Introduction
“You are going to go to sleep now” is an oft-repeated colloquialism in every anesthesiologist’s daily practice. The phrase might be useful in allaying the fears of nervous patients, but does general anesthesia actually mimic sleep? Do they travel on the same neural pathways? To what degree does the comparison accurately reflect the underlying mechanisms involved?
Sleep, especially non-rapid eye movement (NREM) sleep, and anesthesia may use common neuronal and genetic substrates. Anesthetics act through sleep neural circuits but not necessarily in the same way.
Arousal pathways
In order to promote and sustain cortical arousal, neuronal pathways have developed two parallel ascending neuronal pathways. The first branch activates the thalamic relay neurons that are crucial for transmission of information to the cortex. Cholinergic signaling originating from the laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei and the basal forebrain promote the cortically activated states of wakefulness and rapid eye movement (REM) sleep. The second branch bypasses the thalamus, activating neurons in the lateral hypothalamic area and basal forebrain (BF), and throughout the cortex. This pathway originates from monoaminergic neurons in the upper brainstem and caudal hypothalamus. The locus coeruleus (LC) provides norepinephrine-mediated inhibition of the ventrolateral preoptic (VLPO) nucleus in the hypothalamus1,2. Therefore, γ-aminobutyric acid (GABAA)-mediated and galanin-mediated inhibition of the ascending arousal circuits by the VLPO nucleus is inhibited and the awake state is promoted.
Sleep pathways
Sleep is under control of two processes, a circadian clock that regulates the appropriate timing of sleep and wakefulness across the 24-h day and a homeostatic process (“sleep homeostasis”) that regulates sleep need and intensity according to the time spent awake or asleep3. Sleep is a non-homogenous state that can be divided into NREM sleep and REM (“paradoxical”) sleep. The brain areas identified as important in sleep fall into two general groups, those with an arousing influence and active during wakefulness: LC, dorsal raphe (DR), tuberomammillary (TMN) and BF; and those active primarily during sleep: VLPO. The MPA contains both wake- and sleep-active neurons.
Discrete neurochemical changes accompany the different types of sleep with cholinergic (in brain stem and forebrain), noradrenergic (LC) and serotonergic (DR) all becoming less active in NREM sleep while cholinergic activity increases in REM sleep4. Activity in the VLPO is increased in NREM sleep and the GABAergic/galanin input from VLPO inhibits the histaminergic TMN nucleus. Orexinergic pathways from the perifornical nucleus are inactive during NREM sleep (figure 1).
Figure 1.
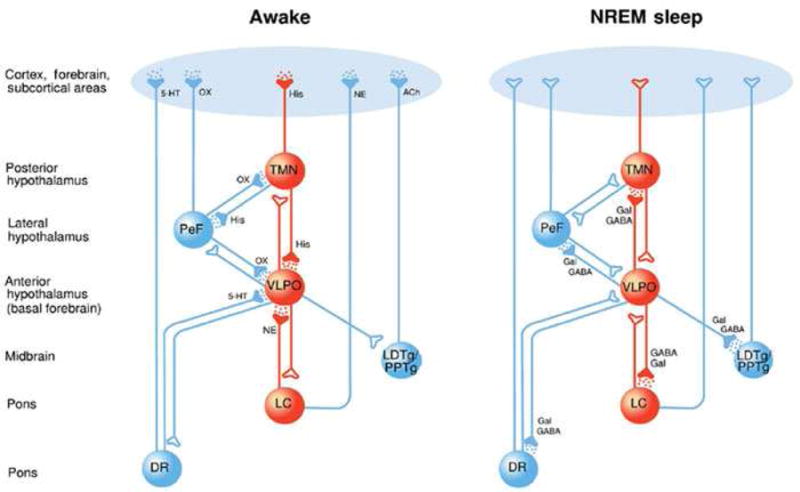
The hypnotic effects of GABAA and αλπηα2 adrenoceptor agonists involve different neural networks: a schematic demonstrating some important neural nuclei involved in producing the sedative state. Active nuclei are depicted in red and inactive nuclei are depicted in blue. (a) In the awake state, certain “awake-active” neural nuclei, including the noradrenergic locus ceruleus (LC), the orexinergic perifornical nucleus (PeF) and the histaminergic tuberomamillary nucleus (TMN), provide excitatory input to higher centres such as the cortex. When awake a “sleep-active” nucleus the venterolateral preoptic nucleus (VLPO) is silent. (b) During GABAergic sedation, potentiated inhibitory actions of the VLPO reduce neural activity in both the PeF and TMN but allow activity to proceed unimpeded in the LC (resulting in intact noradrenergic signaling; active signaling shown with a dotted red line). (c) During α2 adrenoceptor agonist sedation activity is reduced in the LC and TMN while activity is enhanced in the inhibitory VLPO. Activity in the PeF is unaffected by α2 adrenoceptor agonist sedation (resulting in intact orexinergic signaling; active signaling shown with a dotted red line).
