Abstract
Kidney transplantation has become more resource intensive as recipient complexity has increased and average donor quality has diminished over time. A national retrospective cohort study was performed to assess the impact of kidney donor and recipient characteristics on transplant center cost (exclusive of organ acquisition) and Medicare reimbursement. Data from the national transplant registry, University HealthSystem Consortium hospital costs, and Medicare payments for deceased donor (N=53,862) and living donor (N=36,715) transplants from 2002–2013 were linked and analyzed using multivariate linear regression modeling. Deceased donor kidney transplant costs were correlated with recipient (Expected Post Transplant Survival Score, degree of allosensitization, obesity, cause of renal failure) donor (age, cause of death, donation after cardiac death, terminal creatinine), and transplant (histocompatibility matching) characteristics. Living donor costs rose sharply with higher degrees of allosensitization, and were also associated with obesity, cause of renal failure, recipient work ability, and 0-ABDR mismatching. Analysis of Medicare payments for a subsample of 24,809 transplants demonstrated minimal correlation with patient and donor characteristics. In conclusion, the complexity in the landscape of kidney transplantation increases center costs, posing financial disincentives that may reduce organ utilization and limit access for higher risk populations.
INTRODUCTION
The practice of kidney transplantation has become more complex as a result of changing recipient demographics, declining organ quality, and broader application of treatments to address allosensitization. In the US, there has been a 250% increase in the proportion of patients older than age 65 years old at the time of transplantation.(1) In the US, elderly patients (>65) now represent over a quarter of all transplant recipients.(2) While clinically successful, kidney transplant in the elderly is associated with a marked increase in the incidence of perioperative complications and extended length of stay.(3, 4) Similarly, the prevalence of obesity, diabetes, coronary artery disease, and peripheral vascular disease have increased as transplant eligibility criteria evolve to reflect the changing nature of the end stage renal disease (ESRD) population. Equally challenging is the increasing prevalence of transplant recipients with high levels of allosensitization. Renal transplant in patients with panel reactive antibody (PRA) or calculated PRA (cPRA) levels greater than 80 than have increased by over 300% from 2000 to 2013, a trend that has accelerated with the new US allocation system.(5) These highly sensitized patients require an increased intensity of peri-operative treatment including the need for plasmapheresis, intravenous immunoglobulin, and high cost induction therapy.(6) Despite a higher incidence of peri-operative complications, post-transplant survival in the highly sensitized patient is excellent and markedly improved compared to patients on dialysis. (6, 7)
Increased resource utilization has also been driven by the ongoing shortage of high quality donor organs. Nationally, the kidney transplant waiting list has expanded to over 100,000 patients, while deceased donor availability has only marginally increased and living donor transplants are diminishing.(1) To meet this demand, transplant programs now use donor kidneys with high terminal creatinine, organs from older donors, and organs donated after cardiac death (DCD).(8, 9) These kidneys are life-saving when used in the appropriate patient population, yet each has been associated with higher rates of delayed graft function, rehospitalization, need for pulsatile perfusion, and use of cell depleting antibody therapy , all of which may add cost to the transplant episode.(10, 11)
Prior economic analyses of kidney transplantation have focused on the global cost effectiveness of renal care. When compared with dialysis, renal transplant has been shown to improve outcomes and reduce long term medical expenditures even for high risk patients.(12–14) These savings, unfortunately, accrue over time and the cost of the initial transplant procedure is borne by the transplant center which does not benefit from long term cost savings. Current reimbursement for kidney transplant under the Medicare program in the United States, which is the largest payer for transplant services in the world, has been generally static over the past ten years. Kidney transplant centers are paid based on a single diagnosis related group (DRG) which, unlike heart or liver transplant, is not adjusted for recipient complexity or donor factors. Consequently, increase in the direct cost of transplant care for higher risk patients unaccompanied by increased payment may threaten the economic viability of many transplant programs.(15, 16)
The purpose of this investigation was to examine the impact of the changing donor and recipient characteristics on cost and payments in a national sample of US kidney transplant programs. Using a novel database incorporating linked cost accounting data, transplant registry data, and third party payer data, we performed the first national analysis of the financial implications of the increasing complexity of kidney transplant care in the modern era.
METHODS
Data Sources
A nationally representative database was created by linking clinical and demographic information from the national transplant registry with financial data provided by the University HealthSystem Consortium (UHC) for renal transplants (N=90,577) performed between 2002–2013. This study used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere (17). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The UHC data is drawn from 105 academic transplant centers within the United States. UHC data includes patient level claims data from administrative billing claims submissions, adjusted to costs using the transplant hospital’s Medicare cost to charge ratio and adjusted for geographic differential in wages. Transplant records were linked using date of transplant, age, and gender, as previously describe. The UHC population is similar to the overall US kidney renal transplant population . To eliminate misclassified cases and clinical outliers, we restricted the analysis to patients with a minimal cost of $30,000 including organ acquisition to eliminate non-transplant admissions, and a maximum cost of $200,000 which represented the 98th percentile for costs. Cost was adjusted to 2013 dollars using the healthcare consumer price index.
Medicare payments were then obtained for patients with Medicare fee for service insurance who underwent kidney transplant between 2002–2013 at a UHC center. To merge OPTN and Medicare data, beneficiary identifiers from Medicare files were linked to OPTN records using Social Security number, gender, and date of birth. The merge and data cohort generation transplant analysis was described in previous publications.(18) For this cohort, we required a minimum payment of $10,000 (excluding organ acquisition cost center [OAC]) to eliminate non-transplant admissions. Because Medicare claims do not include OAC payment (which is paid via the institutional cost report), Medicare payments reflect only the reimbursement obtained through the transplant DRG and any outlier payments. Consequently, direct calculation of hospital margin is not possible using these data as cost accounting systems and allocation of costs to the OAC may vary over time and between institutions. Medicare payment estimates were also adjusted to account for differential payments by Medicare to transplant programs in Maryland due to that state’s Medicare payment waiver by removing costs recorded as related to OAC for these centers.
Variable Definitions
We calculated the Expected-Post Transplant Survival (EPTS) and Kidney Donor Profile Index (KPDI) score as described by the SRTR.(19) Patients were classified on the basis of body mass index (BMI, kg/m2) as underweight (BMI <18.5, normal(18.5 to < 25), overweight (25 to <30), obese (30 to <35), and morbidly obese (> 35). To adjust for the degree of allosensitization over the duration of the study, we incorporated several different measures into the “PRA” variable. When available, we used the calculated panel reactive antibody (cPRA) recorded at the time of transplant. For patients transplanted prior to routine use of cPRA, we incorporated the PRA at the time of transplant when available or the peak PRA if no PRA at transplant was recorded.(20) Other variables including cause of ESRD, cause of donor death, and payer definitions were based on OPTN records.
Cost model regression analysis
Multivariable linear regression was used to estimate the impact of donor and recipient characteristics on center costs and Medicare payment. Total transplant cost inclusive of OAC was examined as this represents the total cost of transplant care delivered at the center. This cost is relevant for centers receiving private insurance reimbursement as reimbursement for OAC is frequently included in the global reimbursement.
