Abstract
Despite improvements in functional outcomes attributable to advances in radiotherapy, chemotherapy, surgical techniques, and imaging techniques, survival in head and neck squamous cell carcinoma (HNSCC) patients has improved only marginally during the last couple of decades, and optimal therapy has yet to be devised. Genomic complexity and intratumoral genetic heterogeneity may contribute to treatment resistance and the propensity for locoregional recurrence. Countering this, demands a significant effort from both basic and clinical scientists in the search for more-effective targeted therapies. Recent genomewide studies have provided valuable insights into the genetic basis of HNSCC, uncovering potential new therapeutic opportunities. In addition, several studies have elucidated how inflammatory, immune, and stromal cells contribute to the particular properties of these neoplasms. In the present review, we introduce recent findings on genomic aberrations resulting from whole-genome sequencing of HNSCC, we discuss how the particular microenvironment affects the pathogenesis of this disease, and we describe clinical trials exploring new perspectives on the use of combined genetic and cellular targeted therapies.
Keywords: head and neck cancer, human papillomavirus, tumor microenvironment, squamous cell carcinoma, target therapy
1. Introduction
Cancers of the upper aerodigestive tract predominantly accounts for squamous cell carcinomas (SCCs), which develop in the epithelial linings of the oral cavity, pharynx, and larynx—the so-called head and neck SCCs (HNSCCs) [1].
HNSCC has been shown to contain unexpected complexity in terms of etiology, pathogenesis, morphological characteristics, clinical features, and natural history. The disease is strongly associated with tobacco use, heavy alcohol consumption, chewing of betel quid and poor oral hygiene [2–4]. However, although these exposures account for the majority of cases of HNSCC globally, specific oncogenic (high-risk) types of human papillomavirus (HPV)—most frequently HPV type 16 (HPV16)—have been shown to be causally related to a subset of oropharyngeal SCCs (OPSCCs) that arise from the crypt epithelium of the palatine tonsils and the base of the tongue [5–8]. Furthermore, cases of HPV-related OPSCC have been increasing dramatically and now account for 50% of cases in Europe and 65% of cases in the US [9]. HPV-positive disease represents a distinct clinical and epidemiological condition that differs in terms of risk factors, molecular genetic alterations, microscopic appearance, and clinical behavior [10].
Treatment of patients with early-stage HNSCC is relatively successful and relies on straight single-modality therapy: either surgery and/or radiation alone. Unfortunately, at the time of diagnosis, the majority of patients present with locally advanced disease and are managed by combined modality treatment strategies that may profoundly affect quality of life [11].
Despite improvements in functional outcomes attributable to advances in radiotherapy, chemotherapy, surgical and imaging techniques [12–14], survival in patients with HNSCC has not satisfactorily improved during the last couple of decades [15]. Genomic complexity [16], intratumoral genetic heterogeneity [17] and field cancerization [18] may contribute to its resistance to treatment and propensity for locoregional or distant recurrence. Additionally, patients with HNSCC often have limited options for reirradiation [19] or salvage surgery [20] and have only modest responses to second-line systemic therapies [21–23]. Moreover, although HPV-positive HNSCCs have better clinical outcomes and more-favorable responses to radiochemotherapy, compared with HPV-negative HNSCCs [24], a subgroup of patients with HPV-positive HNSCCs experienced high rates of distant failure after concurrent chemoradiation [25] and contradictory results in terms of activity have been reported for drugs targeting the epidermal growth factor (EGF)/EGF receptor (EGFR) pathway in these patients [26–28].
The generally poor outcomes in patients with HNSCC demand a significant effort from both basic and clinical scientists in the search for more-effective targeted therapies. Recent genomewide studies [16,29–33] have provided valuable insights into the genetic basis of HNSCC, opening potential new targetable biologic pathways. In addition, several studies have elucidated how inflammatory, immune and stromal cells contribute to the particular properties of these neoplasms [34,35].
In the present review, we pursued the following aims: (1) to discuss the recent findings on genomic aberrations resulting from whole-genome sequencing of HNSCC, (2) to discuss how the particular tumor microenvironment affects the pathogenesis of the disease and (3) to describe clinical trials exploring the use of combined genetic and cellular targeted therapies.
2. Genetic abnormalities in HNSCCs
Genome and exome analysis using advanced technical approaches [16,29,30,32,33,36] have provided a comprehensive view of the genetic alterations in HNSCC and have underlined several significant properties of these tumors. First, HNSCC tumors have remarkable genetic heterogeneity, which appears to be significantly wider than that reported by Chung et al. almost 10 years ago [37]. Second, the gene expression subtypes in HNSCC correspond to the histopathological classification of basal, mesenchymal, atypical and classical variants and may provide a complementary classification tool for HNSCCs [38]. Third, HPV-positive and HPV-negative HNSCCs have different genetic drivers (33, 34) [36], although distinct hits may converge to generate common effects. Finally, HPV-driven tumors have less gross chromosomal aberrations and approximately half the mutation rate of HPV-negative HNSCCs (30). These findings have not reached clinical relevance yet, and HPV-negative and HPV-positive tumors are currently treated the same way.
2.1 The dominant role of cell cycle and survival genes: TP53/RB pathway
The tumor suppressor genes TP53 and CDKN2A and the oncogene CCND1 represent the most commonly mutated genes in HPV-negative HNSCCs [16,29,30,33,39] and in premalignant dysplastic lesions [40]. DNA damage and oncogenic stress activate p53, which translates stress signals into cell cycle arrest or apoptosis. Three major mechanisms of p53 inactivation have been detected in HNSCC cells: (1) TP53 somatic mutations, which do not cause loss of function but do result in atypical dominant-negative p53 mutants [41] (this condition, frequently described as a gain of function, is better specified as “subversion of function,” as proposed by Muller and Vousden [42]); (2) p53 degradation mediated by HPV E6 oncoprotein [43]; (3) p53 proteasomal degradation, which requires binding of p53 with the negative regulator Mdm2 (Hdm2 in humans) [44].
Differentiated cells or basal cells that accumulate defective p53 protein become susceptible to other genetic mutations, acquire a propensity for metastasis [45] and create a favorable environment for the development of multiple independent transforming events in the same patient [46]. This explains why mutations of the TP53 gene correlate with worse prognosis and higher risk of recurrence after definitive locoregional treatment [47]. TP53 mutations in HNSCC are also associated with poor responses to chemotherapy and radioresistance [48], possibly via the inhibition of radiation-induced senescence [49]. Genomic studies have confirmed that other genes involved in cell cycle regulation, such as CDKN2A and CCND1, present high frequency of alterations in HPV-negative HNSCC [16,29,30]. Cyclin D1, the protein produced by CCND1, promotes cell proliferation in association with kinases CDK4 and CDK6. Extensive research led to demonstrate that the cyclin D1-CDK4/6 complex phosphorylates (activates) the retinoblastoma protein (pRB) and allows progression to mitosis. At the end of cell division p16-INK4A (the protein produced by CDKN2A) blocks cyclin D1-CDK4/6 complex, inhibits pRB phosphorylation and arrests cell cycle in G1 [50]. In the absence of p16-INK4A, pRB is continuously phosphorylated, and cell proliferation proceeds [51]. p14-ARF (also called p16-INK4B), an alternate reading frame protein product of the CDKN2A gene, is also involved in cell cycle regulation. p14-ARF inhibits p53-dependent cell cycle arrest by interacting with MDM2 and inducing p53 ubiquitination [52]. p14-ARF block p53 function and deregulates cell cycle control [53]. Structural abnormalities of the CDKN2A gene in HNSCC patients lead to less production or loss of p14-ARF and limitless replicative potential [54]. Similarly, high levels of cyclin D1 due to amplifications in the CCND1 gene result in an uncontrolled cell cycle and strongly predict unfavorable outcomes in patients with HNSCC [55].
