Abstract
Objective
To evaluate seizures and epilepsy incidence in the first decade of life among children born extremely premature (< 28 weeks gestation).
Method
In a prospective, multi-center, observational study, 889 of 966 eligible children born 2002–2004 were evaluated at 2 and 10 years for neurological morbidity. Complementing questionnaire data to determine a history of seizures, all caregivers were interviewed retrospectively for post-neonatal seizures using a validated seizure screen followed by a structured clinical interview by a pediatric epileptologist. A second pediatric epileptologist established an independent diagnosis based on recorded responses of the interview. A third epileptologist determined the final diagnosis when evaluators disagreed (3%). Lifetable survival methods were used to estimate seizure incidence through 10 years.
Results
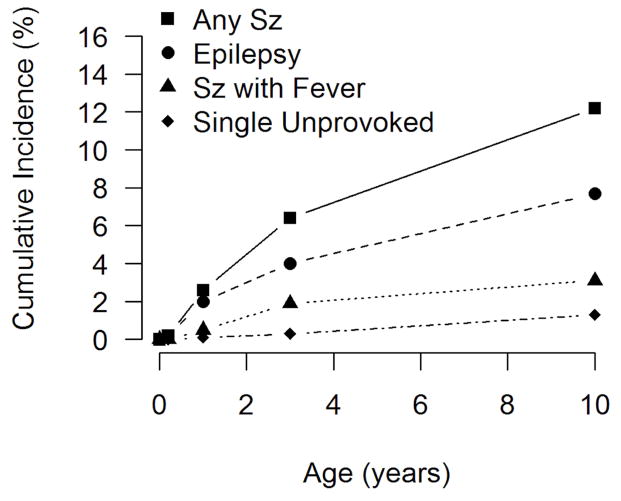
By age 10 years, 12.2% percent (95% CI: 9.8, 14.5) of children had ≥1 seizure, 7.6% (95% CI 5.7, 9.5) had epilepsy, 3.2% had seizure with fever, and 1.3% had a single, unprovoked seizure. Seizure incidence increased with decreasing gestational age. In more than 75% of children with seizures, onset was after one year of age. Seizure incidence was comparable in both sexes. Two-thirds of those with epilepsy had other neurological disorders. One-third of children with epilepsy were not recorded on the medical history questionnaire.
Significance
The incidence of epilepsy through 10 years of age among children born extremely premature is approximately 7 to 14-fold higher than the 0.5–1% lifetime incidence reported in the general pediatric population. Seizures in this population are under-recognized, and possibly under-diagnosed, by parents and providers.
Keywords: extreme prematurity, low gestational age, newborn, risk, rate, epilepsy, seizure incidence
Introduction
Extremely low gestational age newborns (ELGANs, defined by birth before the 28th week of gestation) are at high risk for neurological morbidity including cerebral palsy, autism, and intellectual disability. However, few studies of extremely preterm infants have reported on seizure or epilepsy, and less emphasis has been placed on seizures in these studies than on other neurological morbidities.1–6 Reported epilepsy and seizure rates in extremely premature infants have varied depending on when and how the cohort was identified, seizure ascertainment methods, and length of follow-up, but generally exceed the 1% population epilepsy prevalence.7 In Europe and Canada, rates of epilepsy in children born less than 32 weeks gestation ranged from a low of 0.6% to a high of 8.3%, while more recent reports indicate a prevalence of 3% or less.1,3,5 The prevalence of unprovoked seizures in European children born before 26 weeks gestation appears to have declined from 10% in children born in 1995 to 4% in those born in 2006.4 A single study reported a 17% febrile seizure rate in 60 survivors of premature birth at less than 32 weeks gestation born 1984–1986 in Finland 5, well above the US 3–5% febrile seizure prevalence rate in the general population.8,9 The cumulative incidence of seizures and epilepsy are likely greater in cohorts of children followed beyond 3 years of age, but variability in study designs precludes precise estimation.3,5,6
Methods for seizure identification have not been well defined in most studies of neurological outcomes in ELGANs, often relying on a parent-completed medical history collection form.1–4 Unlike previous studies, we screened children for seizures with a highly sensitive, validated parental questionnaire followed by a structured parent-interview by a pediatric epileptologist to standardize the diagnosis of seizures and epilepsy.10 The aim of this study was to determine the cumulative incidence of epilepsy and seizures occurring after discharge from the neonatal intensive care unit in a large cohort of children born before the 28th week of gestation followed until age 10 years (the ELGAN Study).
