Abstract
The skeleton is unique from all other tissues in the body because of its ability to mineralize. The incorporation of mineral into bones and teeth is essential to give them strength and structure for body support and function. For years, researchers have wondered how mineralized tissues form and repair. A major focus in this context has been on the role of the extracellular matrix, which harbors key regulators of the mineralization process. In this introductory minireview, we will review some key concepts of matrix biology as it related to mineralized tissues. Concurrently, we will highlight the subject of this special issue covering many aspects of mineralized tissues, including bones and teeth and their associated structures cartilage and tendon. Areas of emphasis are on the generation and analysis of new animal models with permutations of matrix components as well as the development of new approaches for tissue engineering for repair of damaged hard tissue. In assembling key topics on mineralized tissues written by leaders in our field, we hope the reader will get a broad view of the topic and all of its fascinating complexities.
Introduction
What is mineralized tissue?
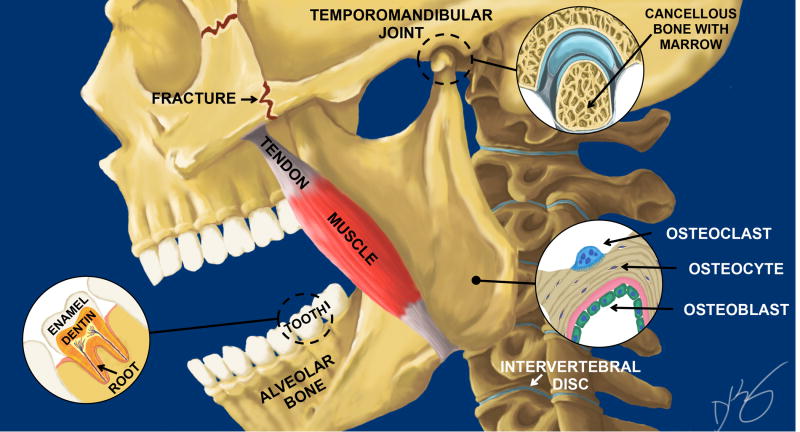
Mineralized tissues come in many “shapes and forms” that are depicted schematically in Fig 1. There are five types of bones that include 1) flat bones that protect internal organs such as the skull shown in Fig. 1, 2) long bones that support weight and facilitate movement with the femur being one example, 3) short bones that are cube shaped and found in wrists and ankles, 4) irregular bones with complex shapes such as the vertebrae and 5) sesamoid bones that are embedded in tendon tissue. Like bone, the tooth is highly mineralized and contains many of the same matrix components, but despite this fact it is very different from bone, as can be seen in Fig. 1. The outer enamel layer of the tooth is “super mineralized”, making it the hardest tissue in the body. Its unique composition gives it astounding strength that protects the tooth from “wear and tear” during eating and chewing. This special issue of Matrix Biology is as diverse as the skeleton itself, with a combination of primary research articles submitted to the journal, along with reviews describing the compostion and ultrastructure of matrix proteins and their role in regulating cell and tissue function. The issue is divided into four chapters that describe the importance of matrix in 1) bones, 2) teeth, 3) tendon, cartilage and cancer and 4) in tissue engineering.
Fig. 1.
Schematic of of a skull depicting key mineralized tissues including a tooth with enamel, dentin and roots, masseter tendon, alveolar bone in the jaw, the temporomandibular joint (TMJ), and the intervertebral disc (IVD). Key cell components of bone are shown in the lower right corner that include the multinuclear osteoclast (blue), the osteocyte (black) and the osteoblast (green). Commonly fractured bones in the face are shown as zigzag lines behind and below the eye socket in the zygomatic arch. Illustration is by David Kirby, co-first author, on the paper in this special edition by Myren et al.