Initial cost modeling was performed using EPTS and KPDI index values. While EPTS was found to correlate with overall cost, the KDPI in aggregate did not (p>.010). However, component variables within KPDI were significant in the multivariate analysis, and were retained in the analysis. Variables were retained in the model if the p value for its association, or for a level of a categorical factor, was less than 0.10 or the variable was part of a set of mutually exclusive options (e.g. diagnosis). Using the cost models to adjust for donor and recipient characteristics, we then estimated the variation in the ratios of observed to expected in cost and profitability for transplant centers nationally.
Data management and analysis was performed using SAS 9.4 software (SAS Institute, Cart, NC) and R 3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Approvals
This study was approved by the Institutional Review Board at Saint Louis University School of Medicine. The project was also reviewed and approved by the Centers for Medicare and Medicaid Services, HRSA, and UHC.
RESULTS
The risk profile of kidney transplant recipients cared for in UHC member hospitals has changed dramatically between 2002–2003 and 2012–2013 (Table 1). Among deceased donor recipients (N=53,862), the median age at transplant has increased from 51 to 55 years, while the percent of patients over than 60 years has increased by 30%. In the more recent era, there are more patients transplanted with diabetes (25.5% vs. 23.0%), and the proportion of patients with more than 5 years of dialysis time has risen (29.0% vs. 22.5%). Consequently, the prevalence of patients with an EPTS score > 85 has increased (26.9% vs. 20.3%). UHC facilities are also transplanting substantially more patients with high levels of allosensitization defined as PRA > 80% (9.2% vs. 5.0%) and > 97% (1.8% vs. 0.9%). Similar changes are evident in the recipients of living donor transplants (N=36,715) who are older (median age 49 vs. 46) and have greater degrees of allosensitization (PRA > 80: 2.5% vs. 1.1%).
Table 1.
Demographic and clinical characteristics of transplant recipients, by donor type, among patients transplant in centers within the University HealthSystem Consortium are presented for the overall population and for patients transplant between 2002–3 and 2012–13.
| UHC Total | 2002–2003 | 2012–2013 | UHC | 2002–2003 | 2012–2013 | |
|---|---|---|---|---|---|---|
| N=53,862 | N=10,558 | N=27,738 | N=36,715 | N=8,415 | N=18,408 | |
| % (Mean+SD) |
% (Mean+SD) |
% (Mean+SD) |
% (Mean+SD) |
% (Mean+SD) |
% (Mean+SD) |
|
| EPTS Score Rank | ‡ | ‡ | ||||
| 0–20 | 24.3 | 29.8 | 23.6 | 44.6 | 31.6 | 30 |
| 21–50 | 33.2 | 34.6 | 33.0 | 33.6 | 23.2 | 21.9 |
| 51–85 | 31.1 | 28.5 | 31.5 | 19.1 | 21.2 | 20.8 |
| 85–100 | 11.4 | 7.2 | 12.0 | 2.7 | 16 | 17.1 |
| Female Gender | 39.4 | 39.5 | 39.1 | 39.8 | 42 | 39.5† |
| Race | ‡ | ‡ | ||||
| Caucasian | 47.3 | 51.7 | 47 | 69.5 | 71.6 | 69.2 |
| African-American | 32.9 | 30.2 | 33.1 | 14.1 | 13.8 | 14.3 |
| Other | 19.8 | 18.2 | 19.9 | 16.3 | 14.6 | 16.5 |
| BMI (kg/m2) | ‡ | ‡ | ||||
| <18.5 | 2.1 | 2.6 | 2.1 | 2.8 | 3.1 | 2.8 |
| 18.5–24.9 | 30.7 | 34.9 | 30.2 | 34.2 | 37.1 | 33.8 |
| 25–29.9 | 33.6 | 33 | 34 | 31.9 | 30.5 | 32.3 |
| 30–35 | 22.1 | 18.8 | 22.4 | 19.8 | 17.4 | 20.2 |
| >35 | 10.7 | 8.3 | 10.7 | 9.5 | 7.7 | 9.7 |
| Unknown | 0.8 | 2.4 | 0.6 | 1.7 | 4.2 | 1.2 |
| Payer Type | ‡ | |||||
| Medicare | 65.2 | 61.6 | 66.5 | 35.5 | 35.8 | 35.3 |
| Private | 29.4 | 32.7 | 28.2 | 59.8 | 59.2 | 60.1 |
| Other | 5.4 | 5.7 | 5.3 | 4.7 | 5.0 | 4.6 |
| Cause of ESRD | ‡ | ‡ | ||||
| Diabetes | 25.3 | 23 | 25.5 | 21 | 21.4 | 20.9 |
| Glomerulonephritis | 19.1 | 19.8 | 18.7 | 25.1 | 24.9 | 25 |
| Hypertension | 25.2 | 23.2 | 25.7 | 16.7 | 14.9 | 17.3 |
| Polycystic kidney disease | 8.1 | 7.8 | 8.2 | 10.9 | 9.7 | 11.1 |
| Other | 22.4 | 26.2 | 22 | 26.3 | 29.2 | 25.8 |
| Peripheral vascular diseases | 4 | 3.5 | 4.4‡ | 3.3 | 3.5 | 3.2 |
| Previous transplant | 12.2 | 11.9 | 12.3 | 10.4 | 10.1 | 10.4 |
| Work Status | ‡ | ‡ | ||||
| Working | 20.6 | 6.2 | 24 | 35.9 | 11.4 | 42.9 |
| Not Working | 60 | 16.6 | 69.6 | 45.1 | 15.8 | 53.5 |
| Unknown | 19.5 | 77.2 | 6.4 | 19 | 72.7 | 3.6 |
| Panel Reactive Antibody (PRA,cPRA) | ‡ | ‡ | ||||
| 0–20 | 77.7 | 85.6 | 77.9 | 90 | 95.1 | 89.8 |
| 21–50 | 7.1 | 5.3 | 7.2 | 4.4 | 2.2 | 4.6 |
| 51–80 | 5.7 | 4.2 | 5.7 | 3.2 | 1.6 | 3.2 |