Reproduced with permission from Sanders RD, Hussell T, Maze M. Sedation & Immunity: Optimisation for Critically Ill Patients. Intense Times 2010;9:2–5
The relatively quiescent LC facilitates a series of changes that includes activation of the galanin/GABA containing neurons of the VLPO nucleus that terminate on and inhibit aminergic neurons within the tuberomammillary nucleus5.
Anesthetic-induced unconsciousness results from specific interactions of anesthetics with the neural circuits regulating sleep and wakefulness.
Where do anesthetics collide in sleep pathways?
The currently available imaging techniques can only indirectly measure neuronal activity, for example through changes in blood flow, glucose metabolism or oxygen concentration. We can then understand the difficulty in fully understanding the mechanisms by which anesthesia induces sleep/unconsciousness. A common finding between NREM sleep and anesthesia in imaging studies is the deactivation of the thalamus leading to cortical inhibition. Anesthetic drugs induce unconsciousness by altering neurotransmission at multiple sites in the cerebral cortex, brain stem, and thalamus. The different actions of the different available anesthetics have made the understanding of the exact mechanism even more difficult. Effects of modern anesthetics on subsequent sleep behavior are known for some, but not all anesthetics. No one electroencephalogram (EEG) pattern characterizes the anesthetized state. Different anesthetics and doses have distinct profiles with respect to the EEG activity.
Although some agents act on excitatory synapses, others act through potentiation of inhibitory synaptic receptors. The GABAA receptors are neurotransmitter-gated chloride channels that exist on cells that may also contain nicotinic acetylcholine receptors, glycine receptors, and serotonin type 3 receptors. Anesthetics such as propofol, etomidate and barbiturates exert their effect through enhancement of GABA-mediated channel activation and prolong post-synaptic inhibitory currents, suppressing neuronal excitability. In brain regions containing neurons that promote wakefulness, GABAergic inhibition has been shown to cause an increase in sleep. These brain regions include the DR nucleus, TMN, medial preoptic area and ventrolateral periaqueductal gray6,7. In a series of studies involving GABAergic agents, it was reported that unlike NREM sleep, these hypnotic agents did not alter noradrenergic activity in the LC8 (figure 1). Instead, these agents converged on the NREM sleep pathway at the level of the hypothalamus9. However, short-term administration of the GABAergic agent propofol permitted normal recovery after a period of sleep deprivation10,11.
At clinical concentrations, drugs like N2O, xenon and ketamine have little or no effect on GABAA receptors. Instead, these anesthetics mainly potently inhibit N-methyl-D-aspartate (NMDA) receptors, which are excitatory cation channels activated by glutamate. These agents reduce excitatory signals in critical neuronal circuits causing unconsciousness. Glutamate levels in the PPT are greater during wakefulness as opposed to NREM and REM sleep12,13. The dissociative state that is produced by ketamine anesthesia can be in part attributed to the different regions to which ketamine promotes glutamate release (nucleus accumbens14, prefrontal cortex15 and anterior cingulate16). It has been shown that isoflurane and sevoflurane reduce glutamate release17–19 and inhibit its uptake20 but few in vivo studies have been published to understand the exact mechanism by which isoflurane modulates glutamatergic transmission.
REM sleep rebound after exposure to volatile anesthetics suggests that these volatile anesthetics do not fully substitute for natural sleep21. In humans, isoflurane anesthesia alone (without surgery) results in no change in subsequent REM or NREM sleep, but a shift in NREM sleep from slow wave sleep to lighter (I and II) stages22. On the other hand, it has been shown that wake-active orexinergic neurons are inhibited by isoflurane and sevoflurane, and that waking up from anesthesia uses neural circuits distinct from those necessary to become anesthetized but similar to the neural circuitry that promotes arousal23. Furthermore, isoflurane depresses serotonin levels on hypoglossal motoneurons in dogs24 and on mice hippocampus25.
The molecular targets for dexmedetomidine are central α2 adrenergic receptors. It has been shown that α2 agonists transduce its hypnotic response after binding to the α2A receptor subtype26 in the LC27. Through signaling processes that involve both pertussis toxin-sensitive G proteins28 and effector mechanisms that include inhibition of adenylyl cyclase28 and ligand-gated calcium channels as well as activation of inwardly-rectifying potassium channels29, the noradrenergic neurons become hyperpolarized and are less likely to achieve an action potential. α2 agonists, like dexmedetomidine, are associated with similar changes in neuronal activity as is seen in deeper stages of NREM sleep2,30 apart from the absence of inhibitory effect on the orexinergic neurons in the perifornical nucleus9. A functional magnetic resonance study showed that a thalamic nucleus, that receives afferent input from orexinergic neurons, is activated during an arousal stimulus in α2- sedated subjects31. Children sedated with dexmedetomidine exhibited an EEG pattern that was identical to that seen in stage 2 NREM sleep32.