Bones
Type I collagen
At least 27 different collagen types have been identified so far [1], many of which are found in the skeleton. The most abundant species in mineralized tissue is Type I collagen, long known to have vital roles in regulating skeletal integrity. The production and processing of collagen is highly orchestrated [1] involving a multitude of chaperones and enzymes that modify and crosslink collagen during its assembly into a triple helix and ultimately into fibrils [2]. Our issue begins with an in-depth overview by P. Trackman, of enzyme-dependent collagen crosslinking with a focus on lysyl oxidases (LOX). The LOX family is composed of 5 members that include LOX, and the lyslyl oxidase-like enzymes LOXL1-LOXL4. The review tells us what is currently known about the biochemical reactions dependent on LOX and, further, the consequences to bone tissue formation when these enzymes are depleted. The multiple cell and molecular functions of LOX members beyond collagen cross-linking are discussed including their potential role in osteogenic differentiation, angiogenesis and in bone healing.
It is generally believed that collagen orients proteins that serve as a nidus for minerals to localize and accumulate, therefore serving a key function in mineralized tissues [3]. Testimony of the importance of type I collagen in mineralized tissue formation comes from patients with mutations in the type I collagen gene (referred to as the Col1A1 and Col1A2 genes), who are afflicted with severe skeletal deformaties in a condition known as osteogenesis imperfecta (OI, or brittle bone disease)[4]. Interestingly, many lethal mutations in OI are located in the triple helical domain of collagen in a region that aligns with binding sites for other ECM components [5] including proteoglycans [6]. This finding emphasizes the importance of the potential synergy between ECM components where one ECM member can affect the function of another [7]. Further studies are needed to delineate the ECM interplay in this mineralized tissue disease.
One of the current treatments for OI is bisphosphonates, and while bisphospohnates have proven effective in reducing fracture rates they do not completely elimate them [8]. The paper by Berman et al. used a mouse model of OI known as OIM with a frameshift mutation in the Col1a2 gene of alpha2 chain of type I collagen to ask the question: could the selective estrogen receptor modulator (SERM), rolaxifene, be a novel treatment for OI and, if so, how would it work? Their study showed that rolaxifene increased the mechanical properties of bone when tested both in vivo and ex vivo, providing a foundation for the development of new therapies using this SERM to reduce bone fragility in patients with OI. A second paper in this issue by Mertz et al. used a different OI mouse model with a Gly610Cys mutation in the alpha 2 chain of type I collagen to show how this mutation disrupts bone modeling mineralization and overall bone toughness. Such studies are needed to understand the tissue and cell basis for the mineralized tissue abnormalities observed in OI.
Non-collagenous proteins and proteoglycans
The importance of collagen in bone mineralization presents a conundrum: how do tissues that do not make collagen (like enamel) control the mineralization process? In this context, it must further be questioned: why does skin that is rife with type I collagen not mineralize? There must be extracellular matrix components other than collagen that are involved in regulating the mineralization of hard tissues. To address some of these points, a review by Boskey et al. describes the mineralization process and its relationship to a family of proteins called Small Integrin-Binding Ligand N-linked Glycoproteins (SIBLINGs). A biochemical characteristic of the SIBLINGs is that they are highly acidic, which is likely one reason they have affinity for the basic hydroxypapatite that makes up the mineral composition of bone. What is interesting about the SIBLINGs is that they are intrinsically “disordered”, meaning that they can adapt to many shapes presumably to give them flexibility and versatility in function. All members of this family have an Arg-Gly-Asp (RGD) cell attachment site that may be used for integrin binding and regulation of cell function. The members include: bone sialoprotein (BSP), dentin matrix protein (DMP), dentin sialophosphoprotein (DSPP), enamelin, MEPE, and osteopontin (OPN). The latter has been directly implicated in the pathophysiology of cancer [9]. Among the SIBLINGs, BSP is the most restricted to mineralized tissue and was, therefore, presumed to have signficant effects on bone. As predicted, BSP-deficient mice have defective osteogenic cell differentiation and skeletal mineralization, which is described in a paper by Bouleftour et al. A new and surprising finding about BSP is that its presence in the local bone marrow microenvironment appears to affect both hematopoiesis and vascularization of bone.
