Abstract
Archaeological and palaeoecological studies throughout the Americas have documented widespread landscape and environmental transformation during the pre-Columbian era. The highly dynamic Formative (or Neolithic) period in northern Chile (ca. 3700–1550 yr BP) brought about the local establishment of agriculture, introduction of new crops (maize, quinoa, manioc, beans, etc.) along with a major population increase, new emergent villages and technological innovations. Even trees such as the Algarrobos (Prosopis section Algarobia) may have been part of this transformation. Here, we provide evidence that these species were not native to the Atacama Desert of Chile (18–27°S), appearing only in the late Holocene and most likely due to human actions. We assembled a database composed of 41 taxon specific AMS radiocarbon dates from archaeobotanical and palaeoecological records (rodent middens, leaf litter deposits), as well an extensive bibliographical review comprising archaeobotanical, paleoecological, phylogenetic and taxonomic data to evaluate the chronology of introduction and dispersal of these trees. Although Algarrobos could have appeared as early as 4200 yr BP in northernmost Chile, they only became common throughout the Atacama over a thousand years later, during and after the Formative period. Cultural and natural factors likely contributed to its spread and consolidation as a major silvicultural resource.
Introduction
The impact of humans on the surface of the Earth over recent centuries has been so significant that there are concrete initiatives to formally define this recent timeframe as a new geological epoch or even era, aptly termed the “Anthropocene” [1–3]. Despite some consensus regarding its causes, the academic community remains divided on when this epoch began. Most authors consider a late onset by the Industrial Revolution [1, 2] and others after the Great Acceleration by the mid-20th century [4]. In contrast, some argue for a much older onset, first by hunter-gatherers followed by an intensification after the Formative period (the regional Neolithic epoch dated at 3700–1550 yr BP) when food production systems (e.g. husbandry and agriculture) rose in importance along with more permanent settlements [5–9].
We examined available evidence to determine whether Algarrobo trees belonging to Prosopis, Algarobia section (from here on we will use the term Algarrobo to include all the described species of this section) could have been introduced by humans from the Chaco Region (in modern Paraguay, Bolivia and Argentina) into the Atacama Desert during the late Holocene. This hypothesis challenges the current idea that this iconic tree was native in Chile, an assertion based on the presence and visibility of these resources from the Formative until the present [10–16]. Their importance for pre-Columbian groups has been emphasized by calling them “the people of Chañar and Algarrobo” [17]. These species, however, were largely absent before the Formative period [18–23]. A rapid spread from a single or several introductions would have transformed the desert landscape significantly. Such a spread could have been further facilitated by natural and/or cultural vectors in a context of increasing moisture availability [16, 24–26] along with major cultural transformations [27, 28].
Taxonomy, distribution and biogeography of Prosopis L
The genus Prosopis (Fabaceae-Mimosoidea) is amphitropical and found throughout the arid and semiarid regions of the world. Around 44 species have been described, most of them native to the Americas with one from tropical Africa and three from Southeast Asia [29, 30]. Over 80% of these species occur in South America, grouped into three sections: Strombocarpa (eight species), Algarobia (30 species) and the monospecific Monilocarpa section (Prosopis argentina) in Argentina [29].
The disjunct distribution of Prosopis in the Americas has led to contrasting hypotheses regarding its evolutionary history. Most scholars agree that the main centre of diversity and polymorphism is the Argentine–Paraguayan–Chilean region. A secondary diversity centre has also been proposed in the Texan–Mexican region [29, 31–36]. The paleotropical hypothesis postulates that Prosopis spread from Africa to North America during the Paleogene, and then subsequently dispersed into South America [37]. In contrast, Simpson [36] proposed that ancestral Prosopis species crossed from Africa to South America and propagated into North America before the radiation of major known lineages.
Under this vicariant scenario, Prosopis experienced subsequent convergent evolution. Pasiecznik et al. [33], suggests that the P. pallida/P. juliflora complex occupies the middle ground between the Texan–Mexican and Argentinean–Paraguayan–Chilean centres; these dispersed to North America via the Andes from Argentina to Bolivia and from there into the hyperarid coast of Chile and southern Peru, before spreading northwards. In contrast, Bessega et al. [38], proposed ancestral areas for the American species in western USA, Mexico, Peru, Ecuador as well as central-northern Argentina, with further lineage fragmentations brought about by successive vicariance events.
The American main sections differ in morphological traits and phytogeographic history [30, 31, 39, 40] (Fig 1). For instance, the Strombocarpa section, divided into Strombocarpae and Cavenicarpae series can produce wood, shade, fuel and ‘screwbeans’ or “tornillos” seed pods that provide good fodder, but are not edible to humans. In contrast, the diverse Algarobia section includes valuable trees with a wide variety of uses such as shade, fuel, food and forage for wildlife, livestock and human consumption [29, 33, 41]. Hence, multiple social and economic benefits of the Algarobia section have motivated their deliberate introduction into different countries [33, 42, 43]. Burkart [29] divided this section into six series: Sericanthae, Ruscifoliae, Humiles, Denudantes, Pallidae and Chilenses. Their taxonomy, however, is confounded by elevated intraspecies phenotypic variation, low genetic differentiation, and frequent hybridization [33, 38, 40, 44]. Molecular clocks show that both sections diverged during the Oligocene, with a more recent event between the extant series during the Miocene. The diversification of Algarobia into the Ruscifoliae, Chilenses and Pallidae series likely occurred during the late Pliocene [32].
Fig 1. Prosopis sections seed pods collected by the authors in northern Chile.
Scale bar represents 1 cm. a) cf. Prosopis alba; b) cf. Prosopis flexuosa; c) cf. Prosopis chilensis; d) Prosopis tamarugo.