Though acetylcholine (ACh) plays a primary role in generating the brain-activated states of wakefulness and REM sleep, cholinergic receptors are not a main target of common anesthetics. Nonetheless, ACh interacts with other transmitter systems that are targets of sleep pharmacology, for example the GABAergic agents. The clinical finding that physostigmine (acetylcholinesterase inhibitor) reverses propofol sedation, causing arousal, suggests that propofol produces unconsciousness, in part, by disrupting cholinergic neurotransmission33. In vitro studies showed that propofol, isoflurane, sevoflurane and ketamine inhibit muscarinic and nicotinic ACh receptors34–38, providing support that these agents cause sedation, in part, by inhibiting cholinergic neurotransmission in brain regions that regulate arousal.
Circadian Rhythm
The two-process model of sleep homeostasis as described by Borbely et al integrates sleep debt (“process-s”) and circadian rhythm (“process-c”). This model implicates circadian rhythm and sleep as intertwined, co-dependent processes39. Experimentally distinguishing process-c from process-s presents a challenge in deconstructing causative factors in sleep disorders. Nevertheless, there is a growing body of evidence to suggest that circadian rhythm can be altered independent of sleep deprivation and that anesthetics can specifically change circadian rhythm.
Circadian rhythmicity is thought to be controlled by the suprachiasmatic nucleus and established by processing external cues (zeitgeibers) like light, into systemic mediators such as temperature, adrenergic signaling, and circulating hormones (e.g., cortisol and melatonin). This process serves to maintain a diurnal pattern presumably to coordinate intracellular or intersystem function by resynchronizing intrinsic cellular molecular clocks. Briefly, the molecular core of the circadian clock involves the heterodimerization of CLOCK and BMAL1 which act as the canonical arm of the clock, and the heterodimerization of PER1/2 and CRY1/2 which are critical components of the negative feedback arm19, stabilizing proteins RORα, REV-ERB, DEC, DBP and E4BP4 act as additional repressors or activators of the canonical arm40, and these proteins oscillate through the day and translocate from the cytoplasm to the nucleus in a highly coordinated fashion to provide a reliable rhythm of approximately 24 hours. Disruption of circadian processes is being studied as a relevant contributing factor to multiple human conditions altering, among others, immunity.
Circadian Rhythm and Anesthesia
Initial indications that circadian rhythm is important in anesthetic delivery are the time-of-day variance in susceptibility to general anesthetics41,42. Indeed, the greatest therapeutic effect of general anesthetics in animal models occurs during the animals rest phase for propofol43 and ketamine44. Volatile anesthetic effect may also vary according to a diurnal pattern where previously, halothane administration in rats varied with a lower minimum alveolar concentration requirement during the rest phase compared to the active phase45. Recently, using bees as a model system, 6-hour isoflurane administration during the rest phase failed to alter the circadian activity patterns of the hive, whereas isoflurane administered in the active phase significantly altered the circadian activity of the hive46. Taken together, the time-of-day administration of anesthesia is likely important in both the dose-dependent effect and maintenance of circadian rhythm.
As parameters for outlining circadian rhythm, cortisol and melatonin levels can be used to make assumptions about the effect of general anesthesia on daily cycling in human subjects. Most human studies following these variables involve general anesthesia with the confounding aspect of surgery, and do not incorporate the natural underlying cycling of these hormones adequately. Given these and other significant caveats, with respect to cortisol, a propofol-based anesthetic appears to decrease the amount of plasma cortisol during surgery compared to sevoflurane47. Postoperatively, using a thiopental/sevoflurane or thiopental/isoflurane based anesthetic, cortisol levels are elevated in men who underwent long duration surgery for larynx or pharynx cancer48. Conversely, women undergoing laparoscopic procedures for pelvis surgery, anesthesia with thiopental/sevoflurane reduced amounts of cortisol 2–4 hours after surgery compared with thiopental/isoflurane anesthesia49. Given that these studies were designed to investigate the anesthetic effects on stress responses and not on circadian rhythm directly, there remains only a suggestion that altered cortisol levels may interfere with circadian rhythm after anesthesia and surgery.