A multitude of studies show that the ECM has important roles in regulating growth factor function. Fibrillin-1 is a matrix protein that regulates skeletal tissue by modulating TGF-β activity and subsequently bone marrow stromal cell fate. Mice deficient in fibrillin-1 in Prx-expressing tissues (or osteochondroprogenitors) have increased levels of red blood cells (Smaldone et al.). When the fibrillin-1 deficient mice were treated with TGF-β neutralizing antibodies, the peripheral red blood cell counts, but not hematopoetic stem cell frequency were normalized indicating fibrillin-1 may regulate the erythoblastic microenvironment.
The final paper in this section is a review by A. Bradshaw on SPARC/osteonectin/BM-40, a protein highly expressed in mineralized tissues that was originally believed to be bone-specific with a primary function of linking collagen to mineral [10]. We now know that SPARC is widely expressed in many tissues, making it in some ways like type I collagen: important in both mineralized and non-mineralized tissues, but perhaps for different reasons. SPARC is now considered a “matricellular” protein [11] with the capacity to regulate bone formation and bone turnover [12]. Testimony to the importance of SPARC in bone biology comes from the elegant work from the Delany lab [13] that identified a single nucleotide polymorphism (SNP) in the 3’ end of the SPARC gene that correlates with the occurrence of osteopenia (osteoporosis) in humans. Further studies revealed that this SNP is targeted by the miRNA (miR-433) that down-regulates SPARC levels and subsequently reduces bone mass [13]. The “SPARC story” is a nice demonstration of the broad spectrum approach taken in our field to understand the mineralization process using biochemistry, animal modeling and human genetics to address key questions.
Osteocyte, osteoclast and other bone cells
The most predominant cell in bone is the osteocyte, which arises from osteoblasts that become embedded in lacunae found within the mineralized matrix (Fig 1). It is generally believed the osteocyte has “mechano-sensing” properties that help bones “feel” the force generated from exercise resulting in increased bone mass. A paper by Gardinier et al. shows that the anabolic effector parathyroid hormone (PTH) may mediate perilacunar remodeling in reponse to excercise. This was demonstrated by inhibiting PTH signaling in mice that were subjected to treadmill running. While the molecular mechanisms for this outcome are still not entirely clear, it was noted that the affected mice had a reduction in sclerostin (SOST), the osteocyte specific protein that antagonizes Wnt signaling. The importance of the Wnt pathway came from previous work showing that patients with activating mutations in the Wnt receptor, LRP-6, had increased Wnt signaling accompanied by increased bone mass [14]. This finding clearly established the link between Wnt signaling and bone accrual. Based on this observation, antibodies to SOST (Scl-Ab) have been developed as a new therapy to increase bone mass.
Mice deficient in the SIBLING DMP fail to properly mineralize freshly deposited bone matrix (osteoid) in a condition known as osteomalacia. A study by Ren et al. used Scl-Abs to show that many of the Dmp-KO bone defects could be reversed by this therapy. The surprising finding in this study was that the mechanism of Scl-Ab action did not rely on alterations in osteocyte-produced FGF-23, which was formerly believed to be a key factor in controlling DMP-dependent mineralization.
The ostocyte is not the only important cell in bone. The cell that resorbs bone, the osteoclast, as explained by Rucci and Teti, has a “fatal attraction” to the bone matrix that it ultimately destroys. This is, in essence, a “love-hate” relationship where osteoclasts “love” the ECM but, at the same time, “hate it” by removing it during resorption. Other important skeletal cells include the pericytes that hug vessel walls and the lining cells that are flattened osteoblasts covering inactive bone surfaces that, under certain conditions, can be induced to proliferate and differentiate into osteogenic cells. The role of bone matrix proteins in regulating pericyte or lining cell function has yet to be clearly elucidated. Other important bone derived cells include the bone marrow stromal cells (BMSCs) that are believed to be progenitors of the bone-forming osteoblast which may have stem cell properties influenced by the ECM. This latter point will be discussed in further detail in the chapter on tissue engineering.