Methods
Overview of the ELGAN study
The ELGAN study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in children born extremely preterm.11 A total of 1506 infants born before the 28th week of gestation were enrolled during the years 2002–2004, and 889 (92%) of 966 eligible survivors were followed to age 10 years. Head ultrasound readings were completed in the neonatal period; cerebral palsy and microcephaly were identified as part of a comprehensive evaluation when the child was 2 years old.12,13 At Age 10 years, children were evaluated for general cognitive ability (intellectual quotient-IQ), autism, and seizures.14,15 Enrollment and consent procedures for this follow-up study were approved by the institutional review boards of all participating institutions.
Evaluation for seizures
888 of the 889 patients recruited at age 10 years were evaluated for possible seizure. Prior to detailed seizure screening, each of the parents completed the ELGAN Study Education and Medical History form, which includes the following yes/no question: “Have you ever been told by a doctor or other professional that your child has epilepsy, seizures, or convulsions?” This question assessed parent recall of a prior diagnosis of seizures or epilepsy but did not determine a seizure case. In addition, parents were asked on the medical history form if their child has taken a daily medication for seizures or epilepsy.
Identification of seizures involved a two stage process. At the time the child was brought for the 10-year assessment, a research assistant asked parents of 888 children 11 broad questions about possible seizures; these 11 questions are outlined elsewhere.10 A yes response to any of these questions prompted a structured telephone interview by the epileptologist to determine whether a reported event was a seizure. The structured clinical interview contained more specific questions from part 2 of a previously validated parent seizure screen for children, complementary questions about possible ictal and post-ictal symptoms, and a narrative history of the events.10 Two hundred seventy-three of the 888 screened positive, and 230 were interviewed by telephone (Table 1). A second epileptologist independently reviewed interview responses and similarly rated the event type. When the two physicians disagreed on the presence of seizures, which occurred for only 3% of the children, a third epileptologist reviewed the interview responses and made the final seizure determination. Additionally, for each distinct seizure phenotype, the age at onset was ascertained to have occurred in one of the following time intervals: in the nursery, before one year of age, between 1 and 3 years of age, and after 3 years of age. The occurrence of the last seizure was allocated to one of the following groups: within the last week, within the last month, within the last year, or more than a year ago.
Table 1.
Subject source for seizure case ascertainment
| Cohort enrolled at birth | 1506 |
| Recruited for follow-up at age 10 years | 966 |
| Enrolled at age 10 years | 889 |
| Completed seizure screen | 888 |
| Screen negative | 615 |
| Screen positive | 273 |
| Completed seizure interview | 230 |
| Were unable to be interviewed | 43 |
Seizure case definitions
Epilepsy was defined as two or more seizures after the neonatal period that were not provoked by fever, trauma, or infection of the central nervous system. Seizure with fever was used to describe those children who had seizures with fever. The International League Against Epilepsy’s definition of febrile seizures excludes children who have had neonatal seizures or prior unprovoked seizures.16 We did not routinely collect information about neonatal seizures. In addition, parents were not asked if seizures associated with fever were preceded by unprovoked seizures in those children who had both. A single unprovoked seizure was defined as an isolated seizure that was not precipitated by fever, trauma, or infection of the central nervous system. It was possible for a child to have both seizures with fever and epilepsy.
Ultrasound protocol scans
Routine scans were performed by technicians at all of the hospitals using digitized high frequency transducers (7.5 and 10 MHz). Ultrasound studies always included the six standard quasi-coronal views and five sagittal views using the anterior fontanel as the sonographic window.17 All ultrasound scans were read by two independent readers who were not provided clinical information.
Head circumference
The head circumference was measured as the largest possible occipital-frontal circumference, and examiners underwent videotaped training. Measurements were rounded to the closest 0.1 centimeter when examined at 24-month corrected age. All head circumferences are presented as Z-scores based on standards in the CDC data sets.