| 81–90 | 4.4 | 2.4 | 4.2 | 1 | 0.5 | 0.9 |
| 91–97 | 3.3 | 1.7 | 3.2 | 0.8 | 0.4 | 0.9 |
| 98–100 | 1.8 | 0.9 | 1.8 | 0.7 | 0.3 | 0.7 |
| Age (Mean) | 38.6 (16.8) | 38.1 (16.9) | 38.7 (16.7)* | 41.5 (11.5) | 40.4 (10.9) | 41.6 (11.5)‡ |
| Female Gender | 40.2 | 41.8 | 39.6‡ | 60 | 58.3 | 59.9* |
| Race | ‡ | * | ||||
| Caucasian | 70.8 | 73.4 | 70.4 | 72.3 | 73.4 | 72.1 |
| African-American | 13.9 | 12.2 | 14.3 | 12.3 | 12.7 | 12.4 |
| Other | 15.4 | 14.4 | 15.3 | 15.4 | 14 | 15.5 |
| Diabetes mellitus | 52.7 | 14.7 | 60.3‡ | 0.1 | 0.02 | 0.05 |
| Diabetes mellitus missing information | 47.3 | 85.3 | 39.7‡ | 99.9 | 99.98 | 99.95 |
| Hypertension | 40.3 | 34.1 | 41.6‡ | 2.1 | 0.3 | 2.4‡ |
| Donation after cardiac death | 12.1 | 5.2 | 13.3‡ | -- | -- | -- |
| Donor Cause of Death | ‡ | |||||
| Cerebrovascular/Stroke | 39.5 | 45.5 | 38.1 | -- | -- | -- |
| Anoxia | 22.1 | 12.4 | 23.4 | -- | -- | -- |
| Head Trauma | 36.1 | 40.3 | 36 | -- | -- | -- |
| CNS Tumor | 0.3 | 0.4 | 0.3 | -- | -- | -- |
| Other/Unknown | 2 | 1.6 | 2.1 | -- | -- | -- |
| HLA mismatches | ‡ | ‡ | ||||
| Zero A, B, and DR | 12.3 | 16.6 | 13.1 | 8.8 | 9.9 | 8.4 |
| Zero DR | 15.7 | 16.6 | 15.3 | 17.7 | 17.6 | 17.8 |
| Cytomegalovirus sero-pairing | ‡ | ‡ | ||||
| Donor − / Recipient − | 12.1 | 11.6 | 12.3 | 22.3 | 20.8 | 22.5 |
| Donor − / Recipient + | 24 | 23.4 | 24.1 | 19.6 | 18.5 | 19.4 |
| Donor + / Recipient − | 17.8 | 16.6 | 18.1 | 15 | 13.9 | 15 |
| Donor + / Recipient + | 41.9 | 39.9 | 41.4 | 32.6 | 32.1 | 32 |
| Donor Terminal Creatinine | * | |||||
| Creatinine (Mean) | 1.1 (0.9) | 1.07 (1.01) | 1.14 (0.88)‡ | -- | -- | -- |
| Missing | 0.1 | 0.2 | 0.04‡ | -- | -- | -- |
| Pumped | 40.8 | 26.0 | 43.4‡ | -- | -- | -- |
| Living Donor / Recipient Relationship | ‡ | |||||
| Unrelated | -- | -- | -- | 29.8 | 22.0 | 30.8 |
| Biologically Related | -- | -- | -- | 56.7 | 64.8 | 55.7 |
| Other Related | -- | -- | -- | 13.5 | 13.3 | 13.5 |
| Paired | -- | -- | -- | 4.2 | 0.5 | 4.6‡ |
P-values:
P 0.002–0.04;
P 0.0001–0.001;
P < 0.0001 comparing 2002–3 with 2012–3
During the study period, the characteristics of the donor population and management of immunosuppression also changed, including increased utilization of deceased donor organs with higher risk characteristics including older age, higher creatinine, and a greater proportion of DCDs (P<0.0001). There has also been a dramatic increase in the portion of kidney transplants which were placed on pulsatile perfusion, received cell depleting antibodies for induction, and had a history of diabetes.
Despite the increasing complexity of this population, transplant was highly successful. Unadjusted one-year graft survival increase from 89.7% in 2002–03 to 91.5% in 2012–13 for deceased donor transplant, and from 95.2% to 96.4% for living donor transplants. There was, however, a higher incidence of delayed graft function (DGF) in deceased donor transplants (25.1% vs 22.9%).
Cost Analysis
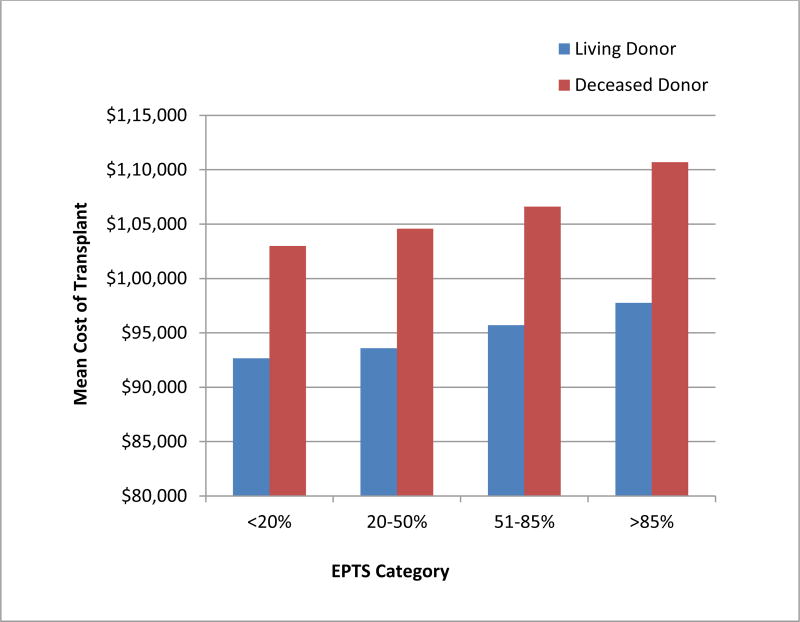
During the study period, the median total reported cost per deceased donor transplant (N= 53,862) performed at a UHC center increased from $97,892 in 2002–2003 to $106,675 in 2012–2013, after adjustment to 2013 dollars. This estimate includes OAC and perioperative care for the initial hospitalization. Univariate analysis revealed a strong association between recipient EPTS and the cost of the transplant procedure (p<0.0001) (Figure 1).
Figure 1.
Univariate analysis of the variation in the median total cost of living donor and deceased donor transplant procedures performed in University HealthSystem Consortium centers across Expected Post Transplant Survival (EPTS) quartiles.
Multivariate modeling confirmed a strong association between recipient characteristics and cost of deceased donor transplant exclusive of organ acquisition cost (Table 2). Recipient EPTS was strongly associated with cost in the multivariate model. Compared to patients with EPTS < 20, EPTS resulted in significant, graded increased in incremental costs (20–50: $1,095; 50–85 $2,292; >85: $5,257; p<0.005 for all). Higher cPRA levels had strong, graded associations with higher transplant costs such that a cPRA 98–100 was associated with $9,097 of incremental expenditure (P<0.0001). Recipient characteristics associated with lower per transplant costs include polycystic kidney disease, female gender, and working at the time of transplant.
Table 2.
