Summary
The ductus arteriosus is an arterial vessel that shunts blood flow away from the lungs during fetal life, but normally occludes after birth to establish the adult circulation pattern. Failure of the ductus arteriosus to close after birth is termed patent ductus arteriosus, and is one of the most common congenital heart defects. Our previous work demonstrated that vascular smooth muscle cell expression of the Jag1 gene, which encodes a ligand for Notch family receptors, is essential for postnatal closure of the ductus arteriosus in mice. However, it was not known what cell population was responsible for receiving the Jag1-mediated signal. Here we show, using smooth muscle cell-specific deletion of the Rbpj gene, which encodes a transcription factor that mediates all canonical Notch signaling, that Notch signal reception in the vascular smooth muscle cell compartment is required for ductus arteriosus closure. These data indicate that homotypic vascular smooth muscle cell interactions are required for proper contractile smooth muscle cell differentiation and postnatal closure of the ductus arteriosus in mice.
Keywords: birth defects, vascular smooth muscle, lateral induction
INTRODUCTION
The ductus arteriosus is an arterial blood vessel that connects the pulmonary artery to the proximal descending aorta, directing blood flow away from the pulmonary circulation during fetal life. After birth the ductus arteriosus is first rapidly, and then permanently occluded, separating the pulmonary and systemic circulations to establish the normal adult circulatory pattern. Failure of the ductus arteriosus to close after birth is termed patent ductus arteriosus (PDA), and is one of the most common human congenital heart defects (Anilkumar, 2013; Coceani and Baragatti, 2012; Schneider and Moore, 2006). PDA patients are at increased risk for pulmonary and cardiac problems such as pulmonary hemorrhage, congestive heart failure, chronic lung disease, sepsis, and necrotizing enterocolitis.
We have shown previously that expression of the Jag1 gene, which encodes a transmembrane ligand for Notch family receptors, is required in vascular smooth muscle cells for postnatal closure of the ductus arteriosus (Feng et al., 2010). Mice with smooth muscle-specific deletion of the Jag1 gene exhibited defects in contractile smooth muscle cell differentiation in the vascular wall of the ductus arteriosus and adjacent aorta, failed to close their ductus arteriosus postnatally, and died during the first few days after birth. It has not been demonstrated, however, what target cell population is required for reception of the Jag1-mediated signal. Here we show, by conditional deletion of the Rbpj gene (that encodes the transcriptional mediator of all canonical Notch signaling), that Notch signal reception is required in vascular smooth muscle cells, indicating that homotypic vascular smooth muscle cell interactions are required for postnatal closure of the ductus arteriosus in mice.
RESULTS AND DISCUSSION
Our previous work demonstrated that expression of the Jag1 gene in vascular smooth muscle cells was essential for postnatal closure of the ductus arteriosus (Feng et al., 2010). To assess whether Notch signal reception also is required in vascular smooth muscle cells, we performed smooth muscle-specific deletion using SM22a-Cre (also known as Tagln-Cre) mice (Holtwick et al., 2002), the same Cre driver line we used in our previous study. We abrogated Notch signal reception in these cells by deleting the Rbpj gene, which encodes a transcription factor that mediates all canonical Notch signaling. SM22a-Cre/+; Rbpjflox/− (hereafter referred to as Rbpj-SMcko, for Rbpj smooth muscle conditional knockout) mice were generated by crossing SM22a-Cre/+; Rbpj+/− male mice to Rbpjflox/flox female mice. Rbpj-SMcko mice were found alive in expected Mendelian ratios when delivered by caesarean section at embryonic day (E) 18.5. However, approximately 50% of the Rbpj-SMcko neonates died by late on postnatal day (P) 0, the day of birth, and most had died by P2 (Table 1). However, one Rbpj-SMcko mouse survived until P6 (Table 1, Figure 1A).
Table 1.
Survival of SM22a-Cre; Rbpjflox/ neonatal mice.
| Age | SM22a-Cre; Rbpjflox/− (number alive) | Control littermates |
|---|---|---|
| E18.5 | 18 (18) | 73 |
| P0 | 12 (6) | 52 |
| P1 | 6 (2) | 29 |
| P4 | 2 (1) | 14 |
| P6 | 1 (0) | 7 |
| Wean | 0 | 89 |
Control: All littermate genotypes other than SM22a-Cre; Rbpjflox/−.
