Abstract
A hexanucleotide repeat expansion in the C9orf72 gene is a common genetic cause of ALS and FTD. The repeats are translated into five different dipeptide repeat proteins (DPRs). In this issue, Lehmer et al (2017) demonstrate that one of these DPRs, poly(GP), can be measured in the CSF of individuals with C9orf72 mutations. In conjunction with the findings from another recent study (Gendron et al, 2017), these DPR biomarkers may prove to be extremely valuable in the quest for effective therapies for C9FTD/ALS.
Subject Categories: Biomarkers & Diagnostic Imaging; Genetics, Gene Therapy & Genetic Disease; Neuroscience
The discovery that GGGGCC expansions in the C9orf72 gene are the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), collectively termed C9FTD/ALS, has transformed our understanding of these diseases (Rohrer et al, 2015). Proposed pathogenic mechanisms include loss of function of C9orf72 protein, and two gain‐of‐function mechanisms: toxicity from C9orf72 repeat RNA or from DPRs produced by repeat‐associated non‐ATG translation, of which there are five, poly(GA), poly(GP), poly(GR), poly(PR) and poly(PA). There has been rapid translation of our understanding of the disease towards developing new therapies, especially for antisense oligonucleotides (ASOs), with human trials planned shortly. ASOs target C9orf72 repeat RNA and can mitigate several C9orf72 repeat‐induced pathologies both in vitro and in vivo (Zhang et al, 2015; Jiang et al, 2016; Gendron et al, 2017).
Although there is great excitement at the prospect of imminent clinical trials, it is critical we apply the lessons from past failures. In ALS, despite numerous Phase II/III clinical trials of potential therapeutics, only one drug, riluzole, has been shown to have a minimal effect on prolonging survival. The reasons for this are multifactorial (Mitsumoto et al, 2014), but one key factor that will facilitate better studies is demonstrating that biological effects are dependent on target engagement in appropriate and robust preclinical models and in subsequent clinical trials. This could be achieved by incorporating validated pharmacodynamic biomarkers, which demonstrate a biological effect in response to a therapeutic intervention, into preclinical and human studies. Importantly, an international effort to update the Guidelines for Clinical Trials in ALS to reflect these concepts is currently underway, with publication of the recommendations expected shortly.
Two key studies, one presented in this issue (Lehmer et al, 2017) and another recent study (Gendron et al, 2017), offer the tantalising prospect of such a biomarker for C9FTD/ALS. Building upon a previous study in a smaller cohort (Su et al, 2014), they validate that CSF poly(GP) is a sensitive and specific biomarker of C9orf72 expansions using Meso Scale Discovery immunoassays, in both asymptomatic and symptomatic individuals.
In cellular and animal models, poly(GR) and poly(PR) are the most toxic DPRs, with poly(GA) also leading to some toxicity. However, both studies chose to measure poly(GP) for several reasons: (i) poly(GP) is one of the most frequent DPRs in C9FTD/ALS central nervous system (CNS) tissue; (ii) it is more soluble than poly(GA), which is the most frequent DPR; (iii) it appears to be a highly stable DPR, as demonstrated by its persistence in cultured iPSC‐derived neurons (Gendron et al, 2017); and (iv) it is produced from both sense and antisense C9orf72 transcripts, although it is worth noting that ASO studies suggest sense transcripts are the major origin.
Interestingly, both studies show that CSF poly(GP) levels are comparable in asymptomatic and symptomatic individuals, although Gendron et al (2017) find a non‐significant trend towards an increase in symptomatic individuals. Further large‐scale, longitudinal studies will help determine if levels increase with symptom onset. Detection of poly(GP) in asymptomatic mutation carriers is in line with pathology studies showing CNS DPR inclusions can be present prior to symptom onset, and suggests that poly(GP) is being released from living neurons, rather than those undergoing neurodegeneration. Neither study finds an increase in CSF poly(GP) with disease progression, either measured longitudinally, or using clinical scores such as the revised ALS Functional Rating Scale and FTLD‐specific Clinical Dementia Rating, or an association with an ALS or FTD phenotype, age of disease onset, or survival. This stability of poly(GP) levels may enhance its value as a pharmacodynamic biomarker. In a clinical trial, for each patient, relative pre‐ and post‐intervention values would be compared; therefore, a stable marker showing normalisation with a therapy is ideal. As an elegant proof of principle of this concept, Gendron et al (2017) show that treating C9orf72 iPSC‐derived neurons and a C9orf72 mouse model with ASOs reduces poly(GP) levels. The levels of intracellular poly(GP) in C9orf72 iPSC neurons correlates with extracellular levels in media bathing the cells, and in a mouse model, CSF poly(GP) correlates with poly(GP) in brain homogenates. This indicates that CSF poly(GP) levels are likely to reflect intraneuronal levels in the CNS. However, it is important to note that in their longitudinal analysis of a small patient cohort, in some individuals CSF poly(GP) naturally decreases over time, which should be accounted for in clinical trials. An emerging theme from trials in Alzheimer's disease (AD) is that administering treatment early in the disease course, before extensive neurodegeneration, may be most effective; therefore, detection of CSF poly(GP) in asymptomatic individuals may have great value for implementing early treatment. Lehmer et al (2017) show that CSF levels of neurofilaments (Nfl and pNfH), markers of axonal damage, are raised in symptomatic but not asymptomatic C9orf72 mutation carriers, in agreement with recent studies of neurofilaments as ALS diagnostic and prognostic biomarkers (Fig 1). The combination of both biomarkers could provide evidence of target engagement and functional rescue.