Angiogenesis
The role of angiogenesis is criticial for bone development and bone healing. An elegant series of experiments by Duan et al. show that vascular endothelial growth factor (VEGF) activity in craniofacial development is controlled by bone progenitor cells rather than cartilage progenitor cells. During bone healing, Berendsen et al. previously showed that the small leucine rich proteoglycan (SLRP) biglycan (Bgn) is important for callus and vessel formation after fracture, suggesting Bgn is needed for neoangiogenesis during the healing process [15]. This paper further showed that Bgn binds directly to VEGF, however, in human umbilical vein endothelial cells (HUVEC), the addition of Bgn was unable to enhance VEGF signaling, leading to the conclusion that other factors besides VEGF may be working with Bgn to regulate angiogenesis [15]. A paper by Myren et al. used three dimensional angiography to confirm the importance of Bgn in angiogenesis during bone healing. Using a HUVEC tube formation assay, Myren et al. suggest an alternative mechanism for Bgn’s control of angiogenesis involves Bgn inhibition of the anti-angiogenic factor, endostatin.
Tooth proteins that help bone
Considering the unique character of teeth, one wonders: could ECM proteins in teeth be used to improve bone? Two ECM proteins that are relatively low in bone but abundant in teeth, odontoblast-produced DSPP and an enamel enriched protein, ameloblastin (Abn), were studied for this purpose. When Dmp-KO mice were mated to transgenic mice that over-express DSPP under the control of the 3.6 Kb Col1a1 promoter, the offspring had increased cortical thickness, bone volume and mineral density and restoration of trabecular thickness. The DSPP-rescued Dmp-KO mice also had less unmineralized osteoid and improvement of the collagen network accompanied by increased Bgn, Bsp and Opn, all of which could be the foundation for the restitution of bone parameters observed. In the second example of “tooth proteins helping bone”, Abn was used. Specifically, a report by Lu et al. shows that Abn-KO mice have defective healing after bone fracture, and when Abn was added back to broken bones it hastened their healing judged by an increase in bone volume and bone mineral density. The concept of a tooth matrix protein helping bone is not new; Emdogain™ (EMD), an extract of porcine enamel, is currently being used to form new bone, cementum and attachment fibers to promote periodontal regeneration [16]. Considering the data presented by Ren and Li, it is tempting to speculate that additional and more effective tooth matrix components will be indentified in the future that aid bone health.
Teeth: Enamel, dentin, root
As mentioned earlier, tooth enamel is the hardest and most highly mineralized tissue in the body. It is 96% mineral and composed of hydroxyapatite crystals that are 1000 times larger than those found in bone. The most abundant protein in enamel is amelogenin, which is encoded by a single gene that produces multiple mRNAs that are differentially spliced resulting in numerous variants made up of multiple combinations of different protein domains. Because enamel is so unique, it has been difficult to examine its formation and function using in vitro systems. To understand the role of the amelogenin variants in enamel, Xia et al. used mice deficient in amelogenin “rescued” with transgenic mice that over express either M180 (full length amelogenin), LRAP (where the -NH2 and -COOH ends of amelogenin are fused) or CTRNC (which is the -COOH truncated form containing the hydrophic core). Their work showed all three regions of amelogenin were needed for structural and functional integrity of mature enamel. Other matrix proteins in enamel were investigated by Simmer et al., who showed that amelotin (AMTN) and kallikrein-4 (KR4) were both essential for proper enamel maturation, but that they function independently. Enamel is very sensitive to changes in pH and can become “demineralized” if the pH falls lower than normal levels. Protons acidify the microenviroment of ameloblasts that require tight pH regulation to prevent disruption of guided crystal growth and enamel formation [17]. A study by Paine et al. examined the spatio-temporal pattern of the enzymes that control cellular pH through proton pump activity and found upregulation of many members of the V-type ATPase subunits in maturation stage amelogenesis. The authors suggest this finding indicates there could be a greater need for lysosomal acidification at this latter stage of enamel formation.
DSPP is highly expressed by the odontoblasts that line dentin tissue (Fig. 1). Beniash et al. find that Dspp-KO mice have accelerated enamel maturation. While this is presumed to arise from the effected dentin that is adjacent to the enamel tissue (Fig. 1), the authors also show for the first time the presence of DSPP mRNA in secretory ameloblasts. Concerning the composition of enamel, a review by Morasso et al. builds a case that keratins, formally thought to be specific to hair, may in fact, be found in mineralized enamel and function to control dental carries in humans.