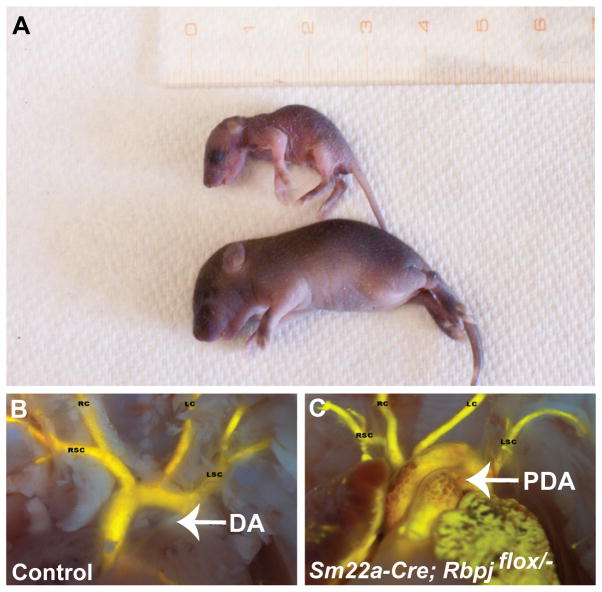
FIGURE 1.
(A) SM22a-Cre; Rbpjflox/− mutant (top) that survived until P6 and control littermate. (B, C) Intracardiac Microfil injections of a control littermate (B) and the SM22a-Cre; Rbpjflox/− mouse that survived until P6 (C). The mutant exhibits a large patent ductus arteriosus, while the ductus arteriosus of the littermate has closed (thin white band of tissue). Abbreviations: DA, ductus arteriosus; LC, left carotid artery; LSC, left subclavian artery; PDA, patent ductus arteriosus; RC, right carotid artery; RSC, right subclavian artery.
All Rbpj-SMcko mice born exhibited patent ductus arteriosus (PDA), with the exception of one Rbpj-SMcko mouse in which closure of the ductus arteriosus could not be evaluated due to the presence of an interrupted aortic arch, a more severe defect of the great arteries in which part of the aortic arch is missing (Table 2). There was a low incidence (3%) of PDA in the control littermates, which consisted of all genotypes other than SM22a-Cre/+; Rbpjflox/−. We had observed a similar low incidence of PDA in control littermate mice in our previous study (Feng et al., 2010). To better visualize PDA, we injected the left ventricles of a subset of the control and Rbpj-SMcko neonates with Microfil injection compound (Figure 1B, C).
Table 2.
Penetrance of patent ductus arteriosus in SM22a-Cre; Rbpjflox/− and control littermate neonatal mice.
| Phenotype | SM22a-Cre; Rbpjflox/− | Control littermates |
|---|---|---|
| PDA | 11/11 (100%)* | 2/52 (3.4%) |
| OTPD | 2/12 (17%) | 1/52 (2%) |
OTPD: outflow tract patterning defects; PDA: patent ductus arteriosus.
One SM22a-Cre; Rbpjflox/ mouse was excluded due to the presence of an interrupted aortic arch.
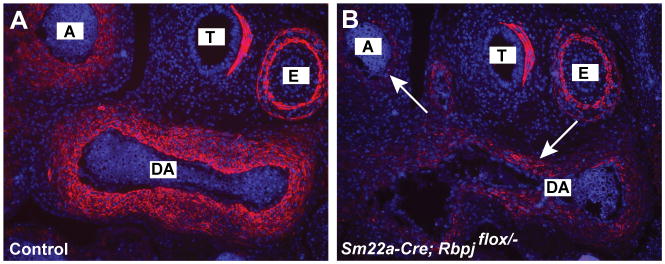
In our previous study of PDA in Jag1 smooth muscle conditional knockout (Jag1-SMcko) mice, we observed defects in contractile vascular smooth muscle cell differentiation in the ductus arteriosus and adjacent aorta (Feng et al., 2010). We assessed expression of smooth muscle actin protein, a marker for contractile smooth muscle cell differentiation, in sections from control littermate and Rbpj-SMcko embryos at E13.5 (Fig. 2). Similarly to the Jag1-SMcko mutants, SM22a-Cre; Rbpjflox/− mutant embryos (Fig. 2B) had greatly reduced expression of smooth muscle actin in the ductus arteriosus and adjacent aorta, but smooth muscle actin expression in the trachea and esophagus was unaffected in the mutants.
FIGURE 2.
Reduced contractile smooth muscle cell differentiation in the aorta and ductus arteriosus of SM22a-Cre; Rbpjflox/− mutants. Immunofluorescent staining of outflow tract vessels in E13.5 embryos stained with antibodies against smooth muscle actin. Sections were counterstained with DAPI. The SM22a-Cre; Rbpjflox/− mutant (B) has greatly reduced expression of smooth muscle actin in the ductus arteriosus (DA) and adjacent aorta (A). Smooth muscle actin expression in the trachea (T) and esophagus (E) is unaffected in the mutant.