Alterations in the p53 and pRB pathways are radically different in HPV-positive tumors [56], where p53 and pRB proteins are inactivated by viral oncoproteins E6 and E7, respectively. Specifically, E6 binds to the cellular protein E6AP, and the E6/E6AP complex is responsible for ubiquitination and proteasome degradation of p53. E7, on the other hand, inactivates pRB, which in turn induces overexpression of p16-INK4A, and cell G1-S phase transition [57].
Outlook and therapeutic challenges
The search for a targeted therapy aimed to modulate p53 has been characterized by limited success, so far. Noteworthy is a single phase III study with adenoviral p53 gene therapy and methotrexate: wild type p53 patients showed better response to gene therapy (probably related to to up-regulation of MDM2), suggesting a role of p-53 profile as predictive biomarker [58].
Therapeutic strategies targeting p16 have not reached clinical trials yet, whereas cyclin D1-CDK 4/6 dual inhibitor is currently being tested in a phase I trial of various advanced cancers (ClinicalTrials.gov identifier: NCT01394016). Another phase I study of a CDK inhibitor in combination with radiotherapy has completed recruitment (ClinicalTrials.gov identifier: NCT00899054). Palbociclib, a target inhibitor of CDK4/6, recently approved for the treatment of breast cancer [59] has been evaluated in combination with cetuximab in a phase I study [60] and a randomized, multicenter, phase II study with palbociclib and cetuximab in HPV-negative recurrent/metastatic HNSCC is also ongoing (ClinicalTrials.gov identifier: NCT02499120).
Regarding HPV positive tumors, preventive and therapeutic anti-HPV vaccines have been developed in an effort to prevent primary or established infections [61]. As prophylactic vaccines are based on L1 viral capsid protein, which is unexpressed in HPV-associated neoplasms and induces only humoral immunity, they are ineffective for established HPV-driven SCCs. Since it stimulates cytotoxic T lymphocytes (CTLs) against infected and transformed cells expressing specific E6 and E7 epitopes specific E6 and E7 epitopes [62], therapeutic vaccination is, conversely, a promising option [63]. Several phase I and II clinical trials are currently investigating the safety and efficacy of therapeutic DNA vaccines (ClinicalTrials.gov identifier: NCT01493154; NCT0216305), protein vaccines (ClinicalTrials.gov identifier: NCT00704041; NCT00257738; NCT00019110), and bacterial vector vaccines (ClinicalTrials.gov identifier: NCT02002182; NCT01598792) for HPV-positive HNSCC, alone or in combination with both chemotherapy and radiation therapy [63–65]
2.2 Genes of cell growth as targets for biological therapy: PI3K/AKT/mTOR pathway
PI3K represents the second most important target gene across human cancers [66], and alterations of the PI3K pathway are common drivers in HNSCC [16,29,30,33,39]. A number of growth factors relay signals through the PI3K signaling cascade. Activated PI3K phosphorylates the second messenger phosphatidylinositols PIP2 and PIP3 and turns on downstream effectors AKT and mammalian target of rapamicin complex 2 (mTORC2). Fine-tuning of PI3K depends on opposing regulators. Phosphatase and tensin homologue (PTEN) shuts off PI3K signaling, whereas PI3K catalytic subunit alpha (PI3KCA) is responsible for complex activation [66]. The oncogene product RAS is also a positive regulator of the PI3K signaling cascade, resulting in cell survival and cell cycle regulation [67].
Abnormal PI3K pathways in HNSCC are derived mostly from gain-of-function mutations of PI3KCA and loss-of-function mutations of PTEN [16,29,30,33,39]. Because the global frequency of mutations affecting various components of the PI3K pathway is very high, and as multiple ligands and receptor tyrosine kinases rely on PI3K, the PI3K pathway has become an elective therapeutic target in HNSCC [16].
Being in the crossroad with RAS and PI3K, MEK, ERK-1 and ERK-2 are also object of several translational studies.
Outlook and therapeutic challenges
The three major classes of PI3K inhibitors—namely, combined PI3K/mTORC, Pan-Class I, and alpha-specific —are currently under clinical evaluation in phase I and II studies, alone or in combination with either chemotherapy or cetuximab (ClinicalTrials.gov identifier: NCT00854152; NCT 01737450; NCT01252628). In particular some controlled trials employing agents that target PI3K isoforms in recurrent/metastatic setting are worthy of mention. Two phase II trials failed to demonstrate benefit when PX-866 (Oncothyreon, Seattle, WA) was added to either docetaxel or cetuximab. In a phase I trial BYL 719 (Novartis Pharmaceuticals), which target alpha isoform of class I PI3K, gave only a partial response and further investigations are ongoing to ascertain its clinical benefit [68–70]
The mTORC inhibitors everolimus and temsirolimus, which are currently used to treat breast cancer, renal cell carcinoma, and pancreatic neuroendocrine tumors, have been also evaluated in combination with erlotinib in platinum-refractory, recurrent/metastatic HNSCC [71]. Both agents showed modest response rate and low tolerability, raising some concern in targeting the EGFR and mTOR pathways together [72,73] Temsirolimus has been tested as single agent in the same setting: although no objective response was recorded, a PFS rate of 40% at 12 weeks has been achieved [74].
Other inhibitors of tyrosine kinase are also under investigation, such as trametinib, a MEK inhibitor used in combination with AKT inhibitors (ClinicalTrials.gov identifier: NCT 01725100), and sorafenib, a multiple tyrosine kinase inhibitor. In a phase II study, treatment with sorafenib has shown poor response rate, but compared favorably with other phase II single agent trials in terms of progression-free and overall survival [75]. Moreover, in vitro experiments indicate that sorafenib might sensitize head and neck squamous cells to ionizing radiation, suggesting the potential to overcome radioresistance mainly through the inhibition of DNA double-strand breaks (DSB) [76].
2.3 EGFR pathway
PI3K signaling is initiated by specific growth factors and coupled receptors, such as the EGF/EGFR. EGFR is part of the ERB family of receptor tyrosine kinases, which includes also ERBB2, ERBB3, and ERBB4. EGF/EGFR complex activates a number of biological functions through downstream PI3K/AKT, Ras/Raf/MAPK, and JAK/STAT. It is also able to translocate to the nucleus and activate transcription, thus producing pleiotropic effects in cellular homeostasis. One of the genes induced by intranuclear EGF/EGFR is the aforementioned CCND1 [77].
EGFR genetic alterations include amplifications and gain-of-function mutations that induce high protein overexpression in a large proportion of HNSCCs and lead to tumor proliferation, angiogenesis, metastasis and consequently poor prognosis of the disease [77,78]. However, EGFR overexpression has not been found to be a predictive biomarker of activity with EGFR targeted therapies [79].
Outlook and clinical challenges
Inhibition of the EGF/EGFR pathway has been the first molecular strategy showing significant prosurvival effect in HNSCC. Inhibitors include recombinant-chimeric (cetuximab) or humanized (nimotuzumab) or fully human (panitunumab and zalutunumab) anti-EGFR monoclonal antibodies. Several controlled clinical studies have confirmed the efficacy of cetuximab in both locally advanced disease (in combination with radiotherapy) and metastatic or recurrent HNSCC (in combination with standard chemotherapy) [14,21].