Investigation into the effect of anesthesia on melatonin cycling, points more directly to a circadian rhythm effect. A comparison of general anesthesia (thiopental/isoflurane) to spinal anesthesia for orthopedic procedures showed a significant reduction in melatonin levels in the first post-surgical night compared to baseline levels, interestingly there was no significant difference in the reduction of melatonin between the experimental groups indicating that the effect was independent of the anesthesia, and pointed to the possibility of a significant surgical influence or post-operative opiate use on melatonin secretion50. In patients undergoing general anesthesia (thiopental/isoflurane/fentanyl) for laparoscopy a modest reduction in 13–hour average melatonin secretion was noted in the evening after surgery compared to the pre-surgical night with a large increase in melatonin secretion on the second night after surgery51. Corroborating these data, in patients undergoing major abdominal surgery with general anesthesia and concomitant use of a thoracic epidural there was a similar modest reduction in basal melatonin secretion on the first postoperative day followed by a significant increase on the second postoperative day52. Following urine metabolites of melatonin (aMT6s), general anesthesia (thiopental/propofol), decreased the maximal concentration and delayed the peak of aMT6s53. Short mask inhalation anesthetics (21 minute average duration) for dilation and curettage showed no difference in melatonin secretion compared to non-surgical controls54. Experimental models allow for more specific examination of melatonin secretion and general anesthesia apart from surgical effect. Rats anesthetized with propofol for 25–30 minutes around the peak of serum melatonin secretion showed a significant reduction in melatonin secretion for the 3 hours following anesthesia, a subsequent increase 20 hours after the anesthetic, and a phase advance of cyclical melatonin secretion of 40 minutes consistent with the approximate duration of anesthesia41. Whether in humans or rats, it seems consistent that melatonin levels are reduced in the immediate post-operative/anesthetic period with a rebound phenomenon observed thereafter, it remains unclear in the human subjects what component of the observed effect can be attributable to either surgery or anesthesia.
The effect of anesthetics on intrinsic molecular circadian clocks is beginning to be explored in experimental models. In rats, 6 hours of sevoflurane anesthesia changed the expression pattern of approximately 1.5% of 10,000 genes surveyed. Interestingly the expression of Per2 was the only circadian protein in the brain that was significantly reduced after the anesthetic55. The effect of reduced expression of Per2 and an auxiliary clock gene Dbp persists for 24 hours56. Both infusions of propofol and dexmedetomidine likewise reduced the expression of Per2 in rat brain 6 hours after anesthetic delivery but the effect persisted for 24 hours only in the dexmedetomidine57. Further investigation demonstrated that a 4-hour sevoflurane anesthetic blunted Per2 mRNA production in the SCN in response to a light stimulus and created a delayed activity rhythm in the anesthetized mice58. Repression of Per2 expression by sevoflurane anesthesia was most significant when administered between the hours of 8am–12pm as compared to other time points, but activity patterns of anesthetized animals were delayed for all time points of anesthetic administration59. Bees anesthetized with a 6-hour course of isoflurane during the day had a reduction in the amplitude of Cry expression and phase delay of both Cry and Per2 expression in whole bee brains compared to bees anesthetized during the evening, leading to alterations in circadian governed homing patterns and foraging times46. With respect to the molecular clock, general anesthesia appears to significantly affect critical clock proteins in a time-of-day dependent fashion and corresponds to changes in activity patterns consistent with circadian disruption.
Circadian Control of Immune Function
A separate line of investigation has focused on the influence of circadian rhythm on immune function. Evidence exists noting that the time of day is important in human subjects in terms of susceptibility to infection60, asthma attacks61 or flares of rheumatic arthritis62, all of which suggest circadian principles underlying these immune mediated processes.
Circulating pools of many immune cells cycle throughout the day indicate the influence of circadian timing. Natural Killer (NK) cells peak in both activity and numbers in the human circulation in the early morning and likely marginate away from circulation during other points of the day63. In mouse models, after lipopolysaccharide (LPS) administration, macrophages circulate cyclically to the spleen64. Importantly, the circadian molecular clock exists and functions in NK cells,65,66 macrophages, dendritic cells, and B cells64,67. Indeed, approximately 8% of the macrophage genome is classified as falling under the control of circadian transcription factors64, and critical immune transcription factors such as signal transducer and activator of transcription family (STATs) and nuclear factor kappa B (NF-κB) are regulated by clock proteins68. Studying the functional consequence of disturbing circadian molecular clock proteins in immune subsets is generating interesting data. Injection of LPS at specific time points caused significantly elevated cytokine production in both macrophage restricted Bmal1 knock-out (KO), and systemic Rev-Erbα KO mice compared to the chronometric 12-hour opposite time point (antiphasic control)69. Cry1/2 double KO mice have elevated NF-κB activity causing increased baseline inflammatory cytokine expression in vitro and generated greater inflammation when challenged with LPS in vitro and in vivo70. Examining lymphocyte function has similarly elucidated at least some aspect of circadian gating. Per2 KO mouse lymphocytes had a robust increase in proliferative capacity after being immunized in vivo when compared to their antiphasic control71. Isolated T cells cultured from mouse lymph nodes proliferated after stimulation in a circadian manner, an effect that was abolished in Clock mutant mice72. Both observationally and experimentally it has been shown that certain immune cells traffick according to apparent circadian parameters, possess oscillating intrinsic molecular clocks, have critical transcription factors controlled by clock proteins, and demonstrate altered function when clock proteins are perturbed.