Multivariate analysis of the costs of transplantation among transplants performed at University HealthSystem Consortium centers (2002–2013)
| Deceased Donor | Living Donor | |||
|---|---|---|---|---|
| Parameter | Estimate | P-Value | Estimate | P-Value |
| Recipient Characteristics | ||||
| EPTS Score Rank | ||||
| 0–20 | Reference | |||
| 21–50 | $1,096 | 0.005 | −$461 | 0.25 |
| 51–85 | $2,292 | <.0001 | $278 | 0.61 |
| 85–100 | $5,257 | <.0001 | $1,312 | 0.23 |
| Female | −$1,589 | <.0001 | −$469 | 0.18 |
| Race | ||||
| Caucasian | Reference | |||
| Black | −$434 | 0.23 | $2,011 | 0.03 |
| Other | $6,588 | <.0001 | −$997 | 0.17 |
| BMI | ||||
| <18.5 | $1,819 | 0.075 | −$95 | 0.93 |
| 18.5–24.9 | Reference | |||
| 25–29.9 | $1,062 | 0.003 | $587 | 0.16 |
| 30–35 | $2,292 | <.0001 | $1,814 | 0.0001 |
| >35 | $2,037 | 0.0001 | $2,139 | 0.001 |
| Unknown | $4,420 | 0.006 | $10,263 | <.0001 |
| Cause of Disease | ||||
| Diabetes | Reference | |||
| Hypertension | $1,280 | 0.004 | $2,582 | <.0001 |
| Glomerulonephritis | −$451 | 0.36 | −$1,980 | 0.0006 |
| Polycystic Kidney Disease | −$1,776 | 0.005 | −$2,105 | 0.002 |
| Other | −$277 | 0.56 | −$690 | 0.21 |
| Peripheral Vascular disease | −$436 | 0.56 | $2,231 | 0.02 |
| Working at Transplant | ||||
| No | Reference | |||
| Yes | −$1,923 | <.0001 | −$2,690 | <.0001 |
| Unknown | −$4,819 | <.0001 | −$7,594 | <.0001 |
| PRA/cPRA | ||||
| 0–20 | Reference | |||
| 21–50 | $3,638 | <.0001 | $4,700 | <.0001 |
| 51–80 | $5,558 | <.0001 | $8,080 | <.0001 |
| 81–90 | $5,002 | <.0001 | $10,355 | <.0001 |
| 91–97 | $8,785 | <.0001 | $13,230 | <.0001 |
| 98_100 | $9,097 | <.0001 | $17,784 | <.0001 |
| Donor Characteristics | ||||
| Age (per year) | $62 | <.0001 | $3 | 0.84 |
| Female | −$717 | 0.02 | −$818 | 0.02 |
| Race | ||||
| Caucasian | Reference | |||
| Black | −$1,511 | 0.001 | $789 | 0.42 |
| Other | $2,595 | <.0001 | $1,426 | 0.05 |
| Diabetes | $3,370 | <.0001 | −$9,713 | 0.19 |
| Hypertension | $665 | 0.04 | $1,610 | 0.17 |
| Donation after Cardiac Death | $6,182 | <.0001 | -- | -- |
| Cause of death | ||||
| Anoxic Injury | Reference | |||
| Cerebrovascular Accident | −$3,040 | <.0001 | -- | -- |
| Head Trauma | −$2,322 | <.0001 | -- | -- |
| CNS Tumor | −$980 | 0.71 | -- | -- |
| Other | −$1,618 | 0.12 | -- | -- |
| HLA 0 Mismatch | −$4,332 | <.0001 | −$3,799 | <.0001 |
| HLA 0-DR Mismatch | −$2,968 | <.0001 | $426 | 0.34 |
| CMV Donor-Recipient Mismatch | ||||
| Recipient Neg / Donor Neg | Reference | |||
| Recipient Pos / Donor Neg | $1,110 | 0.03 | $1,274 | 0.01 |
| Recipient Neg / Donor Pos | $784 | 0.15 | −$894 | 0.11 |
| Recipient Neg / Donor Pos | $67 | 0.90 | −$947 | 0.05 |
| Missing | −$935 | 0.25 | −$826 | 0.19 |
| Terminal Creatinine (mg/dl) | $956 | <.0001 | -- | -- |
| Donor Creatinine Missing | $14,115 | 0.0118 | -- | -- |
| Pumped | −$2,039 | <.0001 | ||
| Donor-Recipient Relationship | ||||
| Unrelated | Reference | |||
| Related Biologically | -- | -- | −$852 | 0.04 |
| Related-Other | -- | -- | −$389 | 0.49 |
| Paired | -- | -- | $3,330 | 0.0002 |
Donor quality also had a significant impact on the expected cost of care. Among deceased donor transplants, older age ($62 per year, p<0.0001), death due to anoxic injury, elevated terminal creatinine ($956 per mg/dl increase, p<0.0001), and DCD donation ($6182 p<0.0001) were independently associated with higher costs. Costs were lower with 0-DR (−$2,968, p<0.0001) and 0-ABDR mismatch total (−$4,332, p<0.0001) HLA antigen mismatches. Finally, transplants using kidneys placed on a pulsatile perfusion pump were $2,039 less expensive (p<0.0001).
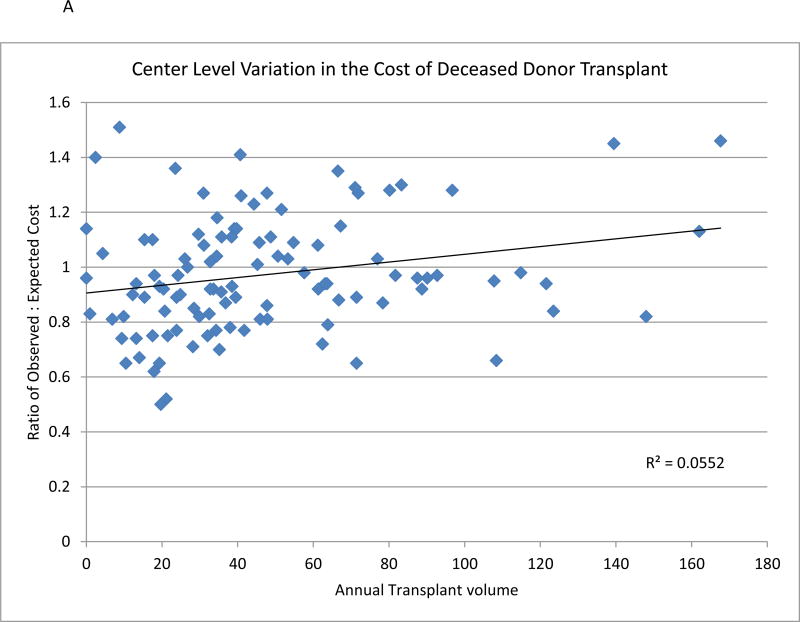
Among UHC centers, the unadjusted total cost per case (median $89,208, 25–75% percentiles: $74,361-$99,746) varied extensively. After adjustment for donor and recipient factors, center level variation persisted with an observed-to-expected cost ratio varied from 0.50 to 1.51 for deceased donors. The ratio of observed-to-expected costs appears to be minimally associated with center volume (P<0.02) (Figure 2A). Analysis at a regional level demonstrated higher than expected costs for deceased donor transplant performed in region 5, 7, and 10. Cost was lower in regions 1, 3, and 11. (Table 3).
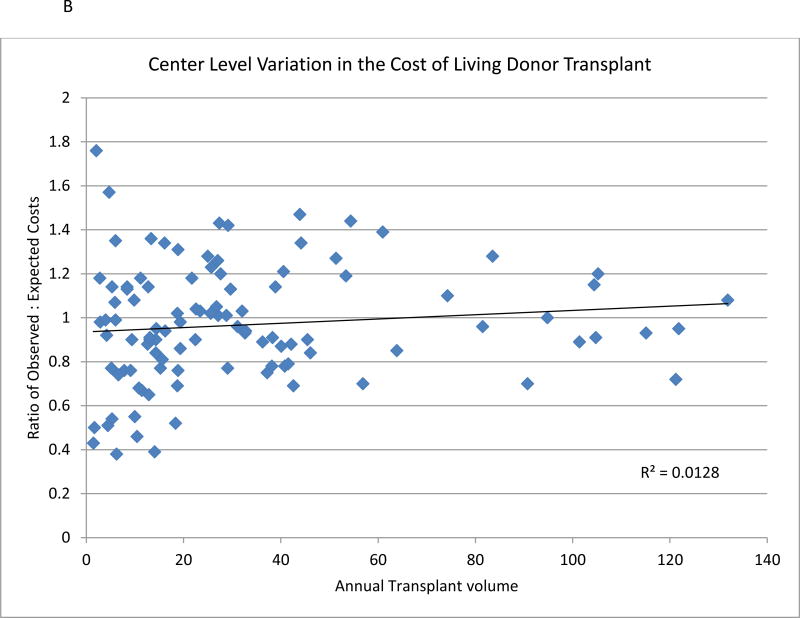
Figure 2.