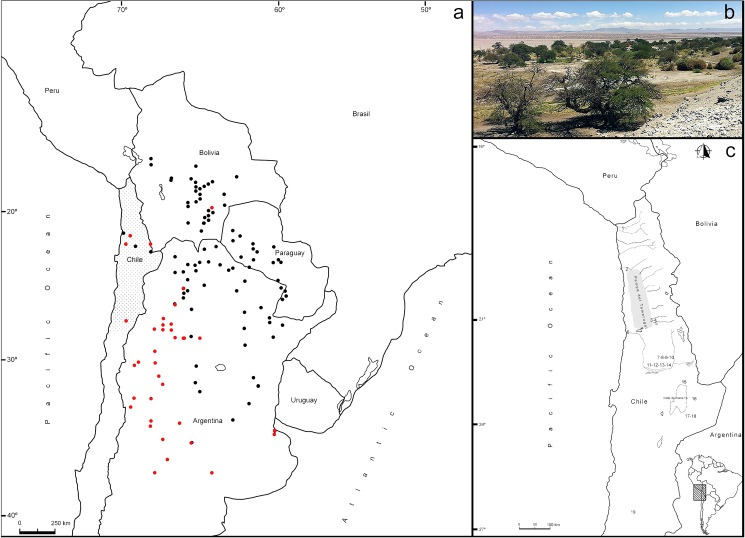
Prosopis species present in the Atacama Desert belong to the Strombocarpa or the Algarobia section. These can be found at up to 3000 masl of elevation throughout the western Andean slope, and are typically confined to discrete zones of groundwater discharge and/or on riverbanks of perennial/ephemeral watersheds from the Pampa del Tamarugal basin to the Salar the Atacama [45–48] (Fig 2A and 2B). Dominant stands in the Pampa del Tamarugal forest are mostly Strombocarpa, including two endemic species Prosopis tamarugo and Prosopis burkartii, as well as Prosopis strombulifera, which is common in northern Chile and northwestern Argentina [29, 49–51].
Fig 2.
(A). The modern distribution of Prosopis alba (black dots) and P. flexuosa (red dots) section Algarobia in central South America (GBIF Secretariat: GBIF Backbone Taxonomy. doi:10.15468/39omei. Accessed via http://www.gbif.org/species/5358452 on 2017-04-10; http://www.gbif.org/species/5358528 on 2017-04-10). Note the widespread distribution in NW Argentina, eastern Paraguay and southern Bolivia compared to the distribution in northern Chile. (B) Algarrobos growing in an oasis of the Atacama Desert (C) Archaeological and palaeoecological sites dated in this study, see also S3 Table (1. Lluta 13; 2. Tiliviche 1B; 3. Guatacondo 4. Ramaditas; 5. Quebrada de Maní 6. Caleta Huelén 42; 7. Loa W3; 8. Loa River; 9. Salado River/El Sifón; 10. Salado River/Las Juntas; 11. Chiu Chiu 200; 12. Confluencia 1; 13. Confluencia 2; 14. Chiu Chiu Cementerio; 15. Calar 1; 16. Talabre Viejo; 17. Tarajne; 18. Vegas de Tilocalar; 19. Finca Chañaral).
Species of the Algarobia section are more widely distributed and range from the Pampa del Tamarugal to the Salar de Atacama basin. There is no consensus as to which species of this section currently grows in the Atacama, although most have been attributed to the Chilenses series (Table 1), [29, 45, 47, 52].
Table 1. Atacama Desert Algarobia section species.
| Series | Species Algarobia section | Synonymns |
|---|---|---|
| Chilenses | P. alba Gris. | P. atacamensis Phil. |
| Chilenses | P. alba var. Panta Gris. | ——- |
| Chilenses | P. flexuosa DC. | P. fruticose Meyen |
| Chilenses | P. chilensis Mol. | P. siliquastrum DC. |
| Chilenses | P. nigra Gris. | ——- |
The biogeography of the Algarobia section is complex. Roig [34] suggested a radiation centre located in the Chaco ecoregion, and subsequent migrations to the southern and western arid regions of South America. As with other legumes common to arid zones, Algarobia could have migrated across the tropics by successfully colonizing one patch after another [35]. According to Bessega et al. [38] and Catalano et al. [32] the diversification and dispersal of the Chilenses series in the Chaco lowlands was closely linked to the onset of aridity in the late Pliocene/Pleistocene, but the timing of dispersion remains controversial. A Pliocene/Pleistocene age implies that Algarobia species expanded into the Atacama Desert after the uplift of the Andes [33, 53, 54], which represents a prominent biogeographical barrier for east-west biotic exchanges in southern South America for at least the past 13 million years.
A rich South American megafauna of frugivores was well suited for long-distance dispersal of seeds [55], and could have facilitated the dispersal of Algarrobo [56]. Though, most of these animals became extinct during the late Pleistocene in the Atacama Desert [57–59], several millennia before Algarrobos appeared there (see results). Endozoocharic dispersal has also been documented for smaller mammals, [29, 60–62] but these would result in radiation rates that are to slow to account for observed genetic distances and disjunct distributions among the Prosopis species from the Algarobia section [38, 63]. Even though several native species such as guanacos and foxes have been reported as seed dispersers [64] their effect is often on a local scale and such mammal vectors are not effective over long distances [65]. There are no records of Prosopis being dispersed by birds [38], or these vectors are otherwise considered minor seed disseminators [33].
Under such a scenario, how and when did the Chilenses series Prosopis species cross over the Andes into the hyperarid Atacama Desert? What dispersal mechanisms were involved? Or perhaps the puzzling biogeography of the Algarobia section points to a human pre-Columbian dispersal event or events?
Material and methods
To establish the arrival and spatial distribution of Algarrobo in the Atacama Desert, we analysed bibliographical references and different geohistorical records from the northern (18–21°S), central (21–24°S) and southern (24–27°S) Atacama Desert (S1 Text).
We also examined in detail the reported presence of Algarobia remains in 17 available pre-Formative and Early Formative archaeological sites from museum collections, as well from data available in archaeological databases (S1 Table). 207 rodent middens (nest deposits built of feces, plant materials, insects, vertebrate remains and encased in urine) collected from 16 different sites nearby archaeological settlements [see 66, 67 for data collection] were also included in our dataset (S2 Table). Finally, we analysed naturally occurring leaf-litter deposits [68] from the southern Pampa del Tamarugal basin that have yielded Algarrobo macrofossils (S2 Table) (i.e. leaves, stems, pods).
All available and purportedly older (than late Holocene) archaeological and palaeoecological remains positively identified as Algarrobo were directly AMS-radiocarbon dated (see Fig 2C). For archaeological samples, specimens found in contexts earlier than ca. 3700 yr BP were given priority for AMS dating. Radiocarbon ages reported here are calibrated using CALIB 7.0.4 at 2-sigma using the Southern Hemisphere calibration curve (SHCal13 Intercept Method); [69] and are given in calendar years before 1950 (cal yr BP).
Results
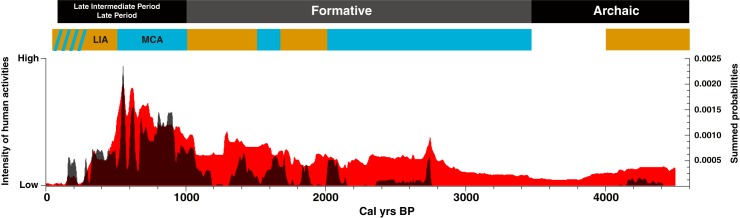
The majority of our direct AMS dates on Algarrobo are from the last 3000 cal yr BP (Fig 3).