Cerebral palsy (CP) evaluation
Those who performed the neurological examinations studied a manual, a data collection form and an instructional CD designed to minimize examiner variability, and demonstrated acceptably low variability.18 The topographic diagnosis of cerebral palsy (CP) (quadriparesis, diparesis, or hemiparesis) was based on an algorithm using these data.19
General cognitive ability evaluation
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales–II (DAS-II) Verbal and Nonverbal Reasoning scales.20 Because DAS-II Verbal and Nonverbal IQ scores were strongly correlated within the sample, the mean of these two measures was used as an estimate of general cognitive ability.
Autism spectrum disorder (ASD) evaluation
All children were screened by parent report with the Social Communication Questionnaire (SCQ) for risk of ASD.21 Children determined to be at risk on the SCQ, were assessed with the Autism Diagnostic Interview – Revised (ADI-R), an in-depth parent interview.22 Children meeting ADI-R criteria for ASD were administered the Autism Diagnostic Observation Schedule-2 (ADOS-2). Children meeting standardized research criteria for ASD on both the ADI-R and ADOS-2 were classified as having ASD.
Attention Deficit Hyperactivity Disorder (ADHD) evaluation
Participants were included in the ADHD symptom group if they met DSM-5 symptom criteria in any two of three contexts. Two contexts were based on having sufficient symptoms among the 18 items specific for ADHD using the Child Symptom Inventory, Fourth Edition (CSI-4), Parent or Teacher Checklist.23,24 A third context was based on the parent’s indication at interview of the child having been diagnosed previously by a clinician to have ADHD. Physician assessment of ADHD precedes treatment with stimulants, and the physician’s diagnosis of ADHD is most often based on parent and teacher inputs more so than their own observations so we regarded this as pertinent to our research classification.25
Data analysis
Our aim was to establish and characterize the cumulative incidence of seizure and epilepsy through age 10 years in the ELGAN Study cohort. Age of first seizure is categorized as in the nursery, before one year, between 1 and 3 years, or between 3 and 10 years, and life table methods were used to estimate cumulative incidence curves for any seizure, epilepsy, seizure with fever (in the absence of epilepsy), and a single unprovoked seizure. Cumulative incidence at age 10 years, from the life table estimates, was presented with 95% confidence intervals. Cumulative incidence was compared for boys vs. girls using the log-rank test, and the association between gestational age and cumulative incidence was tested using the log-rank test for trend. Medication history and prevalence of comorbidities were described for children with seizures.
Forty-three of 273 children who screened at risk for seizures could not be contacted for full evaluation by the epileptologist and so were considered missing data on seizures. Our primary analysis used inverse probability weighting to account for this missing data, with the probability of missingness based on gestational age and the result of the initial seizure screen.26 Secondary analyses used multiple imputation methods to account for missing data, imputing 5 data sets with imputed seizure data based on gestational age and the result of the initial seizure screen.
Results
Ninety-one children had at least one seizure in the interval between discharge from the neonatal intensive care unit and the time of the seizure interview. Cumulative incidence curves for any seizure and by seizure category are presented in Figure 1. The cumulative incidence of any seizure by age 10 years in this cohort was 12.2% (95% CI 9.8,14.5). Fifty-seven children had epilepsy, and the cumulative incidence by age 10 years was 7.6% (95% CI 5.7, 9.5). Ten children, or 1%, had a single unprovoked seizure (Table 2).
Figure 1.
Cumulative incidence of seizures and epilepsy in children born extremely preterm.
Table 2.
Weighted Cumulative Incidence of Seizures by Age 10 in the ELGAN cohort
| n | Cumulative Incidence at age 10 years (95% CI) | |
|---|---|---|
|
| ||
| Any Seizure | 91 | 12.2% (9.8, 14.5) |
| Epilepsy | 57 | 7.6% (5.7, 9.5) |
| Single Unprovoked Seizure | 10 | 1.3% (0.5, 2.2) |
| Seizure with fever* | 24 | 3.2% (1.9, 4.5) |
Isolated seizures with fever (without epilepsy and without single unprovoked seizure)
Twenty-four children had seizures with fever, but did not have unprovoked seizures, a cumulative incidence by age 10 of 3.2% (95% CI 1.9, 4.5). In addition, 17 of the 57 children with epilepsy (30%) and two of 10 children with a single unprovoked seizure (20%) also had seizures with fever.