In platinum-refractory or ineligible patients, cetuximab has shown modest activity as monotherapy [80], but encouraging response rate in combination with paclitaxel in a phase II trial [81]. Benefits achieved with cetuximab were not confirmed for panitunumab in patients with metastatic or recurrent disease [26]. Similarly, disappointing results in terms of overall survival were reported for zalutumumab compared with BSC alone in a phase III study in recurrent/metastatic setting. Nimotuzumab provided survival benefit in inoperable advanced Indian patients in a randomized phase IIb, 5-year study [22,78]. Contrasting these results, inhibitors of tyrosine kinase activity using small molecules, which block the phosphorylation and activation of EGFR (geftinib and erlotinib), have shown limited antitumor activity [82,83] and no additional studies have been planned.
Patients treated with EGFR inhibitors develop high levels of de novo or acquired resistance to therapy. This may be due to activation of other ErbB family receptors, cross-talk with other signaling pathways, nuclear localization of EGFR, or mutant forms of the receptor [84,85]. Therefore, interest is currently shifting to the use of inhibitors that target multiple ERB-family members. Initially, lapatinib, a reversible tyrosine kinase inhibitor of EGFR and ERBB2, has shown promising activity, when used in combination with concurrent chemoradiotherapy in HPV-negative patients [86]. However, in a large adjuvant post-operative phase III study lapatinib added to concurrent chemoradiation and used as long-term maintenance therapy has failed to improve both disease-free and overall survival in high risk HNSCC patients, and has caused additional toxicity compared with placebo [87]. These findings should make us reflect on the opportunity to have reliable data on the effectiveness of targeted therapies before programming large controlled studies. At present, investigation of lapatinib is restricted to a single phase II trial in the advanced setting (ClinicalTrials.gov identifier: NCT01044433). Another ERB-family blocker, afatinib, has shown a response rate similar to that of cetuximab in a phase II randomized trial, with a lack of cross-resistance following sequential EGFR/ErbB therapy [88]. On the basis of these data, afatinib has moved to a phase III trial in recurrent/metastatic setting, confirming its efficacy compared to methotrexate (LUX Head & Neck 1 study), in terms of progression-free survival and patient-reported outcomes [23]. A new trial comparing the efficacy of afatinib with placebo as adjuvant therapy in patients who have received definitive chemoradiotherapy (LUX Head & Neck 2), is currently recruiting participants (ClinicalTrials.gov identifier: NCT01345669). Table 1 summarizes the results of the most relevant clinical trials targeting the EGF/EGFR complex.
Table 1.
Relevant controlled clinical trials targeting EGF/EGFR pathways.
| Agent | Phase II-III trials | Clinical setting | Main results | Reference(s) |
|---|---|---|---|---|
| Cetuximab (Chimeric human anti-EGFR) | Phase III plus RT Phase III plus CT (Extreme) |
Locally advanced Recurrent/metastatic |
Improved OS Improved OS |
Bonner et al. 2010 (Ref. 8) Vermorken et al. 2008 (Ref. 15) |
| Panitunumab (Fully human anti-EGFR mAb) | Phase III plus CT (Spectrum) | Recurrent/metastatic | Negative study, but improved OS in post-hoc analysis in HPV negative | Vermorken et al. 2013 (Ref. 20) |
| Nimotuzumab (Humanized anti-EGFR mAb) | Phase IIb plus CRT or RT | Locally advanced | Improved survival (median not reached for nimotuzumab plus CRT arm) | Reddy et al. 2014 (Ref. 67) |
| Zalutumumab (Fully human anti-EGFR mAb) | Phase III plus BSC/MTX | Platinum refractory recurrent/metastatic | Improved PFS | Machiels et al. 2011 (Ref. 16) |
| Lapatinib (EGFR/HER2 inhibitor) | Phase II plus CRT | Locally advanced | Increased CRR and median PFS in p16-negative disease | Harrington et al. 2013 (Ref. 72) |
| Afatinib (Irreversible ERBB-family blocker) | Phase III trial vs. MTX | Recurrent/metastatic | Improved PFS | Machiels et al. 2015 (Ref. 17) |
BSC, best supportive care; CRR, complete response rate; CRT, concurrent chemoradiotherapy; CT, chemotherapy; mAb, monoclonal antibody; MTX, methotrexate; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
2.4 Genes of squamous cell differentiation: the NOTCH pathway
One important finding of the whole-exome sequencing studies is the high frequency of mutations (up to 15%) in NOTCH1 gene [29,30]. The NOTCH signaling pathway is activated when one cell expressing the appropriate ligand (Jagged or Delta) interacts with a neighbor cell expressing a NOTCH1 receptor. The NOTCH receptor is cleaved by ADAM metalloprotease and γ-secretase complex and the intracellular domain translocates to the nucleus, where it activates transcription of target genes HES1 and HEY1 [89]. In human keratinocytes, NOTCH1 signaling is essential to promote cell differentiation, and down-modulation or loss-of-function mutations of NOTCH1 gene are associated with dysfunctional squamous cell differentiation and development of carcinoma [90]. Fine-tuning of NOTCH signaling depends on a number of regulators. Relevant for cancer development is the reciprocal feedback loop between NOTCH, p53 and p63, which contributes to the balance between self-renewing and differentiation of keratinocytes. Suppression of p53 activity down-regulates NOTCH1, blocks differentiation and promotes uncontrolled cell proliferation [91]. High levels of p63 also inhibit NOTCH1 and suppress differentiation, whereas low levels of p63 and high levels of NOTCH1 result in the opposite effect [92].
NOTCH1 mutations have been detected in a large proportion of HNSCCs, making NOTCH1 the second most frequently mutated gene after TP53 in these tumors [29,30,39]. Several mutations result in NOTCH1 inactivation, suggesting a tumor suppressor function rather than an oncogene function. Only a small subset of patients with HNSCC present with gain-of-function mutations [29,30,93], which are similar to those associated with the leukemia cluster [94]. Mutations of other genes of the NOTCH1 pathway, in the presence of wild-type NOTCH, have also been detected in patients with HNSCC [93].
Outlook and clinical challenges
The NOTCH1 pathway represents a potential new target in cancer therapy, although a therapeutic approach is complicated by the dual nature of tumor suppressor and oncogene of NOTCH1. There are currently no available targeted drugs for this pathway. Inhibitors or activators of the NOTCH1 pathway via block of γ-secretase and histone deacetylase, respectively, are developing.
2.5 MicroRNA (miRNA) in HNSCC
Compelling evidence indicates that the human genome is regulated by microRNAs (miRNAs). miRNAs are short, noncoding RNAs that regulate transcription and translation of their target genes by binding to the highly evolutionarily conserved 3′-untranslated regions of mRNAs [95]. Altered expression of miRNAs correlates with human cancers [96] and several miRNAs are either up-regulated or down-regulated in HNSCC [97].
Up-regulated miRNAs, such as miR-21 (negatively correlated with PTEN) and miR-205 (which targets PTEN), promote cell proliferation by blocking cell cycle inhibitors, whereas down-regulated miRNAs, such as the let-7 family, negatively regulate KRAS [98]. miRNA are also involved in chemoresistance as revealed by levels of expression in resistant HNSCC cell lines [99].