Sleep Disruption and Cognitive Dysfunction in Sedated ICU Patients
Sleep disruption in critically ill patients is a common occurrence in the ICU with the potential to adversely impact patients’ outcome and also provide a direct financial detriment with respect to the length of hospital stay and depletion of healthcare resources. Early polysomnographic studies had revealed extreme sleep disruption in ICU patients with decreases in total sleep-time, altered sleep architecture (predominance of stage 1 and 2 sleep, decreased or absent stages 3 and 4 NREM and REM sleep), and sleep fragmentation73,74; also, up to 50% of the total sleep-time occurred during daytime. Among the possible causes that contribute to sleep disruption in the ICU are those related to the patient’s acute illness and co-morbidities, environmental factors (including noise and inappropriate light), and iatrogenic factors including frequent care-related interruptions and medications prescribed for analgesia and sedation75,76. Among those that are potentially amenable to modification, excessive noise does not contribute as much as was anticipated77 and attention has focused on sedative practices78. Sedative-hypnotic agents are widely used to facilitate sleep in the ICU; however, depending on the sedative agent, it may not produce appropriate sleep hygiene and instead will aggravate the problem by producing less of the restorative properties of natural sleep.
Several studies have now demonstrated the association between the use of benzodiazepine (BZD) and increased incidence79 and duration80 of delirium in medical ICU patients although the relationship of the development and duration of delirium to sleep disruption was not ascertained. Acute withdrawal from long-term sedation with BDZs and opiate narcotics results in profound sleep disruption81. The pivotal work of the MENDs trial79,82,83 indicated the benefits of a specific anesthetic agent, dexmedetomidine in the outcome of ICU population.
Conclusion
Appropriate sleep hygiene is crucial for repair in states of disease and injury and in restoring function especially in the central nervous and immune systems. Lack of sleep hygiene results in cognitive dysfunction, contributes to the delirium that is prevalent in patients within the ICU, adversely affects immunity, and independently increases both morbidity and mortality. Anesthetics used in hospital care have different action targets and ultimately different consequences. Those which act by modulating the GABAA receptor converge at the level of the hypothalamus while α2 adrenergic agonists converge on sleep pathways within the brainstem. Thus, thoughtful attention must be made in selecting an anesthetic agent that best mimics natural sleep. Future studies will further elucidate the benefits of dexmedetomidine as a good anesthetic/sedative candidate to mimic natural sleep.
While the fields of investigation into the anesthetic effect on circadian rhythm and the circadian influence on immune function remain disparate to our knowledge, there exists the plausible concatenation that anesthetics, by altering circadian rhythm (possibly independent of sleep deprivation), affect immune function. Given that anesthetics are often employed adjunctively to facilitate therapeutic interventions, it would be worthwhile to elucidate whether more precise applications of anesthesia could improve immunologic outcomes for patients who require them.
Anesthesia is not the same as sleep. The actions of anesthetics on sleep pathways and the restorative properties of natural sleep for the central nervous system are undeniable and essential, yet they also advance a concomitant advantage to the immune system with fewer infections and greater likelihood of survival from sepsis.
Key Points.
Anesthetic drugs induce unconsciousness by altering neurotransmission at multiple sites.
α2 agonists, like dexmedetomidine, are associated with similar changes in neuronal activity as is seen in deeper stages of NREM sleep.