Fig 3. The intensity of human activities in the Atacama Desert (red curve, from [70]) compared to a summed probability distribution of all AMS radiocarbon dates on Prosopis Algarobia remains from archaeological and paleoecological contexts (dark curve).
The top bar indicates the major cultural periods and paleoclimate variations. Blue bars (orange) represent past wet (dry) climate anomalies. MCA: Medieval Climate Anomaly. LIA: Little ice Age.
A total of 18 specimens from 11 archaeological sites were found and sampled, with a mean of 1036 cal yr BP (S3 Table). The archaeological literature shows scant macroscopic evidence for the presence of Algarrobo before the Formative period [18–23, 71, 72] whereas it became very abundant afterwards [10–15] (see S4 Table). Some preceramic sites (we could not obtain access either Tulan or Cañamo), exhibit an even younger taxon specific 14C chronology, with a mean of 581 cal yr BP. This demonstrates the importance of direct dating when tracking the introduction of specific archaeological features. Preliminary microfossil analyses on processing instruments also indicate that Prosopis species are absent before the onset of the Formative [20]. From a total of 207 scanned rodent middens, only 13 contained Algarrobo endocarps. These were all directly dated and gave a mean age of 970 cal yr BP (S3 Table). Previous plant macrofossils from rodent middens, show that Algarrobo appeared as recently as 700 cal yr BP in the Rio Salado, a tributary of the Loa River [e.g. 66]. Pollen records from rodent middens collected in the Loa basin and oases of the Salar de Atacama (2000–2400 masl) show that Prosopis pollen appears after 400 cal yr BP (Maldonado personal communication 2013).
Late Pleistocene (14600–14400 cal yr BP) leaf litter deposits at Lomas de Sal reveal a dense sub-fossil tree community of the endemic Prosopis tamarugo (Strombocarpa section) as well as other hygrophytic and phreatophytic trees (Schinus molle, Escallonia angustifolia, Myrica pavonis, etc.) [24, 68, 73]. In contrast, leaves and stems retrieved from late Holocene leaf-litter beds slightly further north (at Quebrada Maní) are Algarobia species (cf. Prosopis alba) that date to as early as ~2000 cal yr BP [24, 73] with a mean within of 892 yr cal. BP (S3 Table).
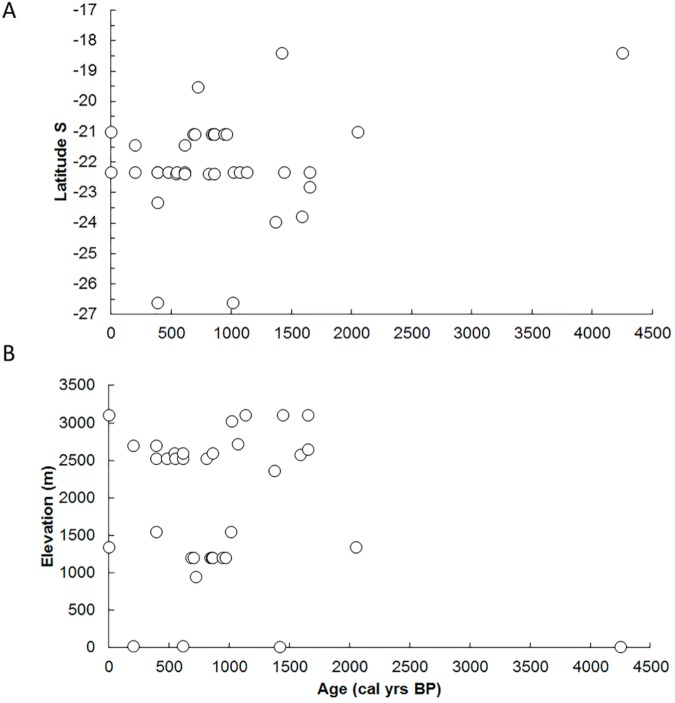
The earliest record of Algarrobo in our sample is a single charred cf. Prosopis endocarp which appears at 4250 cal yr BP at our northernmost site along the coast (Lluta 13). Some 2200 years later (at 2050 cal yr BP), Algarrobo appears more than 3° further south at Ramaditas (an archaeological site in Pampa del Tamarugal basin). Algarrobo remains then appear several hundred years later at the higher elevations and increased latitudes of the central Atacama Desert (22–24°S). The earliest presence in the Calama basin is at 1650 cal yr BP (Río Salado/Las Juntas- a rodent midden site) and appears slightly later in the middle Rio Loa and in the Salar de Atacama basin at ca. 1590 cal yr BP. The youngest ages for Algarrobo appearance in our dataset are from the southernmost sites in the Atacama at ca. 1000 cal yr BP (Finca de Chañaral). The remainder of the samples dated all fall within last 1440 cal yr BP or are modern (Fig 4A and 4B).
Fig 4.
(A) A taxon-specific AMS date distribution of the occurrence of Algarrobo remains versus latitude. (B) A taxon-specific AMS date distribution of the occurrence of Algarrobo remains versus elevation.
Discussion
Literature reviews, macrofossil analyses from palaeoecological and archaeobotanical archives combined with direct dating of Algarobia remains, provide elements for understanding and revaluate the long-term history of Algarrobo in ecosystems of the Atacama over the past 15000 cal yr BP (S3 Table). The Archaic age (4250 cal yr BP) from Lluta 13 in the northernmost sector of the Atacama is an interesting outlier (Fig 4A and 4B) within the site and for the region. One possibility is that it corresponds to Prosopis pallida from the Peruvian coast, rather than the nowadays common species in the Atacama (P. flexuosa, P. alba). Prosopis pallida is part of the Pallidae Series of the Algarobia section and though its origin is intriguing, its distribution is clearly in Peru [33]. In fact, such Prosopis remains are ubiquitous in archaeological contexts since the early Holocene along the northern and central coast of Peru [74–77]. Given the paucity of our remains (in contrast to abundant Prosopis remains in all our archaeological contexts), only further analyses will confirm if this specimen is part of a wider chronological distribution in the area, or if it is part of the Atacama species (and not from Peru e.g. P. pallida). Currently there is no information on early archaeobotanical remains in the Argentinian or Bolivian Chaco, although for the northwestern Argentinean Puna, Algarrobo pods are known since 10000 yr BP [78].