Outlook and clinical challenges
Further studies are warranted to investigate the use of miRNAs as diagnostic, prognostic, or therapeutic markers of HNSCC, but the high rate of abnormalities detected by genomic studies points to a previously unexpected role of these molecules in HNSCC. The tumor suppressor let-7c has been found to be altered in 40% of HPV-negative and 17% of HPV-positive HNSCCs [16], and specific miRNAs have also been associated with a propensity for metastasis and poor outcomes [100].
3. A new perspective in cancer treatment: targeting the tumor microenvironment
3.1 The tumor microenvironment in HNSCC
Numerous studies have demonstrated the essential role that the tumor microenvironment plays in the acquisition of hallmark capabilities [101]. The particular properties of the tumor microenvironment play a prevalent role in progression of HNSCC and represent potential targets for new therapeutic approaches, along with conventional or new molecular-driven therapies. The mucosa of the nasopharynx, oropharynx, and hypopharynx progressively changes from pseudostratified respiratory epithelium to a nonkeratinized stratified squamous layer. The oropharyngeal trait contains tonsillar lymphoid follicles in which the mucosa extends deep into crypts and alternates stratified squamous cells and reticulated spongelike layers [102]. Reticulated patches associated with discontinuous basement membrane collect pathogens hiding in the crypts [103]. The mucosa is also enriched with basal cells localized near the basal lamina. Under normal conditions, these cells contribute to the slow turnover of the epithelium, but they may convert into cancer stem cells (CSCs) responsible for tumor initiation and progression (see [104] [105] for an exhaustive review of CSCs in HNSCC).
The tumor microenvironment of HNSCC, particularly the oropharyngeal trait, contains a predominance of nonepithelial cells, which provide support for growth factors, cytokines, and chemokines to promote invasiveness and chemoresistance (Figure 1). These cells include lymphocytes, macrophages, dendritic cells, vascular cells, and stromal cells. Hereafter, we will discuss how some of these cells exert a suppressive role in the antitumor immune response.
Figure 1.
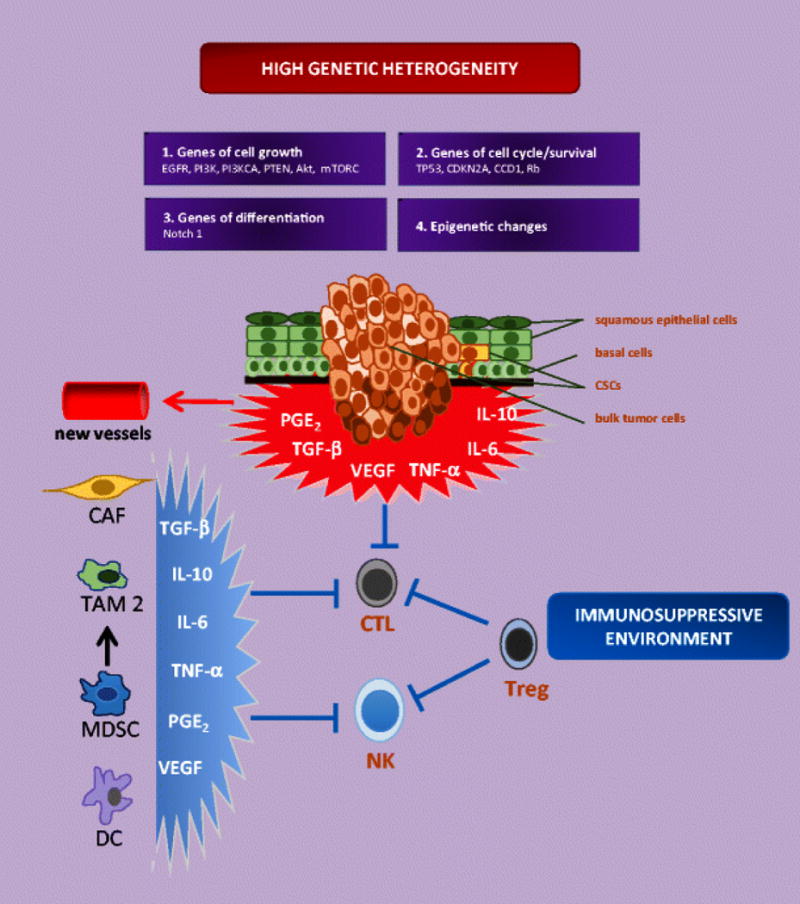
Genetic heterogeneity and the immunosuppressive microenvironment characterize HNSCCs. Genes regulating cell cycle and survival, cell growth, and differentiation are frequently mutated or amplified in HNSCC. Epigenetic changes have also been identified as drivers of tumor progression. Cancerogenesis produces a bulk of heterogeneous cells, including cells with invasion and metastatic capacity. The tumor microenvironment is characterized by an abundance of cytokines and growth factors produced by tumors cells (escape mechanisms) and inflammatory, stromal, and antigen-presenting cells. Collectively, these cells provide an unfavourable milieu that inhibits the immunological response and promotes tumor growth and survival. CAF, cancer-associated fibroblast; CSC, cancer stem cell; CTL, cytotoxic T lymphocyte; DC, dendritic cell; MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage; Treg, regulatory T lymphocyte.
3.2 ROS, inflammation and immunity
Tobacco use, alcohol consumption, and HPV infection trigger inflammatory and immune activation. Oxidative stress is a major effector in this process, as chemical carcinogens produce such a high level of reactive oxygen species (ROS) and reactive nitric species (RNS) that scavenging by antioxidants is always inefficient. Compelling experimental and clinical evidence indicates that ROS produce a broad range of effects, from genomic instability and changes in signaling pathways to activation of inflammation, tissue repair, controlling cell proliferation and survival, affect cell motility and invasiveness, and activate inflammation, tissue repair, de novo angiogenesis [106] and differentiation of basal stem cells [107].
In HNSCC, the cross-talk between tumor and inflammatory cells is multifaceted, as demonstrated by the effect produced by tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) in tumor development. TAM subpopulation M1 promotes inflammation and exerts an antitumor function, whereas TAM subpopulation M2, the predominant variant in malignant proliferations, activates angiogenesis and tissue remodeling and sustains tumor progression [108]. Thus, concomitant to inflammation, monocyte-derived macrophages create a favorable environment for tumor growth by secreting EGF, PDGF, and TGF-β [109]. Macrophages also synthesize the chemotactic factor macrophage inflammatory protein-3α, which drives HNSCC cell migration and invasion [110]. HNSCC patients with high levels of expression of M2 markers CD68 and CD163 present with significantly worse clinical outcomes [111], a finding that provides a rationale for targeting M2 depletion in HNSCC. M2 can also be generated by MDSCs. MDSCs are an intrinsic part of the myeloid lineage and are characterized by the capacity to suppress T cell responses in various ways. MDSCs also produce factors that support tumor growth and angiogenesis, stimulate M2 differentiation, and contribute to the production of an immunosuppressive milieu that favors tumor survival [112].
Head and neck tumor cells are actively eliminated by tumor antigen (TA)–dependent and TA-independent host immune responses. However, immune surveillance breaks down when tumor cells harbor escape mechanisms that allow them to avoid or inhibit the immune system. For example, tumors can co-opt certain immune-checkpoint pathways used by the immune system to maintain self-tolerance, modulate the duration and amplitude of the immune responses and avoid collateral tissue damage. Many of the immune checkpoints are initiated by ligand-receptor interactions, such as Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and programmed cell death protein 1 (PD1).