The basement membrane separating epithelial ameloblasts is very unique from other basement membranes in the body and is considered to be “atypical”. To understand more about the nature of this atypical basement membrane, Antonio et al. used mice deficient in the BM component laminin 332 (LM332) rescued with a humanized LM322 under the control of the cytokeratin 14 promoter. Interestingly, all tissues except enamel could be structurally “rescued”, emphasizing that this atypical BM is indeed unique from all others in the body [18].
In addition to matrix formation, matrix turnover is essential for mineralized tissue homeostasis. Membrane-type matrix metalloproteinase 1 (MT1-MMP) is a transmembrane enzyme that breaks down extracellular components including collagen during post-natal growth and aging. A paper by Foster et al. used conditional ablation of MT1-MMP to show its activity is essential in dental mesenchyme but not in epithelially derived tissue for proper root formation and tooth eruption. Much more information about MMP biological functions can be reamed from the special edition of Matrix Biology edited by Apte and Parks [19] that covers a multitude of topics related to tissue pathology, repair and stem cell activation [20–25].
Matrixvesicles (MV) are structures found in mineralized tissues believed to form by shedding of the plasma membrane. Using odontoblastic cell lines in vitro Chaudhary et al. determined how the formation of MV is regulated during the initiation of the mineralization process. It was also found that mineralization competent MV are distinct from exosomes and, further, that phosphate is critical to the mineralization process.
Tendon, cartilage and cancer
The majority of this special issue focuses on factors that promote tissue mineralization. Ectopic mineralization is a well studied phenomenon that leads to deposition of calcium phosphate complexes in the extracellular matrix, particularly affecting dermis and arterial blood vessels [26]. A central question is: what prevents ossification in soft tissues? In certain conditions such as trauma, soft tissues such as tendons and ligaments, can ectopically ossify (EO). Motimi et al. show that one basis for EO with trauma could be from over-active BMP signaling, and that mice treated with BMP receptor kinase inhibitors have reduced injury induced tendon mineralization. The precise matrix components that regulate EO are not completely known, but could be one or more of the SLRPSs [27, 28]. A critically important region of the tendon is the enthesis that connects the tendon to bone. A paper by Goldberg et al. shows that the SIBLING, BSP, is abundant at the enthesis insertion site and, further, that Bsp-KO mice have “weak” tendons judged by their biomechanical properties, implying that BSP could have further functions in tendon biology. Another common site of ectopic bone formation occurs during advanced stages of osteoarthritis (OA) in structures known as osteophytes. In this context, Koyama et al. show that Prg4-KO mice have early onset OA in the temporomandibular joint (TMJ) (Fig. 1) and that the EO structures develop from safranin O positive cartilage tissue. Interestingly the EO tissue has high levels of hedgehog (HH) signaling that could be reduced with application of an HH inhibitor.
Like the TMJ, the intervertebral disc of the spine (IVD) (Fig. 1) is very complex, having similar but unique structural configurations compared to bone. A review by Risbud et al. describes the essential functions of the heparan sulfate-containing proteoglycan syndecan-4 on cell, tissue and growth factor homeostasis in the IVD with further discussion on its role in cartilage.
A review by Lord et al. provides an overview of the growth plate of long bones explaining how the fundamental processes governing the cartilage to bone transition rely on multiple factors in the ECM including collagen, proteoglycans, growth factors and enzymes. Understanding the factors controlling the bone:cartilage interface is of primary importance for future translational medicine geared to repair defective aging joints [29]. The final paper in this chapter by Yang et al. reviews the expression and function of SIBLINGS and other ECM components in cancer metastasis. The interest in bone matrix proteins in this context began with observations that many cancers that metastasize to bone such as prostate, breast and lung also produce proteins normally made by bone forming cells. It has been suggested that acidic SIBLINGS, which are acidic (with affinity to bone), and contain RGD sequences could be the molecular foundation for the tropism of these cancers to mineralized tissues.