Compared with the epileptologists’ interview, the parents’ response on the Educational-Medical History form completed at the 10-year assessment failed to identify 38 of the 91 (42%) children diagnosed with seizures and 19 of the 57 (33%) children with epilepsy.
The incidence of seizures and epilepsy was similar in girls and boys (p=0.671 for any seizure, p=0.407 for epilepsy, log-rank test). 12.7% (95% CI 9.6, 15.8) of boys and 11.7% (95% CI 8.7, 14.7) of girls had any seizure by age 10 years.
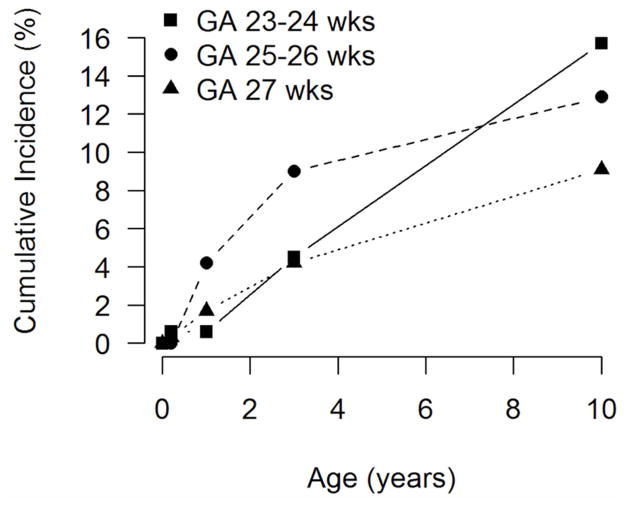
Cumulative incidence of any seizure, by gestational age, is presented in Figure 2, and cumulative incidence by age 10 years for any seizure, epilepsy, seizure with fever, and single unprovoked seizure by gestational age are listed in Table 3. Children born at earlier gestational ages had a higher incidence of any seizure (p=0.034 by the log-rank test for trend). The associations between gestational age and epilepsy, and between gestational age and seizure with fever, were not significant. Children born at earlier gestational ages were more likely to have single unprovoked seizures (p=0.008).
Figure 2.
Cumulative incidence of any seizure in children born extremely preterm, by gestational age category.
Table 3.
Weighted prevalence of seizures by gestational age
| Gestational Age | ||||
|---|---|---|---|---|
|
| ||||
| 23–24 wks (n=172) | 25–26 wks (n=379) | 27 wks (n=292) | Signif** | |
|
| ||||
| Any Seizure | 15.4 (9.8, 21.1) | 12.8 (9.2, 16.3) | 9.4 (5.8, 12.9) | 0.058 |
| Epilepsy | 9.0 (4.5, 13.5) | 8.6 (5.6, 11.6) | 5.5 (2.7, 8.2) | 0.147 |
| Single Unprovoked | 3.9 (0.8, 6.9) | 0.6 (0.0, 1.4) | 0.8 (0.0, 1.9) | 0.039 |
| Seizure with Fever* | 2.6 (0.1, 5.1) | 3.6 (1.6, 5.6) | 3.1 (1.0, 5.2) | 0.777 |
Seizure with fever for those without epilepsy or single unprovoked seizure
Test for trend in prevalence by gestational age through logistic regression
The majority of children had their first seizure after age one year: 77% of children with any seizures, 74% of children with epilepsy, 83% of children with isolated febrile seizures without epilepsy, and 90% of children with a single afebrile seizure (Table 4). More than half (53%) of the children with epilepsy had experienced at least one seizure in the past year. None of the children with isolated febrile seizures had seizures in the year prior to the seizure interview. Forty percent of children with a single afebrile seizure had presented in the year prior to the interview.
Table 4.