The fact that such a multipurpose and invasive species such as Algarrobo does not appear profusely in either the archaeological or palaeoecological records in the Atacama until late in the Holocene has been explained either by seasonality [19, 79, 80], taphonomic biases or a possible range contraction of Algarrobo during a proposed mid-Holocene arid phase [66, 81–83]. A late Holocene appearance would be consistent with known biogeographical data, which points to an origin east of the Andes with a late Pliocene/Pleistocene speciation in the Chaco Region. Its appearance in the Atacama was also contemporaneous with multiple and supra-regional cultural exchanges across the Andes during the Formative. “With introductions made before recorded history, known human population movements, changes in land use and the spread of other plants and animals can be used as indicators of possible spread of Prosopis” [33].
In the Atacama, the spread of Algarrobo was contemporaneous with the introduction by humans of other crops such as quinoa, maize, manioc, and sweet potatoes from the high Andes and the tropical forest [84, 85] and with intensified camelid caravan traffic [28]. New settlement patterns in the Atacama Desert might be another indicator for the introduction of these trees. For instance, in the Pampa del Tamarugal the most prominent Formative sedentary villages such as Guatacondo, Ramaditas, Pircas, Caserones and Quebrada Maní (3000–1000 yr BP) arose in previously unoccupied locations [14, 16, 24, 86]. This is also true for sites in the Loa and Salar de Atacama basins, such as Chiu Chiu 200 and Tulor 1 (ca. 3000–1400 yr BP), which shifted away from settlements located in ravines during the Archaic, to the more expansive oases from the Formative onwards [10, 12, 87, 88]. In all of these Formative settlements, the presence of Algarrobo in domestic and burial sites as well as in buried forests in the surroundings is pervasive [11, 12, 15, 88–91].
Whether intended or not, the introduction of given plants is hard to examine in pre-Columbian times [92]. Indeed, a combination of both is possible. The intended cultivation of Algarrobo has been acknowledged by Palacios and Brizuela [93] who stated that “the presence and similarities between Algarrobos from different localities in the Americas are a consequence of their cultivation, and this appears to be a case for domestication that, as with other American crops, did not persist with the arrival of European settlers” (translated from Spanish by the authors). Furthermore, there are several examples of prehistoric tree management [56, 94–98]. Regarding Algarrobo in Atacama, the Spanish historian Oviedo and Valdes [99] in his 1535 chronicle states that Algarrobo woodlands from the San Pedro de Atacama basin oasis were the product of long-lasting farming activities developed by native inhabitants; and Palacios and Brizuela [93] highlight the considerable exo-morphological similarities between these trees and Prosopis alba specimens found in the Salta District (NW Argentina). This has led several authors to suggest that Prosopis alba may have been introduced from Argentina into the Atacama during pre-Hispanic times [51, 100–102].
As for unintended consequences, Prosopis trees are highly invasive, so once brought in they can colonize extensive areas even from single introductions [33]. This could have been facilitated by climate, which became more hospitable in the area after 2500 yrs BP (14–18). In South Africa, the spread of Prosopis species followed periods of high rainfall [103, 104] possibly due to improved conditions for germination and establishment or perhaps increased seed dispersal events. A long-term study in northern India found that Prosopis juliflora is a pioneer species of denuded or abandoned ravines [105]. In the Atacama, Homero Altamirano (a former national parks ranger) has seen how seeds sprout after being carried by flash floods and it is also common to see fox feces full of seeds [106]. The role that these forests could have had for camelid fodder or other silviopastoralist activities has not been addressed for the Atacama, but has been discussed for prehistoric studies from coastal Peru [107]. In North America, various studies document the encroachment of these trees in grassland ecosystems when herbivores are introduced [108], and although such a mechanism could have preceded humans, it would have intensified when they arrived at suitable areas with domestic and wild animals.
The biogeographical, archaeological and palaeoecological data shown here provides evidence that Algarrobo appeared in the Atacama late in the Holocene (ca. 4000 yr BP or later), and that the most likely vectors were humans. The rapid spread within the Atacama Desert was probably the result of a combination of both natural (geo-dispersal, endozoochary) and cultural actions (either intended as management and cultivation, or unintended such as encroachment of trees due to domestic camelids spreading their seeds near settlements). By ca. 1000 yr BP, these trees became a main resource and colonized landscapes across a wide range of sites and in multiple contexts.
Further dating is needed as new remains appear in different contexts. A critical review of the biogeographic, phylogenetic and taxonomic studies together with better phylogeographic data will also increase our understanding of the presence of this genus in Chile and adjacent areas. Moreover, the relevance of these trees in archaeological and ethnographical contexts implies that we need to better establish the nature of human-Algarrobo interactions. Such assessments will provide new knowledge and challenges for upcoming contingences, as well as a comprehensive approach to indigenous silviculture that succeeded for thousands of years.
Supporting information
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Dates are referential to the sites.
(XLSX)
Acknowledgments
We thank the Anthropology Department of the Universidad de Antofagasta; Anthropology Department of the Universidad de Chile; Herbarium Museo Nacional de Historia Natural de Chile; Herbarium Forestry and Nature Conservation Department, Universidad de Chile; Herbarium of the Botany Department of the Universidad de Concepción; and the Iquique Regional Museum. We also thank multiple researchers and museum or herbarium staff, for their help with information or procurement of the specimens analyzed for this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by FONDECYT grants 3150638 (V.M), 1160744 (C.M.S., C.L. and E.M.G) and 11150210 (E.M.G), FONDAP 1511009 (to CR2), CONICYT/PIA Anillo project SOC1405 (V.M, E.M.G., C.L and C.M.S), CONICYT PFB-23 to the IEB (C.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crutzen PJ. Geology of mankind. Nature. 2002;415(6867):23 doi: 10.1038/415023a [DOI] [PubMed] [Google Scholar]
- 2.Crutzen PJ, Stoermer EF. The Anthropocene. Global Change Newsletter. 2000;41:17–8. [Google Scholar]
- 3.Steffen W, Leinfelder R, Zalasiewicz J, Waters CN, Williams M, Summerhayes C, et al. Stratigraphic and Earth System approaches to defining the Anthropocene. Earth's Future. 2016;4(8):324–45. [Google Scholar]
- 4.Zalasiewicza J, Watersb CN, Williamsa M, Barnoskyc AD, Cearretad A, Crutzene P, et al. When did the Anthropocene begin? A mid-twentieth century boundary level is stratigraphically optimal. Quaternary International. 2015;383:196–203. [Google Scholar]
- 5.Braje TJ, Erlandson JM, Aikens CM, Beach T, Fitzpatrick S, Gonzalez S, et al. An anthropocene without archaeology—should we care? The SAA Archaeological Record 2014;14(1):26–8. [Google Scholar]
- 6.Ellis EC, Fuller DQ, Kaplan JO, Lutters WG. Dating the Anthropocene: Towards an empirical global history of human transformation of the terrestrial biosphere. Elementa: Science of the Anthropocene. 2013;1(1):000018. [Google Scholar]
- 7.Ruddiman WF. The anthropogenic greenhouse era began thousands of years ago. Climatic change. 2003;61(3):261–93. [Google Scholar]
- 8.Ruddiman WF, Fuller DQ, Kutzbach JE, Tzedakis PC, Kaplan JO, Ellis EC, et al. Late Holocene climate: Natural or anthropogenic? Reviews of Geophysics. 2015;54(1):93–118. [Google Scholar]
- 9.Smith BD, Zeder MA. The onset of the Anthropocene. Anthropocene. 2013;4:8–13. [Google Scholar]
- 10.Agüero C, Uribe M. Las sociedades Formativas de San Pedro de Atacama: Asentamiento, cronología y proceso. Estudios Atacameños Arqueología y Antropología Surandinas. 2011;42:53–78. [Google Scholar]
- 11.García M, Vidal A, Mandakovic V, Maldonado A, Peña MP, Belmonte E. Alimentos, tecnologías vegetales y paleoambiente en las aldeas de la Pampa del Tamarugal: dos expresiones del periodo Formativo en Tarapacá (ca. 900 a.C.-800 d.C.). Estudios Atacameños Arqueología y Antropología Surandina. 2014;47:33–58. [Google Scholar]
- 12.Llagostera A, Barón AM, Bravo L. Investigaciones arqueológicas en Tulor 1. Estudios Atacameños. 1984;7:105–15. [Google Scholar]
- 13.Mostny G, González R, Obenhauser F. Peine un Pueblo Atacameño: Instituto de Geografía Universidad de Chile; 1951.