The effects of anesthetics on circadian rhythm possibly lead to immune deregulation
Thoughtful attention must be made in selecting an anesthetic agent that best mimics natural sleep.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002 Oct;5(10):979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005 Oct;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 3.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999 Dec;14(6):557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004 Sep 30;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003 Feb;98(2):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. In: Pfaff DW, Kieffer BL, editors. Molecular and Biophysical Mechanisms of Arousal, Alertness, and Attention. Vol. 1129. Malden: Wiley-Blackwell; 2008. pp. 275–286. [Google Scholar]
- 7.Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007 Mar;145(3):1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002 Oct;5(10):979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 9.Zecharia AY, Nelson LE, Gent TC, et al. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009 Feb 18;29(7):2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004 Jun;100(6):1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesthesia and analgesia. 2001 May;92(5):1232–1236. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol-Regul Integr Comp Physiol. 2001 Mar;280(3):R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 13.Kodama T, Honda Y. Acetylcholine and glutamate release during sleep-wakefulness in the pedunculopontine tegmental nucleus and norepinephrine changes regulated by nitric oxide. Psychiatry Clin Neurosci. 1999 Apr;53(2):109–111. doi: 10.1046/j.1440-1819.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 14.Razoux F, Garcia R, Lena I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007 Mar;32(3):719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- 15.Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117(3):697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 16.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: A 4-T proton MRS study. Am J Psychiat. 2005 Feb;162(2):394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 17.Lingamaneni R, Birch ML, Hemmings HC. Widespread inhibition of sodium channel-dependent glutamate release from isolated nerve terminals by isoflurane and propofol. Anesthesiology. 2001 Dec;95(6):1460–1466. doi: 10.1097/00000542-200112000-00027. [DOI] [PubMed] [Google Scholar]
- 18.Larsen M, Valo ET, Berg-Johnsen J, Langmoen IA. Isoflurane reduces synaptic glutamate release without changing cytosolic free calcium in isolated nerve terminals. Eur J Anaesth. 1998 Mar;15(2):224–229. [PubMed] [Google Scholar]
- 19.Moe MC, Berg-Johnsen J, Larsen GA, Roste GK, Vinje ML. Sevoflurane reduces synaptic glutamate release in human synaptosomes. J Neurosurg Anesthesiol. 2002 Jul;14(3):180–186. doi: 10.1097/00008506-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Liachenko S, Tang P, Somogyi GT, Xu Y. Concentration-dependent isoflurane effects on depolarization-evoked glutamate and GABA outflows from mouse brain slices. Br J Pharmacol. 1999 May;127(1):131–138. doi: 10.1038/sj.bjp.0702543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pick J, Chen YH, Moore JT, et al. Rapid Eye Movement Sleep Debt Accrues in Mice Exposed to Volatile Anesthetics. Anesthesiology. 2011 Oct;115(4):702–712. doi: 10.1097/ALN.0b013e31822ddd72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moote CA, Knill RL. ISOFLURANE ANESTHESIA CAUSES A TRANSIENT ALTERATION IN NOCTURNAL SLEEP. Anesthesiology. 1988 Sep;69(3):327–331. doi: 10.1097/00000542-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jan;105(4):1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandes IF, Zuperku EJ, Stucke AG, Hopp FA, Jakovcevic D, Stuth EAE. Isoflurane depresses the response of inspiratory hypoglossal motoneurons to serotonin in vivo. Anesthesiology. 2007 Apr;106(4):736–745. doi: 10.1097/01.anes.0000264750.93769.99. [DOI] [PubMed] [Google Scholar]
- 25.Whittington RA, Virag L. Isoflurane decreases extracellular serotonin in the mouse hippocampus. Anesthesia and analgesia. 2006 Jul;103(1):92–98. doi: 10.1213/01.ane.0000221488.48352.61. [DOI] [PubMed] [Google Scholar]
- 26.Lakhlani PP, MacMillan LB, Guo TZ, et al. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1997 Sep 2;94(18):9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992 Jun;76(6):948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Correa-Sales C, Reid K, Maze M. Pertussis toxin-mediated ribosylation of G proteins blocks the hypnotic response to an alpha 2-agonist in the locus coeruleus of the rat. Pharmacol Biochem Behav. 1992 Nov;43(3):723–727. doi: 10.1016/0091-3057(92)90400-a. [DOI] [PubMed] [Google Scholar]
- 29.Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994 Dec;81(6):1527–1534. doi: 10.1097/00000542-199412000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Huupponen E, Kulkas A, Tenhunen M, Saastamoinen A, Hasan J, Himanen SL. Diffuse sleep spindles show similar frequency in central and frontopolar positions. J Neurosci Methods. 2008 Jul 15;172(1):54–59. doi: 10.1016/j.jneumeth.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Coull JT, Jones ME, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. Neuroimage. 2004 May;22(1):315–322. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Mason KP, O’Mahony E, Zurakowski D, Libenson MH. Effects of dexmedetomidine sedation on the EEG in children. Paediatric anaesthesia. 2009 Dec;19(12):1175–1183. doi: 10.1111/j.1460-9592.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- 33.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000 Sep;93(3):708–717. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Nagase Y, Kaibara M, Uezono Y, Izumi F, Sumikawa K, Taniyama K. Propofol inhibits muscarinic acetylcholine receptor-mediated signal transduction in Xenopus oocytes expressing the rat M1 receptor. Jpn J Pharmacol. 1999 Mar;79(3):319–325. doi: 10.1254/jjp.79.319. [DOI] [PubMed] [Google Scholar]