A and B: Association between observed cost of renal transplant center and risk adjusted expected costs for deceased donor kidney transplant (Figure 2A) and living donor (Figure 2B) compared with average center annual volume of kidney transplant.
Table 3.
Variation in the ratio of Observed:Expected (O:E) cost of deceased and living donor kidney transplant by UNOS region. Cost was adjusted for donor, recipient, and transplant factors using multivariate modeling.
| Deceased Donor | Living Donor | |||
|---|---|---|---|---|
| Regions | Predicted Cost | O:E Cost | Predict Cost | O:E Cost |
| Region 1 | $106,486 | 0.83 | $93,553 | 0.90 |
| Region 2 | $105,474 | 1.05 | $94,048 | 1.17 |
| Region 3 | $103,108 | 0.93 | $93,785 | 0.93 |
| Region 4 | $106,384 | 0.96 | $94,598 | 0.93 |
| Region 5 | $108,441 | 1.08 | $92,964 | 0.98 |
| Region 6 | $105,743 | 1.02 | $91,422 | 1.02 |
| Region 7 | $105,480 | 1.07 | $93,666 | 1.01 |
| Region 8 | $105,846 | 1.01 | $93,132 | 1.10 |
| Region 9 | $105,585 | 0.93 | $93,677 | 0.85 |
| Region 10 | $104,288 | 1.13 | $95,109 | 1.14 |
| Region 11 | $104,144 | 0.85 | $93,704 | 0.86 |
Nationally, 36,715 living donor transplants were performed in UHC centers between 2002–2013. The average cost increased over the period from $86,914 in 2002–2003 to $95,463 in 2012–2013. Higher EPTS was associated with a non-statistically significant increase in costs. Recipient factors associated with higher cost included live donor transplantation included African American race, higher BMI and increased cPRA. (Table 2b). Increasing allosensitization dramatically increases costs, such that a cPRA of 98–100 was associated with $17,784 in additional cost compared with transplant in patients with a PRA of 0–20 (p<0.0001). Polycystic kidney disease and glomerular disease (compared with diabetes), working at the time of transplant, and 0-ABDR mismatch were all associated with lower costs. Female living donors were associated with modest decreases in costs. Substantial variation in average risk-adjusted cost was also noted among living donor transplant (Figure 2B). Regionally, the ratio of observed to expected living donor transplant cost was highest in region 2 (Michigan, Ohio, Indiana) and lowest in region 11 (Southeast US) (Table 3).
Payment
Medicare payment data were available for 24,765 deceased donor kidney recipients, representing 45.9% of the transplants performed at a UHC hospital. Median CPI adjusted payment for renal transplant, not including organ acquisition payment, decreased from 2002–2003 ($40,222) to 2012–2013 ($34,227). Multivariate modeling demonstrated some association in Medicare payments and recipient characteristics. (Table 4A) Risk adjusted payment was $1,626 higher for patients with EPTS 51–85, and $2,475 dollars greater for EPTS greater than 85. Payments were increased for African Americans and those of other race/ethnicities. There was no association between transplant payments and cPRA. Medicare payments were higher for recipients with donors who were older or had elevated terminal creatinine ($279 per mg/dl, P=0.01), but not significantly different for recipients of DCD organs (p=0.34). They were lower for recipients of kidneys from pumped organs (−1,768, P<0.0001) and diabetic donors (−$1046, P<0.0001).
Table 4.
Association of donor and recipient characteristics with Medicare payments for kidney transplantation (2002–2013)
| Deceased Donor | Living Donor | |||
|---|---|---|---|---|
| Parameter | Estimate | P-Value | Estimate | P-Value |
| Recipient Characteristics | ||||
| EPTS Score Rank | ||||
| 0–20 | Reference | |||
| 21–50 | $925 | 0.0008 | $588 | 0.29 |
| 51–85 | $1,626 | <.0001 | $1,198 | 0.06 |
| 85–100 | $2,475 | <.0001 | $1,104 | 0.29 |
| Female | −$123 | 0.55 | $1,133 | 0.01 |
| Race | ||||
| Caucasian | Reference | |||
| Black | $1,218 | <.0001 | $1,685 | 0.09 |
| Other | $1,454 | <.0001 | −$823 | 0.34 |
| BMI | ||||
| <18.5 | $1,571 | 0.02 | $932 | 0.46 |
| 18.5–24.9 | Reference | |||
| 25–29.9 | −$369 | 0.13 | $595 | 0.26 |
| 30–35 | −$189 | 0.49 | −$278 | 0.65 |
| >35 | $526 | 0.13 | $808 | 0.33 |
| Unknown | $4,969 | <.0001 | $17,872 | <.0001 |
| Cause of Disease | ||||
| Diabetes | Reference | |||
| Hypertension | $71 | 0.81 | $190 | 0.78 |
| Glomerulonephritis | $1,646 | <.0001 | $587 | 0.42 |
| Polycystic Kidney Disease | −$135 | 0.76 | −$1,258 | 0.22 |
| Other | $2,137 | <.0001 | $3,000 | <.0001 |
| Peripheral Vascular disease | $542 | 0.26 | −$1,096 | 0.28 |
| Working at Transplant | ||||
| No | Reference | |||
| Yes | $89 | 0.74 | $1,221 | 0.03 |
| Unknown | $7,128 | <.0001 | $6,360 | <.0001 |
| PRA/cPRA | ||||
| 0–20 | Reference | |||
| 21–50 | −$231 | 0.54 | $123 | 0.90 |
| 51–80 | $0 | 0.99 | $1,983 | 0.07 |
| 81–90 | −$631 | 0.19 | $899 | 0.65 |
| 91–97 | −$110 | 0.83 | $1,829 | 0.37 |
| 98_100 | −$70 | 0.92 | $4,709 | 0.01 |
| Donor Characteristics | ||||
| Age (per year) | $26 | 0.0002 | $31 | 0.11 |
| Female | −$343 | 0.10 | −$860 | 0.05 |
| Race | ||||
| Caucasian | Reference | |||
| Black | $724 | 0.01 | $912 | 0.39 |
| Other | $1,201 | <.0001 | $2,263 | 0.01 |
| Diabetes | −$1,046 | <.0001 | −$1,331 | 0.88 |
| Hypertension | −$209 | 0.32 | $307 | 0.84 |
| Donation after Cardiac Death | $299 | 0.34 | -- | -- |
| Cause of death | ||||
| Anoxic Injury | Reference | |||
| Cerebrovascular Accident | $673 | 0.01 | -- | -- |
| Head Trauma | −$149 | 0.58 | -- | -- |
| CNS Tumor | $6,585 | 0.0003 | -- | -- |
| Other | $637 | 0.36 | -- | -- |
| HLA 0 Mismatch | −$269 | 0.43 | −$1,165 | 0.17 |
| HLA 0-DR Mismatch | −$943 | 0.0006 | $588 | 0.30 |
| CMV Donor-Recipient Mismatch | ||||
| Recipient Neg / Donor Neg | Reference | |||
| Recipient Pos / Donor Neg | $571 | 0.11 | $328 | 0.64 |
| Recipient Neg / Donor Pos | $5 | 0.99 | $744 | 0.34 |
| Recipient Neg / Donor Pos | $796 | 0.01 | $1,104 | 0.09 |
| Missing | $1,855 | 0.001 | $666 | 0.44 |
| Terminal Creatinine (mg/dl) | $279 | 0.01 | -- | -- |
| Donor Creatinine Missing | $3,174 | 0.42 | -- | -- |
| Pumped | −$1,768 | <.0001 | ||
| Donor-Recipient Relationship | ||||
| Unrelated | Reference | |||
| Related Biologically | -- | -- | $296 | 0.58 |
| Related-Other | -- | -- | $2,397 | 0.003 |
| Paired | -- | -- | $5,956 | <.0001 |
Medicare claims were reviewed for 8,165 living donor recipients, representing 22.2% of all living donor transplants performed at UHC centers. Medicare payments were uncorrelated with recipient EPTS score (Table 3B). Characteristics associated with higher payments included female gender and working at transplant. Medicare payments did increase with PRA, such that the highest PRA (98–100) was associated with an incremental payment of $4,709. Donor factors associated with higher payments included age of the donor, donors of “other race”, and participation in paired donor exchange ($5,956, P<0.0001).