Characteristics of children with seizures. These are column percents
| Percent by Seizure Category | |||||
|---|---|---|---|---|---|
| Any (n=91) | Epilepsy (n=57) | With Fever (n=24)* | Single Unprovoked (n=10) | ||
| Age at first seizure | Neonatal | 2.2 | 3.5 | 0 | 0 |
| > | 19.8 | 22.8 | 16.7 | 10 | |
| 1–3 years | 30.1 | 26.3 | 45.8 | 20 | |
| > 3 years | 47.3 | 47.4 | 37.5 | 70 | |
| Seizure in past 1 year | Yes | 37.4 | 52.6 | 0 | 40 |
| No | 62.6 | 47.4 | 100 | 60 | |
| Sex | Boys | 52.7 | 56.1 | 41.7 | 60.0 |
| Girls | 47.3 | 43.9 | 58.3 | 40.0 | |
| Seizure Medication | Currently | 40.7 | 50.9 | 8.3 | 60 |
| In the past | 9.9 | 12.3 | 4.2 | 10 | |
| Never | 49.6 | 36.8 | 87.5 | 30 | |
Isolated seizures with fever (without epilepsy and without single unprovoked seizure)
Fifty-one percent of the children with epilepsy were taking anti-seizure medicine at the time of the interview, while 12% had taken it in the past. However, 37% of the children with epilepsy were never treated with an anti-seizure medicine. Among the children who experienced seizures with fever, 88% never took an anti-seizure medicine. Seven of the ten children who experienced a single afebrile seizure were placed on medicine either in the past (10%) or were taking it at the time of the interview (60%).
Two-thirds of children with epilepsy had one or more additional neurological morbidities, while one-third had no other neurological impairment. This contrasts with the 68% of the entire cohort of ELGAN children that was free of any major neurological morbidity. Children with seizures were more likely than children without seizures to have other neurological morbidities (i.e. CP, IQ<70, ASD, ADHD, microcephaly, and white matter disease); these differences are presented in Table 5.
Table 5.
Number (%) with neurological morbidities, by seizure status
| No Seizure (n=783) | Seizure other than Epilepsy (n=34) | Epilepsy (n=57) | p-value1 | p-value2 | |
|---|---|---|---|---|---|
|
| |||||
| CP | 59 (7.5) | 11 (32.4) | 18 (31.6) | <0.001 | <0.001 |
| IQ<70 | 48 (6.1) | 6 (17.6) | 22 (38.6) | 0.008 | <0.001 |
| ASD | 45 (5.7) | 6 (17.6) | 8 (14.0) | 0.005 | 0.013 |
| ADHD | 123 (15.7) | 7 (20.6) | 18 (31.6) | 0.446 | 0.002 |
| Microcephaly | 78 (10.0) | 7 (20.6) | 16 (28.1) | 0.047 | <0.001 |
| WMD | 87 (11.1) | 10 (29.4) | 21 (36.8) | 0.001 | <0.001 |
p-value comparing comorbidity in those with seizures other than epilepsy vs. no seizure
p-value comparing comorbidity in those with epilepsy vs. no seizure
Results of secondary analyses using multiple imputation to include children who screened at-risk for seizure on the initial seizure screen but were not fully evaluated were similar to results reported above using inverse probability weighting to account for missing data. The estimated cumulative incidence of any seizure by age 10 was 12.4% (95% CI 10.2, 14.6), and associations between gestational age and any seizure (p=0.034 log-rank test of trend) and a single unprovoked seizure (p=0.021 log-rank test of trend) remained significant in imputed analyses.
Discussion
Our major finding is that at age 10 years, children born extremely premature are at a 10–20 fold higher risk of having developed seizures and a 7–14 fold higher risk of having developed epilepsy than the general population of children. In our cohort, the cumulative incidence of seizures was 12.2% and of epilepsy was 7.6%. Additionally, a substantial number of children born extremely preterm were not recognized prior to the seizure interview at age 10 years to have had seizures or epilepsy, and one-third of those with epilepsy had not received anti-seizure medications. Finally, only one-third of those with epilepsy were free of other major neurological morbidity.