- 35.Flood P, RamirezLatorre J, Role L. alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but alpha 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997 Apr;86(4):859–865. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Furuya R, Oka K, Watanabe I, Kamiya Y, Itoh H, Andoh T. The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P-2X purinoceptors in PC12 cells. Anesthesia and analgesia. 1999 Jan;88(1):174–180. doi: 10.1097/00000539-199901000-00033. [DOI] [PubMed] [Google Scholar]
- 37.Coates KM, Flood P. Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha 7 and alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol. 2001 Oct;134(4):871–879. doi: 10.1038/sj.bjp.0704315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheller M, Bufler J, Schneck H, Kochs E, Franke C. Isoflurane and sevoflurane interact with the nicotinic acetylcholine receptor channels in micromolar concentrations. Anesthesiology. 1997 Jan;86(1):118–127. doi: 10.1097/00000542-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Borbely AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Human neurobiology. 1982;1(3):205–210. [PubMed] [Google Scholar]
- 40.Ueda HR, Hayashi S, Chen WB, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genetics. 2005 Feb;37(2):187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 41.Dispersyn G, Pain L, Challet E, Touitou Y. General Anesthetics Effects on Circadian Temporal Structure: An Update. Chronobiology International. 2008;25(6):835–850. doi: 10.1080/07420520802551386. 2008. [DOI] [PubMed] [Google Scholar]
- 42.Chassard D, Duflo F, Bouvet L, Boselli E. Chronobiology of postoperative pain: It’s time to wake up! Canadian Journal of Anaesthesia-Journal Canadien D Anesthesie. 2007 Sep;54(9):685–688. doi: 10.1007/BF03026864. [DOI] [PubMed] [Google Scholar]
- 43.Challet E, Gourmelen S, Pevet P, Oberling P, Pain L. Reciprocal relationships between general (propofol) anesthesia and circadian time in rats. Neuropsychopharmacology. 2007 Mar;32(3):728–735. doi: 10.1038/sj.npp.1301081. [DOI] [PubMed] [Google Scholar]
- 44.Rebuelto M, Ambros L, Waxman S, Montoya L. Chronobiological study of the pharmacological response of rats to combination ketamine-midazolam. Chronobiology International. 2004;21(4–5):591–600. doi: 10.1081/cbi-200026466. 2004. [DOI] [PubMed] [Google Scholar]
- 45.Munson ES, Martucci RW, Smith RE. Circadian variations in anesthetic requirement and toxicity in rats. Anesthesiology. 1970 Jun;32(6):507–514. doi: 10.1097/00000542-197006000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Cheeseman JF, Winnebeck EC, Millar CD, et al. General anesthesia alters time perception by phase shifting the circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2012 May 1;109(18):7061–7066. doi: 10.1073/pnas.1201734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ledowski T, Bein B, Hanss R, et al. Neuroendocrine stress response and heart rate variability: A comparison of total intravenous versus balanced anesthesia. Anesthesia and analgesia. 2005 Dec;101(6):1700–1705. doi: 10.1213/01.ane.0000184041.32175.14. [DOI] [PubMed] [Google Scholar]
- 48.Nishiyama T, Yamashita K, Yokoyama T. Stress hormone changes in general anesthesia of long duration: isoflurane-nitrous oxide anesthesia oxide vs sevoflurane-nitrous. Journal of Clinical Anesthesia. 2005 Dec;17(8):586–591. doi: 10.1016/j.jclinane.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Marana E, Annetta MG, Meo F, et al. Sevoflurane improves the neuroendocrine stress response during laparoscopic pelvic surgery. Canadian Journal of Anaesthesia-Journal Canadien D Anesthesie. 2003 Apr;50(4):348–354. doi: 10.1007/BF03021031. [DOI] [PubMed] [Google Scholar]
- 50.Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta anaesthesiologica Scandinavica. 2002 Jan;46(1):30–36. doi: 10.1034/j.1399-6576.2002.460106.x. [DOI] [PubMed] [Google Scholar]
- 51.Ram E, Vishne TH, Weinstein T, Beilin B, Dreznik Z. General anesthesia for surgery influences melatonin and cortisol levels. World Journal of Surgery. 2005 Jul;29(7):826–829. doi: 10.1007/s00268-005-7724-1. [DOI] [PubMed] [Google Scholar]