CTLA-4 receptor is expressed on T cells and attenuates T-cell immune response through its ligands CD80 and CD84. PD-1 receptor is also expressed in activated T cells, APCs and NK cells and inhibits T-cell activation through its ligands PD-L1 and PD-L2 [113]. The ligands PD-L1 and PD-L2 have broad expression ranging from T, B and NK cells to some tumor cells, including those of HNSCC [34,114]. PD-L1/2-Pd-1 interaction results in progressive exhaustion of the immune response. Ultimately, tumor immune evasion is mainly due to PD-1 positive T cells that infiltrate tumor bulks expressing high PD-L1 levels.
In HPV-positive HNSCC, immunosuppression is increased further by viral infection, which may explain why, paradoxically, these tumors commonly develop within the immune tissue of tonsillar lymphoid follicles, an anatomic site that should favor immunologic antitumor response. Here, HPV blocks interferon-alpha, inhibits CTLs, activates suppressor T lymphocytes, and down-regulates expression of MHC complex I [115]. The immunosuppressive milieu produced by inflammatory cytokines maintains latent infection and favors tumorigenesis, which is initiated when the viral DNA integrates into the host genome and drives genomic instability. Once infected and transformed by HPV, tumor cells activate additional mechanisms to escape the immune system by preventing exposure of tumor antigens and promoting apoptosis of effector T lymphocytes and down-regulation of NK cells [116].
Outlook and clinical challenges
In principle, many of the immune checkpoints can be blocked or modulated by monoclonal antibodies in order to release cytotoxic T cells from anergy and tolerance [113].
Ipilimumab, a monoclonal antibody against CTLA-4, was the first biological drug of this class to obtain FDA approval for its relevant clinical benefit in metastatic melanoma [117]. Since then, a number of immunotherapies have been also proposed for HNSCC, although the prognostic and predictive role of the expression of immune checkpoint biomarkers in HNSCC is still under debate [118–120].
Pembrolizumab, an anti-PD-1 monoclonal antibody, has been tested in the phase Ib Keynote 012 trial in recurrent/advanced HNSCC expressing PD-L1. In this study, response rate was nearly 20% based on RECIST criteria, regardless of HPV status, and clearly correlated with PD-L1 expression level [121]. In an expansion cohort of the same study, tumor shrinkage was reported in 57% of the patients with a response rate of nearly 25% and acceptable toxicity [122] Responses were durable, remarking the novelty of these results, compared to earlier experiences with cetuximab; longer follow-up is needed to assess survival.
Two phase III trials have been planned to compare pembrolizumab as single agent or in combination with chemotherapy) with standard treatment in recurrent/metastatic HNSCC (ClinicalTrials.gov identifier: NCT02252042 – NCT02358031).
Another phase III trial of nivolumab (a fully human antibody targeting the PD-1 receptor) in comparison to standard treatment in recurrent/metastatic HNSCC, has been prematurely discontinued for the evidence of a superior survival for the nivolumab arm (ClinicalTrials.gov identifier: NCT02105636).
Promising results have been also reported in a multiarm dose expansion study employing the PD-L1 inhibitor durvalumab (MEDI4736). In 54 metastatic HNSCCs, not preselected for PD-L1 expression, the response rate was 14%, reaching 50% in the subset of PD-L1 expressing tumors [123]. A phase III open label study of durvalumab with or without tremelimumab (fully human monoclonal antibody targeting CTLA-4) versus standard of care in recurrent/metastatic HNSCC is ongoing (ClinicalTrials.gov identifier: NCT0255159).
To sum up, important and innovative features make the checkpoint inhibitors the current most promising therapeutic strategy in HNSCC for the relative high percentage of durable responses and the favorable toxicity profile.
Other immunotherapies have been designed to target immunological mechanisms involved in tumor progression. This is an area of intensive translational research with both promising successes and persistent disappointments. Besides the already illustrated line of research on checkpoint inhibitors, four other major strategies have been translated from basic research to clinical trials (Table 2) [124–137]: (1) conventional therapies that display immunomodulatory effects; (2) targeted therapies that, beyond the function of targeting oncoproteins, may play a role in tumor-mediated immunosuppression; (3) therapeutic vaccines used to stimulate an active immune response against a specific MHC-bound TA-derived peptide, and (4) autologous T cells engineered to produce special receptors (chimeric antigen receptors) that allow the T cells to recognize specific proteins on tumor cells.
Table 2.
Selected basic science and clinical evidence on immunotherapy for HNSCC.
| Therapeutic approach | Major evidence | Reference(s) |
|---|---|---|
| Conventional therapies | ||
| Cisplatin | Collateral immunomodulatory effects: upregulation of MHC class I, recruitment of T cells and TAMs, downregulation of TREGs and MDSCs | de Biasi et al. 2014 (Ref. 137) |
| Taxanes | Collateral activation of DCs, NK cells, CTLs; upregulation of mannose-6-phosphate tumor cell receptors with increase of permeability to granzyme-B | Chang et al. 2013 (Ref. 135) |
| 5-fluorouracil | Collateral increase of IFN-gamma production by CD8 T cells | Tsuchikawa et al. 2012 (Ref. 133) |
| Radiotherapy | Increase in type I IFNs with enhancement of both intratumor concentration of CXCR3 chemokine and activity of CD8 T cells | Lim et al. 2014 (Ref. 140) |
| Targeted therapies | ||
| Cetuximab | Collateral upregulation of MHC II and costimulatory factors on DCs. Increase of immune responses: complement-dependent cytotoxicity, NK-mediated antibody dependent cytotoxicity, macrophage-mediated antibody dependent cellular phagocytosis. |
Vannemann & Dranoff 2012 (Ref. 134) Srivastava et al. 2013 (Ref. 136) Kumai et al. 2014 (Ref. 139) |
| Bevacizumab | Collateral enhancement of differentiation of DCs and blockade of MDSCs | Alfaro et al. 2009 (Ref. 131) |
| Sunitinib | Blockade of secretion of IL-10 and TGF-b and enhancement of production of IFN-gamma by tumor T cells | Alfaro et al. 2009 (Ref. 131) Ozao-Choi et al. 2009 (Ref. 132) |
| Cancer vaccines | ||
| Multiagent vaccines | Specifically target TAs: Ly6k (lymphocyte antigen 6 complex locus), CDCA1, IMP3 (insulin-like growth factor II m-RNA-binding protein). Phase II trial: Improvement in OS in HLA*24:02+ advanced HNSCC patients | Yoshitake et al. 2015 (Ref. 144) |
| DC-based wild-type p53 peptide vaccine | Induction of antitumor response by T cells. Phase I trial: Treatment safe, with promising clinical outcome | Schuler et al. 2014 (Ref. 141) |
| CAR therapies | ||
| Targeted CAR therapy | LMP1/CAR (latent membrane protein) CSPG-4 CAR (chondroitin sulfate proteoglycan-4). Promising results in preclinical evaluations. |
Tang et al. 2014 (Ref. 142) Geldres et al. 2014 (Ref. 138) |
| Immune checkpoint target therapies | ||
| Monoclonal antibodies | Anti-PD1 – Anti PD-L1 Treatment safe. Remarkable results in preliminary phase I studies. Up to date, no definitive results in terms of clinical outcome in HNSCC. Pembrolizumab and nivolumab under evaluation in a phase III trial. |
Swanson et al. 2015 (Ref. 143) Seiwert et al. 2014 (Ref. 128) Seiwert et al. 2015 (Ref. 129) Segal et al. 2014 (Ref. 130) |
CAR, chimeric antigen receptor; CTLs, cytotoxic T lymphocytes; DCs, dendritic cells; IFN, interferon;IL, interleukin;MDSCs, myeloid-derived suppressor cells; MHC, major histocompatibility complex; NK, natural killer; OS, overall survival; TA, tumor antigen; TAMs, tumor associated macrophages; TGF, tumor growth factor; TREGs: regulatory T cells.