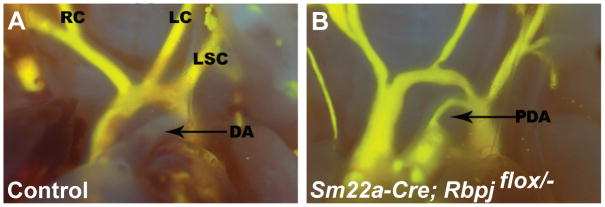
Prostaglandins are key regulators of ductus arteriosus patency and closure. The cyclooxygenases 1 and 2 (COX1 and 2; formally known as prostaglandin-endoperoxidase synthases 1 and 2) are rate-limiting enzymes involved in the conversion of arachidonic acid to prostaglandins. Non-steroidal anti-inflammatory drugs such as indomethacin and ibuprofen inhibit the formation of prostanoids by the COX enzymes (Mitchell and Warner, 2006). In premature infants exhibiting patent ductus arteriosus, COX inhibitors are used clinically as a first line treatment to induce ductus arteriosus closure by reducing prostaglandin levels, thereby inducing constriction of the ductus arteriosus and closure of its lumen. We found in our previous study that postnatal injection of indomethacin was able to rescue ductus arteriosus closure in a subset of the Jag1-SMcko mutants. When we injected indomethacin postnatally into control littermate and Rbpj-SMcko mutants, ductus arteriosus closure was rescued in only 1 of 9 Rbpj-SMcko mutants (Table 3, Figure 3). The enhanced resistance to ductus arteriosus closure after indomethacin administration exhibited by the Rbpj-SMcko mutants, compared to the Jag1-SMcko mutants, may indicate that more than one Notch ligand may be involved in regulating smooth muscle cell differentiation in the ductus arteriosus. Further work will be needed to assess that possibility.
Table 3.
Indomethacin injection in SM22a-Cre; Rbpjflox/ mutant neonates.
| Phenotype | SM22a-Cre; Rbpjflox/ | Control littermates |
|---|---|---|
| Closed DA | 1/9 (11%) | 29/29 (100%) |
| Patent DA | 8/9 (89%) | 0/29 (0%) |
Indomethacin (6 mg/kg) was injected subcutaneously into pups within 12 hours of birth on day 0, or after caesarean delivery on day E18.5. After six hours, pups were euthanized and the ductus arteriosus scored for patency or closure. Mice from four litters are included in this table. DA: ductus arteriosus.
FIGURE 3.
Failure of indomethacin injection to rescue patent ductus arteriosus in a SM22a-Cre; Rbpjflox/− mutant (B). Abbreviations as in Figure 1.
The Notch signaling pathway plays multiple roles during vascular development in mammals and other vertebrates (Gridley, 2010; Kume, 2012), including vascular smooth muscle cell differentiation (Boucher et al., 2012; Lin and Lilly, 2014). We showed previously that expression of the Notch ligand Jag1 in vascular smooth muscle cells is essential for postnatal closure of the ductus arteriosus. However, the Jag1 gene is expressed in both vascular smooth muscle cells and in endothelial cells (Villa et al., 2001), making the recipient cell population for the Jag1-mediated signal unclear. In this study, we show that the Jag1-mediated signal must also be received by the vascular smooth muscle cell compartment.
While this manuscript was in preparation, Baeten and colleagues published an elegant study demonstrating that mutation of the Notch2 and Notch3 receptors caused patent ductus arteriosus in mice (Baeten et al., 2015). In their study, they used smooth muscle-specific deletion of the Notch2 gene (using a different Cre driver line, Myocardin-Cre) together with a constitutive Notch3 deletion to produce mice with different combinations of mutant and wild type Notch2/3 alleles in vascular smooth muscle cells. They found that all of the Notch2-smooth muscle null, Notch3-heterozygous mice exhibited PDA, and approximately 40% of Notch2-smooth muscle null mice with wild type alleles of the Notch3 gene also had PDA.
Our previous results (Feng et al., 2010) fit a model in which Jag1 expression, first in endothelial cells and subsequently in vascular smooth muscle cells, initiated a process of lateral induction in which Jag1 expression and contractile smooth muscle cell differentiation was induced in neighboring cells (Feng et al., 2010). Manderfield and colleagues reached similar conclusions concerning the role of Jag1 expression and Notch signal reception during assembly of arterial vessel walls (Manderfield et al., 2012). Our current results, as well as those of Baeten and colleagues (Baeten et al., 2015), confirm that Jag1-mediated homotypic intercellular interactions are essential for contractile smooth muscle cell differentiation and ductus arteriosus closure in mice. Interestingly, PDA has been identified in some case reports (Harris et al., 2002; Sanchez-Angulo et al., 1997) of Alagille syndrome, which is most commonly caused by JAG1 haploinsufficiency (Turnpenny and Ellard, 2012), suggesting that similar mechanisms likely occur in humans.