- 52.Gogenur I, Ocak U, Altunpinar O, Middleton B, Skene DJ, Rosenberg J. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World Journal of Surgery. 2007 Feb;31(2):290–298. doi: 10.1007/s00268-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 53.Gogenur I, Middleton B, Kristiansen VB, Skene DJ, Rosenberg J. Disturbances in melatonin and core body temperature circadian rhythms after minimal invasive surgery. Acta anaesthesiologica Scandinavica. 2007 Sep;51(8):1099–1106. doi: 10.1111/j.1399-6576.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- 54.Fassoulaki A, Kostopanagiotou G, Meletiou P, Chasiakos D, Markantonis S. No change in serum melatonin, or plasma beta-endorphin levels after sevoflurane anesthesia. Journal of Clinical Anesthesia. 2007 Mar;19(2):120–124. doi: 10.1016/j.jclinane.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto A, Imai J, Nishikawa A, et al. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene. 2005 Aug 15;356:39–48. doi: 10.1016/j.gene.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi K, Takemori K, Sakamoto A. Circadian gene expression is suppressed during sevoflurane anesthesia and the suppression persists after awakening. Brain Research. 2007 Dec 14;1185:1–7. doi: 10.1016/j.brainres.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida H, Kubota T, Krueger JM. A cyclooxygenase-2 inhibitor attenuates spontaneous and TNF-alpha-induced non-rapid eye movement sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2003 Jul;285(1):R99–109. doi: 10.1152/ajpregu.00609.2002. [DOI] [PubMed] [Google Scholar]
- 58.Ohe Y, Iijima N, Kadota K, Sakamoto A, Ozawa H. The general anesthetic sevoflurane affects the expression of clock gene mPer2 accompanying the change of NAD(+) level in the suprachiasmatic nucleus of mice. Neuroscience Letters. 2011 Mar 3;490(3):231–236. doi: 10.1016/j.neulet.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 59.Kadota K, Iijima N, Ohe-Hayashi Y, et al. Time-dependent repression of mPer2 expression in the suprachiasmatic nucleus by inhalation anesthesia with sevoflurane. Neurosci Lett. 2012 Aug 10; doi: 10.1016/j.neulet.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 60.Pollmacher T, Mullington J, Korth C, et al. Diurnal variations in the human rest response to endotoxin. Journal of Infectious Diseases. 1996 Nov;174(5):1040–1045. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]
- 61.Ferraz E, Borges MC, Terra Filho J, Martinez JAB, Vianna EO. Comparison of 4 AM and 4 PM bronchial responsiveness to hypertonic saline in asthma. Lung. 2006 Dec;184(6):341–346. doi: 10.1007/s00408-006-0017-0. [DOI] [PubMed] [Google Scholar]
- 62.Cutolo M, Straub RH. Circadian rhythms in arthritis: Hormonal effects on the immune/inflammatory reaction. Autoimmunity Reviews. 2008 Jan;7(3):223–228. doi: 10.1016/j.autrev.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010 Apr;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 64.Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009 Dec 15;106(50):21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arjona A, Sarkar DK. Circardian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. Journal of Immunology. 2005 Jun 15;174(12):7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 66.Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behavior and Immunity. 2006 Sep;20(5):469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behavior and Immunity. 2012 Mar;26(3):407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bozek K, Relogio A, Kielbasa SM, et al. Regulation of Clock-Controlled Genes in Mammals. PloS one. 2009 Mar 16;4(3) doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gibbs JE, Blaikley J, Beesley S, et al. The nuclear receptor REV-ERB alpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jan 10;109(2):582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jul 31;109(31):12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silver AC, Arjona A, Walker WE, Fikrig E. The Circadian Clock Controls Toll-like Receptor 9-Mediated Innate and Adaptive Immunity. Immunity. 2012 Feb 24;36(2):251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fortier EE, Rooney J, Dardente H, Hardy M-P, Labrecque N, Cermakian N. Circadian Variation of the Response of T Cells to Antigen. Journal of Immunology. 2011 Dec 15;187(12):6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- 73.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985 Apr 6;290(6474):1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hilton BA. Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976 Nov;1(6):453–468. doi: 10.1111/j.1365-2648.1976.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 75.Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001 Feb;7(1):21–27. doi: 10.1097/00075198-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Krachman SL, D’Alonzo GE, Criner GJ. Sleep in the intensive care unit. Chest. 1995 Jun;107(6):1713–1720. doi: 10.1378/chest.107.6.1713. [DOI] [PubMed] [Google Scholar]
- 77.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001 Feb;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 78.Bourne RS, Mills GH. Sleep disruption in critically ill patients–pharmacological considerations. Anaesthesia. 2004 Apr;59(4):374–384. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 79.Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin. 2006 Apr;22(2):313–327. vii. doi: 10.1016/j.ccc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 80.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009 Jan;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cammarano WB, Pittet JF, Weitz S, Schlobohm RM, Marks JD. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998 Apr;26(4):676–684. doi: 10.1097/00003246-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients - The MENDS randomized controlled trial. JAMA-J Am Med Assoc. 2007 Dec;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 83.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Critical care (London, England) 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]