- 14.Rivera MA, Dodd JP. Domesticando el desierto: medio ambiente y ocupaciones humanas en Ramaditas, Desierto de Atacama. Diálogo andino. 2013;(41):45–60. [Google Scholar]
- 15.Vidal A. Patrones de uso de los recursos vegetales durante el periodo Formativo (1000 aC-500 dC) en San Pedro de Atacama: oasis y quebradas [Unpublished undergraduate thesis]. Santiago: Universidad de Chile; 2007.
- 16.Adán L, Urbina S, Pellegrino C, Agüero C. Aldeas en los bosques de prosopis. Arquitectura residencial y congregacional en el período Formativo tarapaqueño (900 AC-900 DC). Estudios Atacameños Arqueología y Antropología Surandinas. 2013;45:75–94. [Google Scholar]
- 17.Martínez JL. Pueblos del Chañar y el Algarrobo, los Atacama en el siglo XVI. Santiago: Dirección de Bibliotecas Archivos y Museos, Universidad de Chile, Centro de Investigaciones Diego Barros Arana; 1998.
- 18.Druss M. Medio ambiente, economía de subsistencia y patrones de asentamiento del Complejo Chiu-Chiu (ca. 3000 a 2000 AC), norte de Chile. Estudios Atacameños. 1976;4:19–24. [Google Scholar]
- 19.Holden TG. Evidence of prehistoric diet from northern Chile: coprolites, gut contents and flotation samples from the Tulán Quebrada. World Archaeology. 1991;22(3):320–31. doi: 10.1080/00438243.1991.9980149 [DOI] [PubMed] [Google Scholar]
- 20.McRostie VB. The Role of Plant Production in Subsistence and Cultural changes during the Formative Period in the Atacama Puna, Southern Andes, Chile (1400BC-500AD). A Re-evaluation based on the Analyses of Microfossils Attached to Hoes and Grinding Tools, and Isotopic Analyses of Human Bones [Ph.D. Disertation]. London: University College London; 2013.
- 21.Núñez L, Moragas C. Una ocupación con cerámica temprana en la secuencia del distrito de Cáñamo (costa desértica del norte de Chile). Estudios Atacameños. 1977;5:21–49. [Google Scholar]
- 22.Núñez L, McRostie VB, Cartajena I. Consideraciones sobre la recolección vegetal y la horticultura durante el Formativo Temprano en el sureste de la cuenca de Atacama. Darwiniana. 2009;47(1):56–75. [Google Scholar]
- 23.Williams LR. Analysis of coprolites recovered from six sites in northern Chile In: Meighan CW, True DL, editors. Prehistoric Trails of Atacama: Archaeology of Northern Chile. Monumenta Archaeologica. Los Angeles: The University of California; 1980. p. 195–204. [Google Scholar]
- 24.Gayo EM, Latorre C, Santoro CM, Maldonado A, De Pol-Holz R. Hydroclimate variability on centennial timescales in the low-elevation Atacama Desert over the last 2,500 years. Climate of the Past. 2012;8:287–306. [Google Scholar]
- 25.Barón AM. Tulor: posibilidades y limitaciones de un ecosistema. Chungara. 1986;16–17:149–58. [Google Scholar]
- 26.Santoro CM, Gayo EM, Capriles JM, de Porras ME, Maldonado A, Standen VG, et al. Continuities and discontinuities in the socio-environmental systems of the Atacama Desert during the last 13,000 years. Journal of Anthropological Archaeology. 2016. doi: doi: 10.1016/j.jaa.2016.08.006 [Google Scholar]
- 27.Uribe M. El Formativo: ¿progreso o tragedia social? Reflexiones sobre evolución y complejidad social desde Tarapacá (norte de Chile, Andes Centro Sur) In: Acuto FA, Zarankin A, editors. Sed Non Satiata II. Córdoba: Encuentro Grupo Editor; 2008. p. 257–77. [Google Scholar]
- 28.Muñoz I, ero C, Valenzuela D. Poblaciones prehispánicas de los valles occidentales del norte de Chile: desde el período Formativo al Intermedio Tardío (ca. 1.000 a.c.– 1.400 d.c.) In: Falabella F, Uribe M, Sanhueza L, Aldunate C, Hidalgo J, editors. Prehistoria en Chile desde sus Primeros Habitantes hasta los Incas. Santiago: Editorial Universitaria y Sociedad Chilena de Arqueología; 2016. p. 181–237. [Google Scholar]
- 29.Burkart A. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). Journal of the Arnold Arboretum. 1976;57(3):219–49. [Google Scholar]
- 30.Hunziker JH, Saidman BO, Naranjo CA, Palacios RA, Poggio L, Burghardt AD. Hybridization and genetic variation of Argentine species of Prosopis. Forest Ecology and Management. 1986;16(1–4):301–15. [Google Scholar]
- 31.Burkart A, Simpson BB. The genus Prosopis and annotated key to the species of the world Mesquite: Its Biology in two Desert Scrub Ecosystems Stroudsburg: Dowden, Hutchinson and Ross Inc; 1977. p. 201–35. [Google Scholar]
- 32.Catalano SA, Vilardi JC, Tosto D, Saidman BO. Molecular phylogeny and diversification history of Prosopis (Fabaceae: Mimosoideae). Biological Journal of the Linnean Society. 2008;93(3):621–40. [Google Scholar]
- 33.Pasiecznik NM, Felker P, Harris PJC, Harsh LN, Cruz G, Tewari JC, et al. The Prosopis Juliflora—Prosopis Pallida Complex: A Monograph. Coventry, UK: HDRA; 2001. [Google Scholar]
- 34.Roig FA. Aportes a la etnobotánica del género Prosopis. In: IADIZA, editor. Contribuciones Mendocinas a la Quinta Reunión Regional para América Latina y el Caribe de la Red de Forestación del CIID Conservación y Mejoramiento de Especies del Género Prosopis. Mendoza: Unidades de Botánica y Fisiología Vegetal, IADIZA; 1993. p. 99–119.
