
- 80.Rosol TJ, Tannehill-Gregg SH, Corn S, Schneider A, McCauley LK. Animal models of bone metastasis. Cancer Treat Res. 2004;118:47–81. doi: 10.1007/978-1-4419-9129-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holzapfel BM, Thibaudeau L, Hesami P, Taubenberger A, Holzapfel NP, Mayer-Wagner S, Power C, Clements J, Russell P, Hutmacher DW. Humanised xenograft models of bone metastasis revisited: novel insights into species-specific mechanisms of cancer cell osteotropism. Cancer Metastasis Rev. 2013;32:129–45. doi: 10.1007/s10555-013-9437-5. [DOI] [PubMed] [Google Scholar]
- 82.Groen RW, Noort WA, Raymakers RA, Prins HJ, Aalders L, Hofhuis FM, Moerer P, van Velzen JF, Bloem AC, van Kessel B, Rozemuller H, van Binsbergen E, Buijs A, Yuan H, de Bruijn JD, de Weers M, Parren PW, Schuringa JJ, Lokhorst HM, Mutis T, Martens AC. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120:e9–e16. doi: 10.1182/blood-2012-03-414920. [DOI] [PubMed] [Google Scholar]
- 83.Vaiselbuh SR, Edelman M, Lipton JM, Liu JM. Ectopic human mesenchymal stem cell-coated scaffolds in NOD/SCID mice: an in vivo model of the leukemia niche. Tissue Eng Part C Methods. 2010;16:1523–31. doi: 10.1089/ten.tec.2010.0179. [DOI] [PubMed] [Google Scholar]
- 84.Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P, Brown TD, Dalton PD, Power CA, Hollier BG, Hutmacher DW. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Model Mech. 2014;7:299–309. doi: 10.1242/dmm.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]