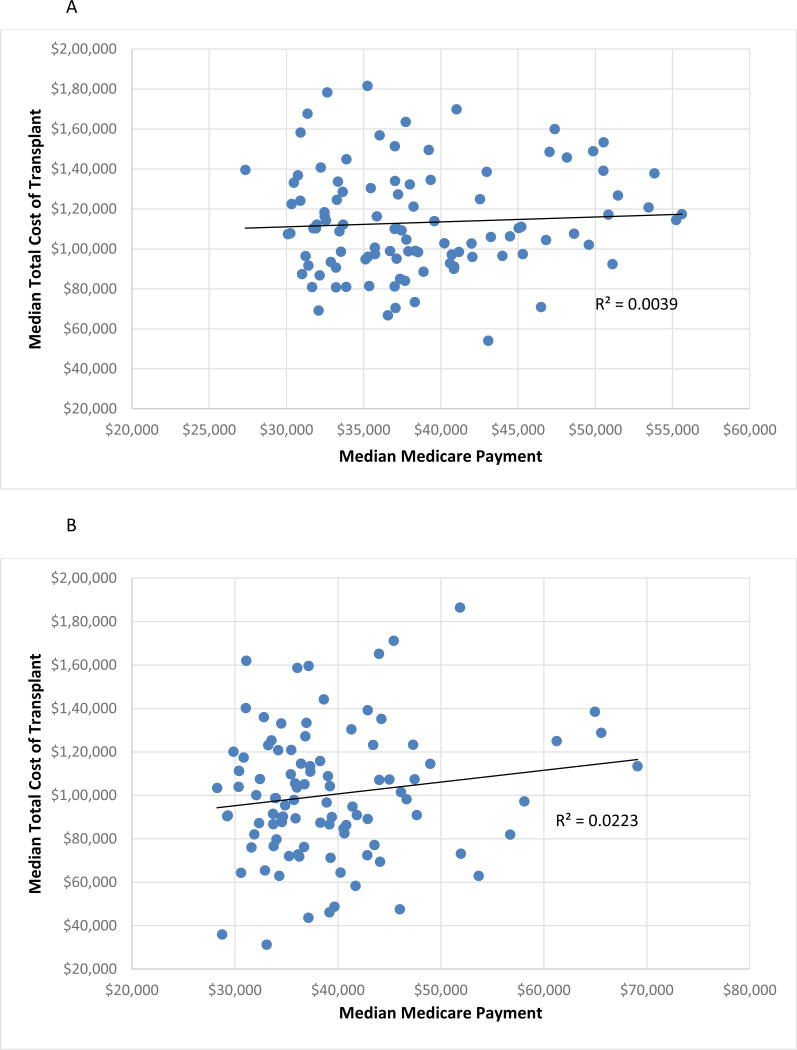
Analysis of regional variation in Medicare payments demonstrated higher than expected payments in three UNOS regions ( 5, 7, and 10) and lower than expected payments in two regions (1 and 11). Average payment for a deceased donor transplant varied by 18.9% ($37,019 to $45,570) across centers. Living donor transplant payment varied by 13.7% nationally $38,700-$44,868). Average payment and total cost of transplant were not correlated for either deceased or living donor transplant (Figure 3).
Figure 3.
Comparison of median center total cost of the transplant episode including organ acquisition cost and Medicare payment exclusive of organ acquisition cost for deceased (Figure 3A) and living (Figure 3B) donor transplant.
Discussion
This national cohort analysis of the changing financial aspects of kidney transplant demonstrates the tremendous financial challenge facing US transplant programs as a result of the changing composition of the renal transplant population. Patients are older, have a greater burden of comorbid conditions, present with more dialysis time, and have higher levels of allosensitization. As a result, the prevalence of patients with elevated EPTS scores has increased dramatically. Similarly, donor organs clearly have higher risk characteristics including DCD donation, high terminal creatinine, donor diabetes, and longer cold ischemic times. This study provides the first national, risk adjusted data which quantifies the association between hospital costs and these donor and recipient factors. We demonstrate a marked increase in hospital costs for transplantation of non-standard donor organs and high risk recipients. Furthermore, there was a very poor correlation between Medicare payments and high cost characteristics as DRG payments are not adjusted for patient or donor risk, unless centers reach outlier status. Consequently, transplant margins will be reduced for centers that transplant these higher risk patients.
Previous clinical economic analyses of renal transplantation have demonstrated a substantial lifetime benefit of renal transplantation, compared with life time dialysis, even using non-standard donor organs. Using Medicare data, Whiting and colleagues demonstrated a cost savings for the Medicare program, although this was mediated in part for donor and recipient characteristics.(21) The economic breakeven point range from 4.4 years for non-expanded criteria donor (ECD)/low- risk donors to 13 years for ECD. Subsequent analyses by Matas and Schnitzler in 2004, have valued the direct cost savings associated with living donor transplant at $94,579, which coupled with a net gain of 3.5 quality adjusted life years (QALYs), suggests an overall benefit of $263,319 per transplant.(22) More recently, a comprehensive economic model suggested that living kidney donation was associated with societal benefits of $1.4 million per transplant, if the economic benefit of improved quality of life, added years in the workforce, and reduced life time dialysis costs were included in the analysis.(14) However, current payment models for transplant centers focus only on the cost of the transplant episode; consequently, the long term cost savings derived from transplantation of higher risk candidates is not shared with transplant programs.