In previous studies of children born extremely premature, seizure and epilepsy rates have varied depending on the defined cohort, length of follow-up, and seizure case ascertainment. Studies differ in the definition of prematurity, some using gestational age and others very low birth weight.1–5 Seizure rates in children born before 26 weeks have varied between 4% and 10%, with the highest rates being reported in those born in 1995 or before.2,4 Epilepsy rates in prior studies have ranged from 0.6% to 3% in children born extremely preterm, and were highest when the cohort was defined by gestational age rather than by birth weight.1,3 Similar to the Epipage study, a longitudinal study of children born in France before 32 weeks gestation between 1997–1998, the cumulative incidence of seizures in the ELGAN cohort was inversely related to gestational age.3
The Epicure study, a longitudinal study of children born before 25 weeks gestation in the United Kingdom and Ireland, found that at age 3 years,10% of those born in 1995 developed unprovoked seizures compared with just 4% of children born in 2006.4,27 In a cohort of children born before 32 weeks gestation between 1984–1986, an even higher rate of seizures was found, 13%.5 Overall, these studies suggest that seizure rates among individuals born very prematurely have declined over the years.4,5,27
Although the children in the ELGAN cohort were born between 2002 and 2004, contemporary to the Epicure cohort, we report a 12.2% cumulative incidence of seizures. The discrepancy between seizure rates in the literature and what we report may be explained by our more sensitive ascertainment method and our follow-up to age 10 years. Published epidemiological studies of very or extremely preterm infants either have not clearly described the methods used to ascertain seizures and epilepsy or have ascertained a history of seizures and epilepsy through a limited parent interview.1,3,4 If we had relied on the ELGAN Study parent-completed Education and Medical History form alone for case ascertainment, more than a third of ELGAN children with epilepsy would not have been identified. The use of a structured and validated screening questionnaire followed by a parent-interview with a pediatric epileptologist likely enhanced the diagnostic yield.10 Also, confirmation of seizure cases required the agreement of two epileptologists, minimizing the likelihood of false positive events. Consequently, we consider our estimates to be as accurate as possible without benefit of supportive electroencephalographic data.
Our seizure ascertainment methods may also partly explain why over one third of children with epilepsy were not treated with anti-seizure medication. It is likely that some of these children had not been identified as having epilepsy by a health care provider in the past.
In a cohort of premature children born less than 32 weeks from 1984–1986, seizures with fever occurred at a rate approximately three times that expected in the general population.5 Nearly two decades later, we found the overall cumulative incidence of seizures with fever in children born extremely premature to be 3.2%, in range of the 2–5% reported in the United States and globally.8,9 Only 3–5% of children with febrile seizures in the general population eventually develop epilepsy, whereas, more than 40% of our cohort who experienced at least one seizure associated with fever developed epilepsy.28 Given the high incidence of epilepsy seen in in our cohort who had experienced at least one seizure with fever, it is probable that their fever-associated seizures were symptomatic of latent epilepsy, unlike most febrile seizures in the general population.9 This view is supported by the observation that more than a third of the children in our cohort experienced their first seizure with fever after 3 years of age, later than expected for children in the general population who develop febrile seizures (peak incidence in the second year of life).9
The higher risk of epilepsy in our cohort of children born extremely premature compared with the general population may be related to the elevated risk for other brain disorders. Neurological morbidity was significantly higher for children in our cohort with seizures or epilepsy than for children without seizures.
Furthermore, each of these might be the consequence of early life inflammation, deficiency of critical endogenous neuroprotective molecules such as brain-derived neurotrophic factor, or epigenetic mechanisms.29–35
Seizures and epilepsy rates increase as children are followed beyond 2–3 years of age.3,5,6 Nearly half (47.3%) of the children who developed seizures in our cohort did so after age 3 years. Given that epilepsy predisposition increases during puberty, the cumulative incidence of seizures is likely to be higher if we re-evaluate the ELGAN cohort after puberty.36 Many infant follow-up clinics do not follow children past 2 years of age. Given the high rates of seizures and epilepsy in children born extremely premature, the window for clinical follow-up should be extended well into childhood, and possibly through puberty.