3.3 Invasion and metastasis
HNSCCs are characterized by their propensity to spread via direct infiltration through lymphatic, haematogenous, or perineural routes. Neck metastatic lymph nodes are quite common at presentation, with survival reduced nearly by half when they are present [138]. Metastatic dissemination involves several steps, most of which are coordinated by epithelial mesenchymal transition (EMT) and remodeling of the extracellular matrix. Cells undergoing EMT shift protein synthesis to overexpress cytoskeletal proteins that detach and invade the extracellular matrix through actin-rich protrusions and focal adhesions. In addition, the invasive borders of HNSCCs are enriched with cells that express matrix metalloproteinases (MMPs) — mainly MMP-9 and MMP-2—and actin-rich structures, called filopodia and invadopodia, that mediate ECM proteolysis [139]. The TGF-β pathway is a key molecular player in EMT. TGF-β, in cooperation with its cognate receptors and transducers (SMAD2, SMAD3, and SMAD4), activates genes of cell motility and down-regulates epithelial genes [140]. TGF-β is secreted by tumor cells and by a number of cells of the tumor microenvironment, including cancer-associated fibroblasts and TAMs.
Another pathway associated with proliferation and migration of tumor cells involves Src, a cytoplasmic tyrosine kinase, activated by a number of growth factors, including EGFR, FGFR and VEGFR [141].
Outlook and clinical challenges
Clinical evidence supporting the targeting of metastatic dissemination in HNSCC has been elusive. Studies using sarcatinib (AZD0530), a small molecule inhibitor of Src, in combination with either the phospholipase C inhibitor U73122 or the EGFR inhibitor gefitinib found reduced cell invasion in vitro [142,143], but clinical trials have failed to demonstrate any significant benefit [144]. Broad-spectrum MMP inhibitors have also been used, with very limited success in most cancers [145].
3.4 Angiogenesis
Angiogenesis is a well-known factor that is necessary for nourishing tumor cells and CSC niches and for promoting metastatic progression [146]. Angiogenesis is supported by hypoxic response or inflammation [147] as well as a variety of factors in the tumor microenvironment, such as VEGFR and NF-kB. VEGF enhances endothelial growth, migration, and differentiation. Its overexpression has been detected in up to 40% of cases of HNSCC and is associated with poor prognosis [148].
Outlook and clinical challenges
Targeted therapies to inhibit angiogenesis include monoclonal antibodies anti-VEGF and multikinases inhibitors, such as sunitinib and the aforementioned sorafenib. Bevacizumab, an anti-VEGFR monoclononal antibody, has been tested in phase II trials in combination with other molecular targeted therapies or chemotherapy and has shown interesting levels of activity [149–151]. Unfortunately, a significant number of bleeding events (some of which were fatal) have been reported, suggesting that evaluation on the dose to be used and patients’ selection has to be reconsidered. Results are also anticipated from trials investigating multiple tyrosine kinase inhibitors. At present, phase II studies have reported stable disease as better response, with an encouraging PFS and toxicity profile for sorafenib [75,152]. Two controlled trials with chemotherapy associated with bevacizumab (ClinicalTrials.gov identifier: NCT00588770) or sorafenib (ClinicalTrials.gov identifier: NCT02035527) are ongoing.
4. Conclusions
HNSCC is an extremely heterogeneous disease with distinct patterns of presentation and biological behavior. Patients with HNSCC are frequently treated with aggressive treatment strategies that may strongly affect quality of life and elicit unpredictable results. The success of EGFR-targeting therapies combined with radiation or chemotherapy covers a limited number of cases. For this reason, it is essential both to explore new multi-strategy approaches, by the use of combined genetic and cellular targeted new therapies, and to investigate potential predictive biomarkers for treatment response. To date, only HPV is a validated independent prognostic indicator and predictive marker of response to treatment. Recent whole-exome sequencing studies have provided a comprehensive view of the genetic alterations and the complexity of gene mutations underlying this malignancy. Although few driver genes are currently targetable, and although the predominance of tumor suppressor gene alterations presents a challenge for the treatment of HNSCC, these investigations, as well as new insights into the tumor microenvironment, have provided a deeper and comprehensive understanding of HNSCC biology establishing a basis for potential molecular recognition–based customized therapeutic approaches. In particular, the immune checkpoint inhibitors represent the most promising strategy for HNSCC in the next future.
Figure 2.
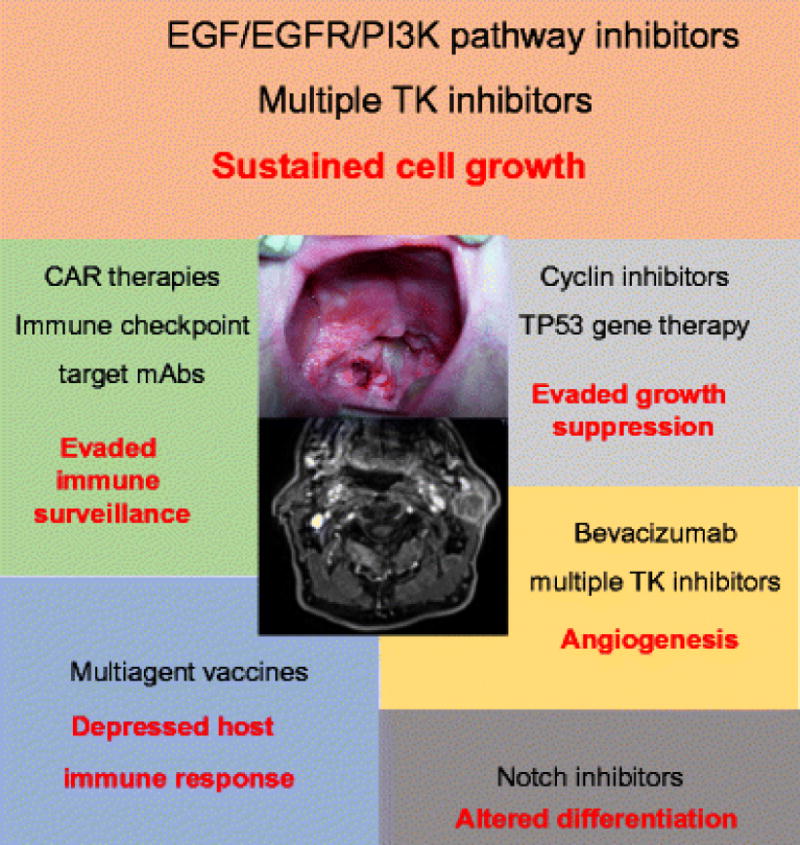
Principal hallmarks of HNSCC (red text) and corresponding therapeutic approaches (black text). Inhibitors of the EGF/EGFR/PI3K pathway are the most validated options in the clinical setting, but other promising therapies are under clinical testing or preclinical design. The image in the central area of the figure refers to locoregionally advanced oropharyngeal squamous cell carcinoma. CARs, chimeric antigen receptors; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; mAbs, monoclonal antibodies; PI3K, phosphoinositide 3-kinase; TK, tyrosine kinase.