While the long term societal benefits of kidney transplant are clear, the implications of the changing nature of the donor and recipient population on transplant center finances is more challenging. Engelsbe and colleagues performed a single center evaluation of transplant finances at the University of Michigan from 1999–2005.(15) In a multivariate analysis, reduced hospital margins were associated with ECD transplant, year of transplant, and the development of delayed graft function. Recipient and donor characteristics including race, gender, history of diabetes, age, and BMI were also associated with lower costs in univariate analysis; however, given the limited sample size, these associations were not significant in multivariate analyses. In a second single center analysis, Saidi et al. reported a marked increase in the incidence of DGF in ECD recipients (35.6% vs. 15.1%) and a corresponding 48% increase in hospital charges due to longer lengths of stay and greater need for inpatient dialysis. More recently, Stahl et al. examined UHC data from 2009–12. In this limited data set, they demonstrated that ECD transplantation was associated with a higher 30 day readmission rate (OR 1.35 p<0.001) and DGF rate (OR 1.33 p<0.001). However, there were no significant differences in adjusted or unadjusted cost of transplant using either ECD or KDPI index to categorize organ quality. The authors conclude that “recipient characteristic may be a more influential predictor of initial costs after transplant”. Neither Saidi nor Stahl considered the interaction of payment and hospital costs, nor did they examine individual components of these indexes on risk adjusted costs. Using the UHC data, we identified higher costs among recipients of deceased donor kidneys from donors who were older, diabetic, had an elevated creatinine, or died from anoxic injuries. All of which are more common in contemporary practice. Pulsatile perfusion may be one method to address the higher cost of using non-standard organs through reduction in rates of DGF and the need for inpatient dialysis.(11) The observed incremental costs associated with lower organ quality are likely conservative estimates of the overall impact of these grafts on transplant finances as used of these grafts is frequently accompanied by higher rates of readmission, for-cause renal biopsy, and post-transplant renal replacement therapy.
In this study, we specifically examined the economic implications of recipient characteristics on transplant center finances. Using the EPTS score as a marker for recipient comorbidity, we demonstrate a strong, linear association between recipient risk and cost in deceased donor transplant. After adjusting for other donor and recipient factors, high EPTS greater than 85 was independently associated with $5,257 in incremental costs for the transplant program. Although, Medicare payments were higher for elevated EPTS patients ($2,474), this is not sufficient to compensate for the higher costs. Consequently, deceased donor transplantation in Medicare recipients with moderately high EPTS patients (51–85) and high EPTS (81–100) is likely to result in a significant expected reduction in hospital margin. This loss is likely to be exacerbated as organs with higher risk characteristics (donor age, terminal creatinine) are appropriately transplanted into willing recipients. In living donor recipients, EPTS did not correlate with cost or payment data. Multiple studies have confirmed the benefits of renal transplant in selected elderly patients as well as the substantial barriers to accessing transplant services faced by these same patients.(3, 23, 24) Elderly patients, diabetics, and re-transplant patients are excluded from transplant waitlists or subsequently removed as too sick to transplant at much higher rates than other candidates.(25–28) These economic data demonstrate that hospital finances are a potential barrier to transplantation of at risk patients and may contribute the observed differences in access for these populations. The lack of appropriate adjustment of payments for highly prevalent and potentially expensive conditions (e.g. advanced coronary artery disease, prolonged cold ischemic time, and low socioeconomic status) is likely to exacerbate financial losses associated with transplantation of these candidates.
Allosensitization has also been shown to decrease access to transplantation among waitlisted candidates despite a rising prevalence of high PRA/cPRA candidates on the waiting list. Transplantation in these patients requires more aggressive induction regimens and the potential need for costly plasma exchange. Despite the early increase in costs, however, desensitization treatments have been shown to be clinically effective and perhaps even cost effective. (7, 29) However, centers performing these procedures may face a significant economic disincentive as the degree of allosensitization was highly correlated with center cost. Compared with patients without alloantibodies, increasing PRA in deceased donor transplant incremental costs between $3,638-$9097 per transplant. Similar findings were demonstrated in the living donor population, in which high PRA candidates were associated with reductions in incremental costs ranging from $4,708-$17,781. Despite the marked increased cost of these transplants, there was no difference in payments for either deceased or living donor transplants performed on Medicare patients. It was not possible to determine if these patients underwent specific desensitization protocols, although the benefits of 0-ABDR mismatch organs were capture for patients that were allocated these organs.
The variation in risk adjusted costs following surgical procedures has been widely reported nationally using health care payment data. Englesbe and colleagues evaluated variation in costs among 43,393 kidney transplant patients, of which 35% were classified by the authors as “high cost” patients based on Medicare payment including outlier payments and readmission.(30) The incidence of high cost patients varied from 5% to 50% between medical centers. The proportion of high cost patients was found to be inversely proportional to risk adjusted outcomes, such that the centers with the best risk adjusted outcomes reported the lowest costs. Among the factors that contribute to higher costs is the incidence of post-transplant complications. The cost of early post-operative complications in kidney transplantation, for example, is significant with sepsis increasing costs by up to $134,000.(31, 32) More recently, Irwin examined the variation in payments to centers with a large private transplant network. Average payment for the transplant episode varied from 54% to 154% of the median payment ($233,532). These differences were driven, in part, by case mix, access to living donors, length of stay, and access to preemptive transplant. This investigation demonstrates a marked variation in the facility reported cost of transplant care among transplant programs even after adjusting for donor and recipient factors. The ratio of observed to expected costs ranged from 0.47 to 1.64 at the center level. The etiology of the marked variation in the risk-adjusted costs differences remains to be completely explained, but likely reflects medical and surgical care patterns including choice of induction agents, underlying socioeconomic differences, and the incidence and management of complications. The study also demonstrates the poor correlation between average center-level cost and actual Medicare payments, reflecting the limited adjustment of payments for differences in patient characteristics, donor quality, and necessary (or potentially unnecessary) clinical care.