Strengths and Limitations
This study is likely more accurate than prior reports of the cumulative incidence by age 10 years of seizures and epilepsy in a large cohort of children born before 28 weeks gestation and followed from birth. Case ascertainment was obtained with a highly sensitive seizure screen, followed by a clinical interview with a pediatric epileptologist, and required confirmation by a second and sometimes third pediatric epileptologist.
Video-EEG in the neonatal period was not implemented, so the occurrence of neonatal seizures could not be verified. Seizure and epilepsy types and the rationale used by primary and specialty care clinicians caring for children with seizures in the ELGAN Study also could not be accurately evaluated without reviewing EEG data. In addition, our ascertainment could have been incomplete because 1) we were not able to complete the seizure interviews for 43 of 273 children who had a positive part 1 seizure screen and 2) parents of children were interviewed in some instances several years after an event concerning for seizure.
Conclusions
In this sample of 10-year-old children born less than 28 weeks gestation, the life-time incidence of seizures was 10–20 times higher and epilepsy 7–14 times higher than in the general population. Among extremely low gestational age children with febrile seizures, epilepsy is much more likely to occur than in the general population, 41% vs 3–5%. Seizures in ELGAN children are under-recognized, and possibly under-diagnosed, by parents and providers. Children born extremely premature need to be followed carefully for signs of seizures throughout childhood. Future studies of this population may lead to a better understanding of mechanisms underlying epileptogenesis.
Acknowledgments
Funding Source: This study was supported by the National Institute of Neurological Disorders and Stroke (grants 5U01NS040069-05 and 2R01NS040069-09).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
Contributor’s Statements:
Dr. Douglass contributed to the conceptualization of the study, personally interviewed families, reviewed and analyzed the data, and drafted the manuscript
Dr. Kuban, Dr. Leviton, Dr. O’Shea, Dr. Heeren, and Ms. Allred each contributed to the conceptualization of the study, analyzed and interpreted the data, assisted in the writing of the manuscript, and approved the final manuscript as submitted.
Dr. Stafstrom contributed to the conceptualization of the study, assisted in the interpretation of the data, reviewed and rated seizure interviews whenever there was a disagreement between the first two raters (Dr. Douglass and Dr. DeBassio), assisted in the writing of the manuscript, and approved the final manuscript as submitted.
Dr. DeBassio reviewed and rated seizure interviews and contributed in the writing of the manuscript, and approved the final manuscript as submitted. Dr. Hirtz contributed to the conceptualization of the study, helped interpret the data, contributed in the writing of the manuscript, and has approved the final manuscript as submitted.
Ms. Rollins: assisted with the collection of seizure interviews, organized the data, contributed to the design of the structured seizure interview, and approved the final manuscript as submitted.
The authors confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Financial Disclosure Statement: None of the authors have relevant financial interests, activities, relationships, or affiliations, and none received support for this project aside from a grant provided by NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aziz K, Vickar DB, Sauve RS, Etches PC, Pain KS, Robertson CM. Province-based study of neurologic disability of children weighing 500 through 1249 grams at birth in relation to neonatal cerebral ultrasound findings. Pediatrics. 1995;95(6):837–844. [PubMed] [Google Scholar]
- 2.De Groote I, Vanhaesebrouck P, Bruneel E, et al. Outcome at 3 years of age in a population-based cohort of extremely preterm infants. Obstet Gynecol. 2007;110(4):855–864. doi: 10.1097/01.AOG.0000284447.43442.55. [DOI] [PubMed] [Google Scholar]
- 3.Marret S, Marchand-Martin L, Picaud JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PloS One. 2013;8(5):e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345(Journal Article):e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrgard EA, Karvonen M, Luoma L, et al. Increased number of febrile seizures in children born very preterm: relation of neonatal, febrile and epileptic seizures and neurological dysfunction to seizure outcome at 16 years of age. Seizure. 2006;15(8):590–597. doi: 10.1016/j.seizure.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer AP, Voss W. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 7.Epilepsy Fast Facts. http://www.cdc.gov/epilepsy/basics/fast-facts.htm.