- 35.Simpson BB, editor. Mesquite, its Biology in two Desert Shrub Ecosystems. Strondsberg: Dowden, Hutchinson & Ross; 1977. [Google Scholar]
- 36.Simpson BB, Tate JA, Weeks A. The biogeography of Hoffmannseggia (Leguminosae, Caesalpinioideae, Caesalpinieae): a tale of many travels. Journal of Biogeography. 2005;32(1):15–27. [Google Scholar]
- 37.Lavin M, Luckow M. Origins and relationships of tropical North America in the context of the boreotropics hypothesis. American Journal of Botany. 1993;80:1–14. [Google Scholar]
- 38.Bessega C, Vilardi JC, Saidman BO. Genetic relationships among American species of the genus Prosopis (Mimosoideae, Leguminosae) inferred from ITS sequences: evidence for long‐distance dispersal. Journal of Biogeography. 2006;33(11):1905–15. [Google Scholar]
- 39.Bessega C, Saidman BO, Vilardi JC. Genetic relationships among American species of Prosopis (Leguminosae) based on enzyme markers. Genetics and Molecular Biology. 2005;28(2):277–86. [Google Scholar]
- 40.Saidman BO, Vilardi JC, Pocovi MI, Acreche N. Genetic divergence among species of the section Strombocarpa, genus Prosopis (Leguminosae). Journal of Genetics. 1996;75(2):139–49. doi: doi: 10.1007/bf02931757 [Google Scholar]
- 41.Saidman BO, Bessega C, Ferreyra LI, J N., Vilardi JC. The use of genetic markers to assess populations structure and relationships among species of the genus Prosopis (Leguminosae). Boletín Sociedad Argentina Botánica. 2000;35(3–4):315–24. [Google Scholar]
- 42.Booth FEM, Sickens GE. Non-timber uses of selected arid zone trees and shurbs in Africa. Rome: FAO Conservation Guide 19. Food and Agriculture Organization of the United Nations; 1988.
- 43.Shackleton RT, Le Maitre DC, Pasiecznik NM, Richardson DM. Prosopis: a global assessment of the biogeography, benefits, impacts and management of one of the world's worst woody invasive plant taxa. AoB Plants. 2014;6:plu027 doi: 10.1093/aobpla/plu027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bessega CF, Pometti CL, Miller JT, Watts R, Saidman BO, Vilardi JC. New microsatellite loci for Prosopis alba and P. chilensis (Fabaceae). Applications in Plant Sciences. 2013;1(5):1200324. doi: org/10.3732/apps.1200324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barros S, Wrann J. El género Prosopis en Chile. Ciencia e Investigación Forestal. 1992;6(2):296–334. [Google Scholar]
- 46.Briner C. Caracterizacion fenotípica de los biotipos de tamarugo en la Pampa del Tamarugal In: Habit MA, editor. Estado Actual del Conocimiento sobre Prosopis tamarugo. Santiago: FAO; 1985. p. 233–7. [Google Scholar]
- 47.Carevic F, Carevic A, Delatorre J. Historia natural del género Prosopis en la Región de Tarapacá. Idesia. 2012;30(3):113–7. [Google Scholar]
- 48.Habit MA, Contreras DT, Gonzalez RH, editors. Prosopis Tamarugo: Fodder Tree for Arid Zones. Santiago: FAO Regional Office for Latin America; 1980. [Google Scholar]
- 49.Chiappa E, Villasenor R, Toro H, Covarrubias R. Táctica reproductiva de Prosopis (Mimosaceae) y asociaciones ecológicas de sus polinizadores, en el desierto del norte de Chile. Multequina. 1997;6:9–20. [Google Scholar]
- 50.Muñoz C. Plantas en Extinción. Santiago: Editorial Universitaria; 1973. [Google Scholar]
- 51.Trobok S. Morfología de frutos y semillas de Prosopis (Fabaceae-Mimosoideae) chilenos In: Habit MA, editor. Estado Actual del Conocimiento sobre Prosopis tamarugo. FAO: Roma; 1985. p. 239–53. [Google Scholar]
- 52.Benoit CI, editor. Libro Rojo de la Flora Terrestre de Chile (Primera Parte). Santiago: Corporacion Nacional Forestal; 1989. [Google Scholar]
- 53.Garzione CN, Hoke GD, Libarkin JC, Withers S, MacFadden B, Eiler J, et al. Rise of the Andes. Science. 2008;320(5881):1304–7. doi: 10.1126/science.1148615 [DOI] [PubMed] [Google Scholar]
- 54.Luebert F, Weigend M. Phylogenetic insights into Andean plant diversification. Frontiers in Ecology and Evolution. 2014;2(27):1–17. doi: doi: 10.3389/fevo.2014.00027 [Google Scholar]
- 55.Long A, Martin PS. Death of American ground sloths. Science. 1974;186(4164):638–40. doi: 10.1126/science.186.4164.638 [DOI] [PubMed] [Google Scholar]
- 56.Fredrickson EL, Estell RE, Laliberte A, Anderson DM. Mesquite recruitment in the Chihuahuan Desert: historic and prehistoric patterns with long-term impacts. Journal of Arid Environments. 2006;65(2):285–95. [Google Scholar]
- 57.Alberdi MT, Prado JL, López P, Labarca R, Martínez I. Hippidion saldiasi Roth, 1899 (Mammalia, Perissodactyla) en el Pleistoceno tardío de Calama, norte de Chile. Revista Chilena de Historia Natural. 2007;80(2):157–71. [Google Scholar]
- 58.Grosjean M, Núñez L, Cartajena I. Palaeoindian occupation of the Atacama Desert, northern Chile. Journal of Quaternary Science. 2005;20(7–8):643–53. PubMed PMID: ISI:000234438900003. [Google Scholar]