Tissue Engineering
In recent years, there has been an exponential interest in the role of matrix in tissue engineering [30]. This chapter contains a combination of reviews and primary research papers that focus on experimental procedures that capitalize on matrix components to engineer mineralized tissue. A paper by Gower et al. describes a potential way to fabricate bone-like biomaterials via biomimetic processing. This concept is taken further in a review by Bellis et al. that explains how bone mimetic scaffolds can be used as a template for matrix proteins, growth factors and cells that mimic the basic biochemistry and structure of bone that are “inspired” by bones natural composition. The mechanobiology of engineered tissues and that relationship to TGF-β action is reviewed by Alliston et al. Finally a paper by Chen et al. shows how devitalized ECM elaborated by bone stem/progenitor cells could be used to retain their “stem-ness”. All the articles in this section show the great potential for the matrix in the exciting field of tissue engineering and mineralized tissue reconstruction in a special issue of Matrix Biology currently in preparation.
About the Cover
The cover of this special issue was painted by an artist named Angela McQuillan while she was studying at Temple University and working as technician in Dr. Renato Iozzo’s lab. She explained this creation was inspired by the color palette she viewed while staining and analyzing histology slides under the microscope. Angela also revealed that in her “down time” she would peruse issues of Matrix Biology and make sketches based on the inspiration from the images she saw that brought her to “another world”. To the guest editor’s eye, the abstract structures on this cover resemble many parts of the skeleton including cartilage, bone, vessels and what could be osteoclasts, osteocytes or osteoprogenitors in bone or even ameloblasts in teeth. This painting and the new cover of the regular issues of Matrix Biology, also painted by Angela, captures the abstract beauty of matrix tissue architecture and the concept of new discovery still to come.
Acknowledgments
Work in Marian Young’s laboratory is supported by the Intramural Program of the NIH, NIDCR.
References
- 1.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. Journal of cell science. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 2.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current opinion in cell biology. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traub W, Arad T, Weiner S. Origin of mineral crystal growth in collagen fibrils. Matrix. 1992;12:251–255. doi: 10.1016/s0934-8832(11)80076-4. [DOI] [PubMed] [Google Scholar]
- 4.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2015 doi: 10.1016/S0140-6736(15)00728-X. [E-pub ahead of print] PMID:2654281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Human mutation. 2007;28:209–221. doi: 10.1002/humu.20429. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer L. Proteoglycans, key regulators of cell-matrix dynamics. Matrix biology : journal of the International Society for Matrix Biology. 2014;35:1–2. doi: 10.1016/j.matbio.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix biology : journal of the International Society for Matrix Biology. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. The Cochrane database of systematic reviews. 2014;7:CD005088. doi: 10.1002/14651858.CD005088.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix biology : journal of the International Society for Matrix Biology. 2014;37:131–41. doi: 10.1016/j.matbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 11.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix biology : journal of the International Society for Matrix Biology. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. The Journal of clinical investigation. 2000;105:1325. doi: 10.1172/JCI7039C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dole NS, Kapinas K, Kessler CB, Yee SP, Adams DJ, Pereira RC, et al. A single nucleotide polymorphism in osteonectin 3' untranslated region regulates bone volume and is targeted by miR-433. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:723–732. doi: 10.1002/jbmr.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix biology : journal of the International Society for Matrix Biology. 2014;35:223–231. doi: 10.1016/j.matbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontology 2000. 2015;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 17.Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML. Regulation of pH During Amelogenesis. Calcified tissue international. 2010;86:91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nature reviews Molecular cell biology. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 19.Apte SS, Parks WC. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:1–6. doi: 10.1016/j.matbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:167–174. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:175–183. doi: 10.1016/j.matbio.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Jiang Q, Uitto J. Ectopic mineralization disorders of the extracellular matrix of connective tissue: molecular genetics and pathomechanisms of aberrant calcification. Matrix biology : journal of the International Society for Matrix Biology. 2014;33:23–28. doi: 10.1016/j.matbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 28.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix biology : journal of the International Society for Matrix Biology. 2015;24:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix biology : journal of the International Society for Matrix Biology. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AH, Kyriakides TR. Matricellular proteins and biomaterials. Matrix biology : journal of the International Society for Matrix Biology. 2014;37:183–191. doi: 10.1016/j.matbio.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]