Figure 1. C9FTD/ALS CSF biomarkers.
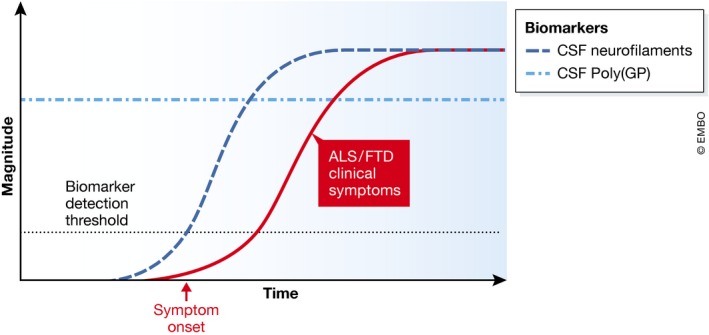
CSF poly(GP) is similar in asymptomatic and symptomatic individuals, whilst CSF neurofilaments are detected in symptomatic individuals.
Lehmer et al (2017) suggest CSF poly(GP) could be used alongside genotyping as a diagnostic biomarker, and were able to reclassify one patient misdiagnosed with AD as C9FTD. Due to somatic instability of GGGGCC repeats, it is possible that patients without an expansion in blood DNA have expansions in the CNS; therefore, the addition of CSF poly(GP) could add diagnostic value. However, it is important to note that both studies describe a small number of false positives, non‐C9orf72 individuals with elevated poly(GP), or false negatives, C9orf72 individuals with non‐detectable poly(GP). Improving the sensitivity and specificity of these assays is critical and may require the use of other potentially more sensitive platforms, such as the Simoa (single molecule array), which was used on a subset of samples by Gendron et al (2017). Lehmer et al (2017) use monoclonal antibodies in their immunoassay, which are less variable and can be produced at higher throughput than polyclonal antibodies, so may provide an additional advantage. Developing similar assays for other more toxic DPRs may also be beneficial, as they may correlate better with progression or prognosis.
In addition to CSF, Gendron et al (2017) specifically detected poly(GP) in peripheral blood mononuclear cells. Detection in blood would be advantageous for repeat measurements in clinical trials. This could mark a significant step forward, but further studies are needed to determine whether blood and CSF poly(GP) levels correlate.
In conclusion, developing promising biomarkers should be integral to clinical trials in C9FTD/ALS, and a biomarker assay should ideally be sensitive, specific, reproducible with low variability, standardised and affordable. These studies now highlight CSF poly(GP) as one such potential candidate.
Acknowledgements
We apologise for not individually citing all relevant original papers due to space constraints. RB is a Leonard Wolfson Clinical Research Training Fellow and is funded by a Wellcome Trust Research Training Fellowship (107196/Z/14/Z). RB was supported by the Motor Neuron Disease Association to participate in the ALS Clinical Trial Guidelines Workshop at Airlie House, Virginia. TGM is funded by the Brain Research Trust. AMI is funded by the Motor Neuron Disease Association, Alzheimer's Research UK and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (648716 ‐ C9ND).
See also: C Lehmer et al (July 2017) and TF Gendron et al (March 2017)
References
- Gendron TF, Chew J, Stankowski JN, Hayes LR, Zhang YJ, Prudencio M, Carlomagno Y, Daughrity LM, Jansen‐West K, Perkerson EA et al (2017) Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72‐associated amyotrophic lateral sclerosis. Sci Transl Med 9: eaai7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis‐Downes M, Seelman A, Stauffer JE, Jafar‐Nejad P, Drenner K, Schulte D et al (2016) Gain of toxicity from ALS/FTD‐linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC‐containing RNAs. Neuron 90: 535–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmer C, Oeckl P, Weishaupt JH, Volk AE, Diehl‐Schmid J, Schroeter ML, Lauer M, Kornhuber J, Levin J, Fassbender K et al (2017) Poly‐GP in cerebrospinal fluid links C9orf72‐associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med 9: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto H, Brooks BR, Silani V (2014) Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol 13: 1127–1138 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Isaacs AM, Mizielinska S, Mead S, Lashley T, Wray S, Sidle K, Fratta P, Orrell RW, Hardy J et al (2015) C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 14: 291–301 [DOI] [PubMed] [Google Scholar]
- Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen‐West K, Belzil VV, Desaro P et al (2014) Discovery of a biomarker and lead small molecules to target r(GGGGCC)‐associated defects in c9FTD/ALS. Neuron 83: 1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S et al (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525: 56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]