- 59.Núñez L, Cartajena I, Grosjean M. Archaeological silence and ecorefuges: arid events in the Puna of Atacama during the Middle Holocene. Quaternary International. 2013;307(September):5–13. [Google Scholar]
- 60.Bray WL. On the relation of the flora of the lower Sonoran zone in North America to the flora of the arid zones of Chili and Argentine. Botanical Gazette. 1898;26(2):121–47. [Google Scholar]
- 61.Mares MA, Enders FA, Kingsolver JM, Neff JL, Simpson BB. Prosopis as a niche component In: Simpson BB, editor. Mesquite Its Biology in two Desert Scrub Ecosystems. Stroudsburg: Dowden, Hutchinson and Ross, Inc.; 1977. p. 123–49. [Google Scholar]
- 62.Mooney HA, Simpson BB, Solbrig OT. Phenology, morphology, physiology In: Simpson BB, editor. Mesquite: Its biology in Two Desert Ecosystems. Stroudsburg: Dowden, Hutchinson and Ross, Inc.; 1977. p. 26–45. [Google Scholar]
- 63.Bessega C, Ferreyra L, Vilardi JC, Saidman BO. Unexpected low genetic differentiation among allopatric species of section Algarobia of Prosopis (Leguminosae). Genetica. 2000;109:255–66. [DOI] [PubMed] [Google Scholar]
- 64.Campos CM, Velez S. Almacenadores y frugívoros oportunistas: el papel de los mamíferos en la dispersión del algarrobo (Prosopis flexuosa DC) en el desierto del Monte, Argentina. Revista Científica de Ecología y Medio Ambiente. 2015;24(3):28–34. doi: doi: 10.7818/ECOS.2015.24–3.05 [Google Scholar]
- 65.Ferreyra LI, Bessega C, Vilardi JC, Saidman BO. Consistency of population genetics parameters estimated from isozyme and RAPDs dataset in species of genus Prosopis (Leguminosae, Mimosoideae). Genetica. 2007;131(3):217–30. doi: 10.1007/s10709-006-9132-3 [DOI] [PubMed] [Google Scholar]
- 66.Latorre C, Betancourt JL, Rylander KA, Quade J, Matthei O. A vegetation history from the arid prepuna of northern Chile (22–23° S) over the last 13,500 years. Palaeogeography Palaeoclimatology Palaeoecology. 2003;194(1–3):223–46. [Google Scholar]
- 67.Latorre C, Betancourt JL, Rech JA, Quade J, Holmgren C, Placzek C, et al. Late Quaternary history of the Atacama Desert In: Smith M, Hesse P, editors. 23° S: The Archaeology and Environmental History of the Southern Deserts. Canberra, Australia: National Museum of Australia Press; 2005. p. 73–90. [Google Scholar]
- 68.Gayo EM, Latorre C, Jordan TE, Nester PL, Estay SA, Ojeda KF, et al. Late Quaternary hydrological and ecological change in the hyperarid core of the northern Atacama Desert (~21˚S). Earth Science Reviews. 2012;113:120–40. [Google Scholar]
- 69.Hoggs AG, Hua Q, Blackwell PG, Niu M, Buck CE, Guilderson TP, et al. SHCal13 southern hemisphere calibration, 0–50,000 years cal BP. Radiocarbon. 2013;55(4):1889–903. [Google Scholar]
- 70.Gayo EM, Latorre C, Santoro CM. Timing of occupation and regional settlement patterns revealed by time-series analyses of an archaeological radiocarbon database for the South-Central Andes (16°–25° S). Quaternary International. 2015;356:4–14. [Google Scholar]
- 71.Moragas C. Manifestaciones rupestres en el tramo bajo de la quebrada de Tambillo, provincia de Iquique, I región. Chungara. 1996;28(1–2):241–52. [Google Scholar]
- 72.Núñez L. Dinámica de grupos pre-cerámicos en el perfil de la costa y altiplano (norte de Chile). Estudios Atacameños. 1975;3:59–74. [Google Scholar]
- 73.Nester PL, Gayo E, Latorre C, Jordan TE, Blanco N. Perennial stream discharge in the hyperarid Atacama Desert of northern Chile during the latest Pleistocene. Proceedings of the National Academy of Sciences. 2007;104(50):19724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dillehay TD, editor. Where the Land Meets the Sea: 14,000 Years of Huamn History on the North Coast of Peru. Austin: University of Texas Press; 2017. [Google Scholar]
- 75.Feldman R. Áspero: Perú Architecture, Subsistence, Economy, and other Artifacts of a Preceramic Maritime Chiefdom. Cambridge-Massachusetts: Harvard University; 1980. [Google Scholar]
- 76.Quilter J, Ojeda B, Pearsall D, Sandweiss D, Jones J, Wing E. Subsistence economy of El Paraíso an early Peruvian site. Science. 1991; 251(4991):227–83. [DOI] [PubMed] [Google Scholar]
- 77.Gorbahn H. The Middle Archaic site of Pernil Alto, southern Peru: The beginnings of horticulture and sedentariness in mid-Holocene conditions. Diálogo Andino. 2013;41:61–82. [Google Scholar]
- 78.Giovannetti MA, Lema VS, Bartoli CG, Capparelli A. Caracterización grano de almidón de Prosopis chilensis (Mol.) Stuntz y P. flexuosa DC, y el análisis de sus restos arqueológicos en Andino América del Sur. Journal of Archaeological Science. 2008;35(11):2973–85. [Google Scholar]
- 79.Druss M. Environment, Subsistence Economy, and Settlement Patterns of the Chiu-Chiu Complex, ca. 2700 to 1600 B.C. of the Atacama Desert, Northern Chile [Unpublished Ph.D. Dissertation]. Columbia: Columbia University; 1977.