References
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. 1988;48:3282–7. [PubMed] [Google Scholar]
- 3.Znaor A, Brennan P, Gajalakshmi V, Mathew A, Shanta V, Varghese C, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. 2003;105:681–6. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]
- 4.Talamini R, Vaccarella S, Barbone F, Tavani A, Vecchia CL, Herrero R, et al. Oral hygiene, dentition, sexual habits and risk of oral cancer. 2000;83:1238–42. doi: 10.1054/bjoc.2000.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. 2005;11:5694–9. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 7.Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, Conradt C, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. 1998;58:5–13. [PubMed] [Google Scholar]
- 8.Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer’s tonsillar ring. 1997;79:595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Stein AP, Saha S, Kraninger JL, Swick AD, Yu M, Lambert PF, et al. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J Sudbury Mass. 2015;21:138–46. doi: 10.1097/PPO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boscolo-Rizzo P, Mistro AD, Bussu F, Lupato V, Baboci L, Almadori G, et al. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33:77–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Boscolo-Rizzo P, Gava A, Marchiori C, Baggio V, Mosto MCD. Functional organ preservation in patients with locoregionally advanced head and neck squamous cell carcinoma treated by platinum-based multidrug induction chemotherapy and concurrent chemoradiotherapy. Ann Oncol. 2011;22:1894–901. doi: 10.1093/annonc/mdq681. [DOI] [PubMed] [Google Scholar]
- 12.Ghi MG, Paccagnella A, Ferrari D, Foa P, Cossu Rocca M, Elena V. Concomitant chemoradiation (CRT) or cetuximab/RT (CET/RT) versus induction Docetaxel/Cisplatin/5-Fluorouracil (TPF) followed by CRT or CET/RT in patients with Locally Advanced Squamous Cell Carcinoma of Head and Neck (LASCCHN). A randomized phase III factorial study ( NCT01086826) J Clin Oncol. 2014:5s. [Google Scholar]
- 13.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 14.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. The LancetOncology. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 15.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. The Oncologist. 2010;15:994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mroz EA, Tward AM, Hammon RJ, Ren Y, Rocco JW. Intra-tumor Genetic Heterogeneity and Mortality in Head and Neck Cancer: Analysis of Data from The Cancer Genome Atlas. PLoS Med. 2015;12:e1001786. doi: 10.1371/journal.pmed.1001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boscolo-Rizzo P, Rampazzo E, Perissinotto E, Piano MA, Giunco S, Baboci L, et al. Telomere shortening in mucosa surrounding the tumor: Biosensor of field cancerization and prognostic marker of mucosal failure in head and neck squamous cell carcinoma. Oral Oncol. 2015 doi: 10.1016/j.oraloncology.2015.02.100. [DOI] [PubMed] [Google Scholar]
- 19.Lartigau EF, Tresch E, Thariat J, Graff P, Coche-Dequeant B, Benezery K, et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013;109:281–5. doi: 10.1016/j.radonc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Putten L, Bree R, Doornaert PA, Buter J, Eerenstein SEJ, Rietveld DHF, et al. Salvage surgery in post-chemoradiation laryngeal and hypopharyngeal carcinoma: outcome and review. Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-Facc. 2015;35:162–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 22.Machiels J-P, Subramanian S, Ruzsa A, Repassy G, Lifirenko I, Flygare A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12:333–43. doi: 10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 23.Machiels J-PH, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–94. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 24.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Vainshtein JM, Spector ME, Ibrahim M, Bradford CR, Wolf GT, Stenmark MH, et al. Matted nodes: High distant-metastasis risk and a potential indication for intensification of systemic therapy in human papillomavirus-related oropharyngeal cancer. Head Neck. 2015 doi: 10.1002/hed.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 27.Vermorken JB, Psyrri A, Mesía R, Peyrade F, Beier F, de Blas B, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25:801–7. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:2940–50. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014;24:185–99. doi: 10.1101/gr.164806.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parfenov M, Pedamallu CS, Gehlenborg N, Freeman SS, Danilova L, Bristow CA, et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc Natl Acad Sci U A. 2014;111:15544–9. doi: 10.1073/pnas.1416074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker TP, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesselring R, Thiel A, Pries R, Fichtner-Feigl S, Brunner S, Seidel P, et al. The complement receptors CD46, CD55 and CD59 are regulated by the tumour microenvironment of head and neck cancer to facilitate escape of complement attack. Eur J Cancer Oxf Engl 1990. 2014;50:2152–61. doi: 10.1016/j.ejca.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 38.Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PloS One. 2013;8:e56823. doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Van Der Wal JE, Snow GB, Leemans CR, et al. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol. 2002;161:1051–60. doi: 10.1016/S0002-9440(10)64266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 42.Muller PAJ, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 43.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 44.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 45.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Peltonen JK, Helppi HM, Paakko P, Turpeenniemi-Hujanen T, Vahakangas KH. p53 in head and neck cancer: functional consequences and environmental implications of TP53 mutations. Head Neck Oncol. 2010;2:36–3284. 2–36. doi: 10.1186/1758-3284-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–61. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrone F, Bossi P, Cortelazzi B, Locati L, Quattrone P, Pierotti MA, et al. TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:761–6. doi: 10.1200/JCO.2009.22.4170. [DOI] [PubMed] [Google Scholar]
- 49.Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–6. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher S, Kefford RF, Rizos H. Enforced expression of p14ARF induces p53-dependent cell cycle arrest but not apoptosis. Cell Cycle Georget Tex. 2005;4:465–72. doi: 10.4161/cc.4.3.1526. [DOI] [PubMed] [Google Scholar]
- 53.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 54.Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto R, Uzawa N, Nagaoka S, Hirata Y, Amagasa T. Prognostic significance of cyclin D1 amplification and overexpression in oral squamous cell carcinomas. Oral Oncol. 2003;39:610–8. doi: 10.1016/s1368-8375(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 56.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. 2002;21:1510–7. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 57.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. 2002;2:910–7. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 58.Nemunaitis J, Clayman G, Agarwala SS, Hrushesky W, Wells JR, Moore C, et al. Biomarkers Predict p53 Gene Therapy Efficacy in Recurrent Squamous Cell Carcinoma of the Head and Neck. 2009;15:7719–25. doi: 10.1158/1078-0432.CCR-09-1044. [DOI] [PubMed] [Google Scholar]
- 59.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 60.Loren A phase I trial of the addition of the CDK 4/6 inhibitor palbociclib to cetuximab in patients with incurable head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. n.d. [Google Scholar]
- 61.Kumar S, Biswas M, Jose T. HPV vaccine: Current status and future directions. 2015;71:171–7. doi: 10.1016/j.mjafi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monie A, Tsen SW, Hung CF, Wu TC. Therapeutic HPV DNA vaccines. 2009;8:1221–35. doi: 10.1586/erv.09.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voskens CJ, Sewell D, Hertzano R, DeSanto J, Rollins S, Lee M, et al. Induction of MAGE-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. 2012;34:1734–46. doi: 10.1002/hed.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hung C-F, Ma B, Monie A, Tsen S-W, Wu T-C. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8:421–39. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cory L, Chu C. ADXS-HPV: A therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccines Immunother. 2014;10:3190–5. doi: 10.4161/hv.34378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. 2011;2:261–74. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimeno A, Shirai K, Choi M, Laskin J, Kochenderfer M, Spira A, et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann Oncol. 2015;26:556–61. doi: 10.1093/annonc/mdu574. [DOI] [PubMed] [Google Scholar]