This study has several limitations common to all studies which examine large data sets. The UHC data were derived from hospital charge masters and adjusted to costs using intermediate product cost-to-charge ratios. This may differ from activity based costing estimates within specific institutions. Second, the tracking and attribution of the organ acquisition cost differed between institutions. These differences are unlikely to impact the estimates of cost or payment data. However, direct estimate of transplant center margins may be inaccurate as this requires that organ acquisition cost payments are added to the hospital payment. Unfortunately, these costs are not reported in standard Medicare analytic files and therefore, we did not analyze individual transplant center margins for this analysis. Third, this analysis focused solely on the technical component of the cost of transplant. There are additional costs associated with high cost recipients (increased professional changes, pharmacy charges for immunosuppression, dialysis for delayed graft function) that may be considered within global contracts but are delivered outside of the inpatient setting. Consequently, the economic implications of recipient severity of illness and donor quality may be greater than that captured in this analysis. Fourth, Medicare data are available for a limited portion of the population (20% of all living donor recipients). This may bias the results slightly as private payers provide better reimbursement. However, the trends identified in this analysis are likely robust as the majority of payers employ minimally adjusted case rates. Finally, with respect to the structure of our economic models, alternatives to ordinary least-squares (OLS) models, such as regressions estimating the determinants of the natural log of Medicare payments, may be more efficient but also may produce biased estimates and are difficult to interpret. Because we have access to cost data for very large samples, we employ the unbiased estimator. Our past work has demonstrated nearly identical results with OLS cost regression and regressions on the natural log of Medicare payments,(33) and OLS has become our standard in analyses of the economic impact of complications in transplantation.(34, 35)
In conclusion, changes in donor and recipient characteristics have increased costs and eroded profitability for renal transplant nationally. Despite the clear long term benefit of transplantation, even among higher risk patients, transplant centers face significant economic disincentives. Policy makers should consider the creation of risk adjusted payment for renal transplant, similar to that of liver and heart transplant, to ensure that access is reserved for expensive but deserving candidates. The inclusion of supplemental payments for providers that successfully utilize marginal donor organs (e.g. KPDI>85) would ensure that financial considerations no longer contribute to organ discard but, instead, drive innovation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Abbreviations
- BMI
Body Mass Index
- cPRA
Calculated Panel Reactive Antibody
- DCD
Donation after Cardiac Death
- ECD
Expanded Criteria Donor
- EPTS
Expected Post Transplant Survival
- KPDI
Kidney Donor Profile Index
- OAC
Organ Acquisition Cost
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel Reactive Antibody
- UHC
University HealthSystem Consortium
Footnotes
Institution at which work was performed: Saint Louis University School of Medicine, St. Louis, MO, USA
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 2.Denecke C, Biebl M, Pratschke J. Optimizing clinical utilization and allocation of older kidneys. Current opinion in organ transplantation. 2015;20(4):431–7. doi: 10.1097/MOT.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 3.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, et al. Frailty and mortality in kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(1):149–54. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83(8):1069–74. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 5.System USRD. Anual Data Report 2015. [Available from: http://www.usrds.org/2015/view/Default.aspx.
- 6.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. The New England journal of medicine. 2016;374(10):940–50. doi: 10.1056/NEJMoa1508380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. The New England journal of medicine. 2011;365(4):318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 8.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(7):1797–807. doi: 10.1111/j.1600-6143.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 9.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(10):2310–6. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 10.Saidi RF, Elias N, Kawai T, Hertl M, Farrell ML, Goes N, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(12):2769–74. doi: 10.1111/j.1600-6143.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan PM, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(11):2391–401. doi: 10.1111/j.1600-6143.2008.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnitzler MA, Lentine KL, Burroughs TE. The cost effectiveness of deceased organ donation. Transplantation. 2005;80(11):1636–7. doi: 10.1097/01.tp.0000179637.37276.5a. [DOI] [PubMed] [Google Scholar]
- 13.Matas AJ, Schnitzler M, Daar AS. Payment for living kidney donors (vendors) is not an abstract ethical discussion occurring in a vacuum. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(8):1380–1. doi: 10.1111/j.1600-6143.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 14.Held PJ, McCormick F, Ojo A, Roberts JP. A Cost-Benefit Analysis of Government Compensation of Kidney Donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 doi: 10.1111/ajt.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englesbe MJ, Ads Y, Cohn JA, Sonnenday CJ, Lynch R, Sung RS, et al. The effects of donor and recipient practices on transplant center finances. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(3):586–92. doi: 10.1111/j.1600-6143.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- 16.Axelrod DA. Economic and financial outcomes in transplantation: whose dime is it anyway? Current opinion in organ transplantation. 2013;18(2):222–8. doi: 10.1097/MOT.0b013e32835f0757. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services. Health Resources and Services Administration. 2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. [Google Scholar]
- 18.Axelrod DA, Dzebisashvili N, Lentine K, Segev DL, Dickson R, Tuttle-Newhall E, et al. Assessing variation in the costs of care among patients awaiting liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(1):70–8. doi: 10.1111/ajt.12494. [DOI] [PubMed] [Google Scholar]
- 19.Recipients SRoT. Technical Methods for The Program-Specific Reports 2015. [Available from: http://www.srtr.org/csr/current/Centers/201512/all_csr_documentation.pdf.
- 20.Nassir BA, Dean CE, Li S, Salkowski N, Solid CA, Schnitzler MA, et al. Variation in Cost and Quality in Kidney Transplantation. Transplantation. 2015 doi: 10.1097/TP.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 21.Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, et al. Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments. Transplantation. 2000;70(5):755–60. doi: 10.1097/00007890-200009150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Matas AJ, Schnitzler M. Payment for living donor (vendor) kidneys: a cost-effectiveness analysis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(2):216–21. doi: 10.1046/j.1600-6143.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Gaylin DS, Held PJ, Port FK, Hunsicker LG, Wolfe RA, Kahan BD, et al. The impact of comorbid and sociodemographic factors on access to renal transplantation. JAMA : the journal of the American Medical Association. 1993;269(5):603–8. [PubMed] [Google Scholar]
- 24.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Seminars in nephrology. 2009;29(6):621–35. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schold JD, Srinivas TR, Howard RJ, Jamieson IR, Meier-Kriesche HU. The association of candidate mortality rates with kidney transplant outcomes and center performance evaluations. Transplantation. 2008;85(1):1–6. doi: 10.1097/01.tp.0000297372.51408.c2. [DOI] [PubMed] [Google Scholar]
- 26.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clinical journal of the American Society of Nephrology : CJASN. 2006;1(3):532–8. doi: 10.2215/CJN.01130905. [DOI] [PubMed] [Google Scholar]
- 27.Heaphy EL, Poggio ED, Flechner SM, Goldfarb DA, Askar M, Fatica R, et al. Risk factors for retransplant kidney recipients: relisting and outcomes from patients’ primary transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(6):1356–67. doi: 10.1111/ajt.12690. [DOI] [PubMed] [Google Scholar]
- 28.Schold JD, Buccini LD, Poggio ED, Flechner SM, Goldfarb DA. Association of Candidate Removals From the Kidney Transplant Waiting List and Center Performance Oversight. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13594. [DOI] [PubMed] [Google Scholar]
- 29.Vo AA, Petrozzino J, Yeung K, Sinha A, Kahwaji J, Peng A, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95(6):852–8. doi: 10.1097/TP.0b013e3182802f88. [DOI] [PubMed] [Google Scholar]
- 30.Englesbe MJ, Dimick JB, Fan Z, Baser O, Birkmeyer JD. Case mix, quality and high-cost kidney transplant patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(5):1108–14. doi: 10.1111/j.1600-6143.2009.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(1):129–39. doi: 10.1111/j.1600-6143.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- 32.Naik AS, Dharnidharka VR, Schnitzler MA, Brennan DC, Segev DL, Axelrod D, et al. Clinical and economic consequences of first-year urinary tract infections, sepsis, and pneumonia in contemporary kidney transplantation practice. Transplant international : official journal of the European Society for Organ Transplantation. 2016;29(2):241–52. doi: 10.1111/tri.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S.wait-listed and transplanted renal allograft recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(5):590–8. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 34.Schnitzler MA, Johnston K, Axelrod D, Gheorghian A, Lentine KL. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91(12):1347–56. doi: 10.1097/TP.0b013e31821ab993. [DOI] [PubMed] [Google Scholar]
- 35.Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, L’Italien G, Lentine KL. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241–9. doi: 10.1097/TP.0b013e318255f839. [DOI] [PubMed] [Google Scholar]