- 80.Núñez L, Cartajena I, Carrasco C, de Souza P. El templete de Tulán y sus relaciones formativas panandinas (norte de Chile). Bulletin de L´Institut FRanccais d´Etudes Andines. 2005;34(3):299–320. [Google Scholar]
- 81.Betancourt JL, Latorre C, Rech JA, Quade J, Rylander KA. A 22,000-year record of monsoonal precipitation from Northern Chile's Atacama Desert. Science. 2000;289(5484):1542–6. PubMed PMID: ISI:000089071700044. [DOI] [PubMed] [Google Scholar]
- 82.Grosjean M, Cartajena I, Geyh MA, Núñez L. From proxy data to paleoclimate interpretation: the mid-Holocene paradox of the Atacama Desert, northern Chile. Palaeogeography, Palaeoclimatology, Palaeoecology. 2003;194(1):247–58. [Google Scholar]
- 83.Maldonado A, Betancourt JL, Latorre C, Villagrán C. Pollen analyses from a 50 000-yr rodent midden series in the southern Atacama Desert (25°30'S). Journal of Quaternary Science. 2005;20(5):493–507. DOI: doi: 10.1002/jqs.936 [Google Scholar]
- 84.Rothhammer F, Santoro CM, Poulin E, Moraga M, Standen VG. Archeological and mtDNA evidence for tropical lowland migrations and cultural change during the Late Archaic / Formative in northern Chile. Revista Chilena de Historia Natural. 2009;82(4):543–52. [Google Scholar]
- 85.Lia VV, Confalonieri VA, Ratto N, Cámara JA, Miante AM, Poggio L, et al. Microsatellite typing of ancient maize: insights into the history of agriculture in southern South America. Proceedings of the Royal Society of London B: Biological Sciences. 2007;274(1609):545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Núñez L. Pircas: ocupación temprana en el norte de Chile. Gaceta Arqueológica Andina. 1984;11:8–10. [Google Scholar]
- 87.Núñez L. La naturaleza de la expansióna aldeana durante el formativo tardío en la cuenca de Atacama. Chungara Revista de Antropología Chilena. 2005;37(2):165–93. [Google Scholar]
- 88.Benavente A. Chiu-Chiu 200. Una comunidad pastora temprana en la Provincia del Loa (II Región). Actas del IX Congreso Nacional de Arqueología; La Serena: Sociedad Chilena de Arqueología; 1982. p. 75–94.
- 89.Adán L, Urbina S. Arquitectura formativa en San Pedro de Atacama. Estudios Atacameños. 2007;34:7–30. [Google Scholar]
- 90.Ramírez de Bryson LM, Bryson RU, Bryson RA. Paleoclimatic and material cultural perspective on the Formative period of northern Chile. Chungara Revista de Antropología Chilena. 2001;33(1):5–12. [Google Scholar]
- 91.Tartaglia LJ. An analysis of cultivated plant remains from Guatacondo, Chile In: Meighan CW, True DL, editors. Prehistoric Trails of Atacama: Archaeology of Northern Chile. Monumenta Archaeologica. Los Angeles: The University of California; 1980. p. 127–33. [Google Scholar]
- 92.Yetman DA, Burquez A. A tale of two species: speculation on the introduction of Pachycereus pringlei in the Sierra Libre, Sonora, Mexico by Homo sapiens. Desert Plants. 1996;2:23–31. [Google Scholar]
- 93.Palacios R, Brizuela M. Prosopis: historia y elementos para su domesticación. Agrociencia. 2005;9(1–2):41–51. [Google Scholar]
- 94.Ford A. Dominant plants of the Maya forest and gardens of El Pilar: Implications for paleoenvironmental reconstructions. Journal of Ethnobiology. 2008;28(2):179–99. [Google Scholar]
- 95.Gillespie A, Bocanegra-Ferguson D, Jimenez-Osornio J. The propagation of Ramón (Brosimum alicastrum SW.; Moraceae) in Mayan homegardens of the Yucatan peninsula of Mexico. New Forests. 2004;27(1):25–38. [Google Scholar]
- 96.Harrison RJ. Arboriculture in Southwest Europe: dehesas as managed woodlands In: Harris D, editor. The Origins and Spread of Agriculture and Pastoralism in Eurasia. Whashington, DC: Smithsonian Institution Press; 1996. p. 363–7. [Google Scholar]
- 97.Chepstow-Lusty A, Jonsson P. Inca agroforestry: Lessons from the past. AMBIO: A Journal of the Human Environment. 2000;29(6):322–8. [Google Scholar]
- 98.Casas A, Caballero J. Traditional management and morphological variation in Leucaena esculenta (Fabaceae: Mimosoideae) in the Mixtec Region of Guerrero, Mexico. Economic Botany. 1996;50(2):167–81. [Google Scholar]
- 99.Fernández de Oviedo Valdés G. Historia Natural y General de las Indias. Sevilla: Juan Cromberger; 1535.
- 100.Altamirano GH. Variedad de Frutos y Semillas en las Especies del Género Prosopis Presentes en Chile. Santiago: Corporación Nacional Forestal; 2012. [Google Scholar]
- 101.McRostie VB. Arboricultura y silvopastoralismo en el período Formativo (1.400 a.c.-500 d.C.) de la cuenca del Salar de Atacama. Chungara Revista de Antropología Chilena. 2014;46(4):543–57. [Google Scholar]
- 102.Rodríguez R, Matthei O, Quezada M. Flora Arbórea de Chile. Concepción: Universidad de Concepción; 1983. [Google Scholar]
- 103.Zimmermann HG. Biological control of mesquite, Prosopis spp.(Fabaceae), in South Africa. Agriculture, Ecosystems & Environment. 1991;37(1–3):175–86. [Google Scholar]
- 104.Csurhes SM, editor. Mesquite (Prosopis spp.) Queensland, Australia: Department of Natural Resources and Mines; 1996.
- 105.Chinnimani S. Ecology of succession of Prosopis juliflora in the ravines of India In: Tewari JC, Pasiecznik NM, Harsh LN, Harris PJC, editors. Prosopis Species in the Arid and Semi-Arid Zones of India; Rajasthan: Henry Doubleday Research Association (HDRA); 1998. p. 21–2. [Google Scholar]
- 106.Altamirano GH. Prosopis chilensis (Molina) Stuntz In: Donoso C, editor. Las Especies Arbóreas de los Bosques Templados de Chile y Argentina. Valdivia: Marisa Cuneo Ediciones; 2006. p. 534–40. [Google Scholar]
- 107.Shimada M, Shimada I. Prehistoric llama breeding and herding on the north coast of Peru. American Antiquity. 1985;50(1):3–26. [Google Scholar]
- 108.Brown JR, Archer S. Woody plant invasion of grasslands: establishment of honey mesquite (Prosopis glandulosa var. glandulosa) on sites differing in herbaceous biomass and grazing history. Oecologia. 1989;80(1):19–26. doi: 10.1007/BF00789926 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Dates are referential to the sites.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.