- 8.Berg AT, Shinnar S. The contributions of epidemiology to the understanding of childhood seizures and epilepsy. J Child Neurol. 1994;9(suppl 2):2S19–2S26. [PubMed] [Google Scholar]
- 9.Syndi Seinfeld D, Pellock JM. Recent research on febrile seizures: A review. J Neurol Neurophysiol. 2013;4(165):1–4. doi: 10.4172/2155-9562.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglass LM, Kuban K, Tarquinio D, et al. A Novel Parent Questionnaire for the Detection of Seizures in Children. Pediatr Neurol. 2016;54:64–69. doi: 10.1016/j.pediatrneurol.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 11.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuban KC, O’Shea TM, Allred EN, et al. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J Child Neurol. 2014;29(12):1692–1698. doi: 10.1177/0883073813513335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuban KC, Allred EN, Oshea TM, et al. Developmental correlates of head circumference at birth and two years in a cohort of extremely low gestational age newborns. J Pediatr. 2009;155:334–349. doi: 10.1016/j.jpeds.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph RM, O’Shea TM, Allred EN. Prevalence and Associated Features of Autism Spectrum Disorder in Extremely Low Gestational Age Newborns at Age 10 Years. Autism Res. 2016 doi: 10.1002/aur.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuban KC, Joseph RM, O’Shea TM, et al. Girls and Boys Born before 28 Weeks Gestation: Risks of Cognitive, Behavioral, and Neurologic Outcomes at Age 10 Years. J Pediatr. 2016;173(Journal Article):69–75. doi: 10.1016/j.jpeds.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34(4):592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuban Karl CK, Allred Elizabeth N, Michael O’Shea T, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuban KC, O’Shea M, Allred E, Leviton A, Gilmore H, DuPlessis A, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–31. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 19.Kuban KCK, Allred EN, O’Shea TM, Paneth N, Pagano M, Leviton A, et al. An algorithm for diagnosing and classifying cerebral palsy in young children. J Pediatr. 2008;153:466–72.e1. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott CD. Differential Ability Scales®-l|(DAS-ll) 2007. [Google Scholar]
- 21.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: 2003. [Google Scholar]
- 22.LeCouteur A, Lord C, Rutter M. Autism Diagnostic Interview – Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 23.Sprafkin J, Gadow KD, Salisbury H, et al. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc. 2002;31(4):513–24. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 24.Gadow KD, Sprafkin J. Child Symptom Inventory–4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 25.Power TJ, Mautone JA, Manz PH, et al. Managing attention-deficit/hyperactivity disorder in primary care: a systematic analysis of roles and challenges. Pediatrics. 2008;121(1):e65–72. doi: 10.1542/peds.2007-0383. [DOI] [PubMed] [Google Scholar]
- 26.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 27.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343(6):378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 28.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35 Suppl 2(Journal Article):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 29.Auvin S, Shin D, Mazarati A, Sankar R. Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia. 2010;51 Suppl 3:34–38. doi: 10.1111/j.1528-1167.2010.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vries EE, van den Munckhof B, Braun KPJ, van Royen-Kerkhof A, de Jager W, Jansen FE. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;63:177–190. doi: 10.1016/j.neubiorev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Ong M-S, Kohane IS, Cai T, Gorman MP, Mandl KD. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014;71(5):569–574. doi: 10.1001/jamaneurol.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Z, Li W, Qu B, et al. Serum brain-derived neurotrophic factor levels in epilepsy. Eur J Neurol. 2014;21(1):57–64. doi: 10.1111/ene.12232. [DOI] [PubMed] [Google Scholar]
- 33.Prince DA, Gu F, Parada I. Antiepileptogenic repair of excitatory and inhibitory synaptic connectivity after neocortical trauma. Prog Brain Res. 2016;226:209–227. doi: 10.1016/bs.pbr.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Marder E, Goaillard J-M. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7(7):563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010;39(1):53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dworetzky BA, Townsend MK, Pennell PB, Kang JH. Female reproductive factors and risk of seizure or epilepsy: data from the Nurses’ Health Study II. Epilepsia. 2012;53(1):1528–1167. doi: 10.1111/j.1528-1167.2011.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]