METHODS
Mice
Rbpjflox and Rbpjnull (the deleted form of the Rbpjflox allele) mice (Tanigaki et al., 2002) were obtained from Dr. T. Honjo. The transgenic SM22a-Cre (official name Tg(Tagln-cre)1Her; MGI ID: 2446975) line (Holtwick et al., 2002) was obtained from the Jackson Laboratory. All experimental procedures on mice were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee at Maine Medical Center.
Histology and Immunofluorescence
Embryos and neonatal mice were fixed in 4% paraformaldehyde. Chest cavities were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For immunofluorescence, sections were dewaxed in a standard xylene and ethanol series, then rehydrated with phosphate buffered saline (PBS). An antigen retrieval step was performed in boiling 10 mM sodium citrate, pH 6.0, for 10 minutes. Slides were then blocked in 5% goat serum/2% BSA/PBST for 2 hours at room temperature before being covered with diluted primary antibodies at 4°C overnight. Antibodies used in this study were polyclonal anti-smooth muscle alpha actin primary antibody (Abcam, 1:200), and Alexafluor-labeled fluorescent secondary antibodies (Molecular Probes). All slides were mounted by using Vectashield mounting medium with DAPI (Vector Laboratories).
Intracardiac Injections
To better visualize the outflow tract and ductus arteriosus, hearts of SM22a-Cre; Rbpjflox/− mutant and control littermate mice were injected with Microfil silicone rubber injection compound (MV-122; Flow Tech Inc.). Pups were isolated at E18.5 by caesarean section, and were euthanized seven hours post surgery. The chest cavities were opened and fixed in 10% neutral buffered formalin. After fixing for one hour, neonates were rinsed and Microfil compound was injected into the left ventricle using a 27 gauge needle, as described previously (Feng et al., 2010).
Indomethacin treatment
To determine whether postnatal indomethacin administration could rescue closure of the ductus arteriosus of SM22a-Cre; Rbpjflox/− mutant neonatal mice, pups from caesarean delivery at embryonic day (E)18.5, or naturally delivered pups at postnatal day (P)0, were injected subcutaneously with indomethacin (6 mg/kg). Pups were euthanized six hours after injection, the chest cavities were opened and closure of the ductus arteriosus was scored visually. In some treated mice, outflow tracts were visualized by Microfil injection.
Acknowledgments
This work was supported by the March of Dimes Birth Defects Foundation, and utilized institutional core services funded by grants 5P30GM103392 and 5P30GM106391. This paper is dedicated to the memory of Luke Krebs, who made pioneering contributions to our understanding of Notch signaling during vascular development.
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
LITERATURE CITED
- Anilkumar M. Patent ductus arteriosus. Cardiol Clin. 2013;31:417–430. doi: 10.1016/j.ccl.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Baeten JT, Jackson AR, McHugh KM, Lilly B. Loss of Notch2 and Notch3 in vascular smooth muscle causes patent ductus arteriosus. Genesis. 2015 doi: 10.1002/dvg.22904. [DOI] [PubMed] [Google Scholar]
- Boucher J, Gridley T, Liaw L. Molecular pathways of Notch signaling in vascular smooth muscle cells. Front Physiol. 2012;3:81. doi: 10.3389/fphys.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36:92–97. doi: 10.1053/j.semperi.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Feng X, Krebs LT, Gridley T. Patent ductus arteriosus in mice with smooth muscle-specific Jag1 deletion. Development. 2010;137:4191–4199. doi: 10.1242/dev.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Cao QL, Waight D, Hijazi ZM. Successful combined orthotopic liver transplant and transcatheter management of atrial septal defect, patent ductus arteriosus, and peripheral pulmonic stenosis in a small infant with Alagille syndrome. Pediatr Cardiol. 2002;23:650–654. [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T. Ligand-dependent Notch signaling in vascular formation. Adv Exp Med Biol. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lilly B. Notch signaling governs phenotypic modulation of smooth muscle cells. Vascul Pharmacol. 2014;63:88–96. doi: 10.1016/j.vph.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125:314–323. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- Sanchez-Angulo JI, Benitez-Roldan A, Castro-Fernandez A, Ruiz-Campos J. Alagille syndrome and isolated persistent ductus arteriosus. Gastroenterol Hepatol. 1997;20:418–421. [PubMed] [Google Scholar]
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251–257. doi: 10.1038/ejhg.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]