- 69.Jimeno A, Bauman JE, Weissman C, Adkins D, Schnadig I, Beauregard P, et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol. 2015;51:383–8. doi: 10.1016/j.oraloncology.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safety, pharmacokinetics, and preliminary activity of the α-specific PI3K inhibitor BYL719: Results from the first-in-human study. J Clin Oncol. n.d. [Google Scholar]
- 71.Nguyen SA, Walker D, Gillespie MB, Gutkind JS, Day TA. mTOR inhibitors and its role in the treatment of head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2012;13:71–81. doi: 10.1007/s11864-011-0180-2. [DOI] [PubMed] [Google Scholar]
- 72.Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. J Clin Oncol. doi: 10.1093/annonc/mdv194. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phase II study of temsirolimus and erlotinib in patients (pts) with recurrent/metastatic (R/M), platinum-refractory head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. doi: 10.1016/j.oraloncology.2012.12.016. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grünwald V, Keilholz U, Boehm A, Guntinas-Lichius O, Hennemann B, Schmoll HJ, et al. TEMHEAD: a single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO) Ann Oncol. 2015;26:561–7. doi: 10.1093/annonc/mdu571. [DOI] [PubMed] [Google Scholar]
- 75.Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:3330–5. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laban S, Steinmeister L, Gleißner L, Grob TJ, Grénman R, Petersen C, et al. Sorafenib sensitizes head and neck squamous cell carcinoma cells to ionizing radiation. Radiother Oncol. 2013;109:286–92. doi: 10.1016/j.radonc.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 78.Reddy BKM, Lokesh V, Vidyasagar MS, Shenoy K, Babu KG, Shenoy A, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50:498–505. doi: 10.1016/j.oraloncology.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Licitra L, Mesia R, Rivera F, Remenar E, Hitt R, Erfan J, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck. EXTREME study. 2011;22:1078–87. doi: 10.1093/annonc/mdq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 81.Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez JJ, et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:1016–22. doi: 10.1093/annonc/mdr367. [DOI] [PubMed] [Google Scholar]
- 82.Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 83.Kirby AM, A’Hern RP, D’Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94:631–6. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:5064–73. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 85.Hama T, Yuza Y, Suda T, Saito Y, Norizoe C, Kato T, et al. Functional mutation analysis of EGFR family genes and corresponding lymph node metastases in head and neck squamous cell carcinoma. Clin Exp Metastasis. 2012;29:19–25. doi: 10.1007/s10585-011-9425-5. [DOI] [PubMed] [Google Scholar]
- 86.Harrington K, Berrier A, Robinson M, Remenar E, Housset M, de Mendoza FH, et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer Oxf Engl 1990. 2013;49:1609–18. doi: 10.1016/j.ejca.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 87.Harrington K, Temam S, Mehanna H, D’Cruz A, Jain M, D’Onofrio I, et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients With Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Oncol 2015:JCO. 2015;61:4370. doi: 10.1200/JCO.2015.61.4370. [DOI] [PubMed] [Google Scholar]
- 88.Seiwert TY, Fayette J, Cupissol D, Campo JMD, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25:1813–20. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 90.Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev. 2009;9:587–95. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong R, Bao R, Faber PW, Bindokas VP, Bechill J, Lingen MW, et al. Notch1 Activation or Loss Promotes HPV-Induced Oral Tumorigenesis. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okuyama R, Ogawa E, Nagoshi H, Yabuki M, Kurihara A, Terui T, et al. p53 homologue, p51/p63, maintains the immaturity of keratinocyte stem cells by inhibiting Notch1 activity. Oncogene. 2007;26:4478–88. doi: 10.1038/sj.onc.1210235. [DOI] [PubMed] [Google Scholar]
- 93.Sun W, Gaykalova DA, Ochs MF, Mambo E, Arnaoutakis D, Liu Y, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014;74:1091–104. doi: 10.1158/0008-5472.CAN-13-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–5. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Büssing I, Slack FJ, Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400–9. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis. 2014;5:e1018. doi: 10.1038/cddis.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Dai Y, Xie C-H, Neis JP, Fan C-Y, Vural E, Spring PM. MicroRNA expression profiles of head and neck squamous cell carcinoma with docetaxel-induced multidrug resistance. Head Neck. 2011;33:786–91. doi: 10.1002/hed.21540. [DOI] [PubMed] [Google Scholar]
- 100.Kimura S, Naganuma S, Susuki D, Hirono Y, Yamaguchi A, Fujieda S, et al. Expression of microRNAs in squamous cell carcinoma of human head and neck and the esophagus: miR-205 and miR-21 are specific markers for HNSCC and ESCC. Oncol Rep. 2010;23:1625–33. doi: 10.3892/or_00000804. [DOI] [PubMed] [Google Scholar]
- 101.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(Pt 1):111–27. [PMC free article] [PubMed] [Google Scholar]
- 103.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunner TB, Kunz-Schughart LA, Grosse-Gehling P, Baumann M. Cancer stem cells as a predictive factor in radiotherapy. Semin Radiat Oncol. 2012;22:151–74. doi: 10.1016/j.semradonc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang C-W, Chen Y-S, Chou S-H, Han C-L, Chen Y-J, Yang C-C, et al. Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Cancer Res. 2014;74:6291–305. doi: 10.1158/0008-5472.CAN-14-0626. [DOI] [PubMed] [Google Scholar]
- 108.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 109.Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Chang K-P, Kao H-K, Yen T-C, Chang Y-L, Liang Y, Liu S-C, et al. Overexpression of macrophage inflammatory protein-3α in oral cavity squamous cell carcinoma is associated with nodal metastasis. Oral Oncol. 2011;47:108–13. doi: 10.1016/j.oraloncology.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Balermpas P, Rodel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer. 2014;111:1509–18. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li S, Labrecque S, Gauzzi MC, Cuddihy AR, Wong AH, Pellegrini S, et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18:5727–37. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- 116.Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, et al. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–64. [PubMed] [Google Scholar]
- 117.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jie H-B, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75:2200–10. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ham Y, Oh HY, Seo S-S, Kim MK. Association Between Health Behaviors and a Family History of Cancer Among Korean Women. Cancer Res Treat Off J Korean Cancer Assoc. 2015 doi: 10.4143/crt.2015.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Badoual C, Hans S, Merillon N, Ryswick CV, Ravel P, Benhamouda N, et al. PD-1-Expressing Tumor-Infiltrating T Cells Are a Favorable Prognostic Biomarker in HPV-Associated Head and Neck Cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 121.A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV– associated head and neck (H/N) cancer. J Clin Oncol. n.d. [Google Scholar]
- 122.Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol. doi: 10.1200/JCO.2016.68.1478. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol. n.d. [Google Scholar]
- 124.Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, Dubrot J, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–9. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsuchikawa T, Md MM, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:1713–9. doi: 10.1245/s10434-011-1906-x. [DOI] [PubMed] [Google Scholar]
- 127.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang C-L, Hsu Y-T, Wu C-C, Lai Y-Z, Wang C, Yang Y-C, et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013;73:119–27. doi: 10.1158/0008-5472.CAN-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie H-B, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:5384–91. doi: 10.1158/1078-0432.CCR-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Geldres C, Savoldo B, Hoyos V, Caruana I, Zhang M, Yvon E, et al. T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:962–71. doi: 10.1158/1078-0432.CCR-13-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumai T, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, et al. Tumor-derived TGF-β and prostaglandin E2 attenuate anti-tumor immune responses in head and neck squamous cell carcinoma treated with EGFR inhibitor. J Transl Med. 2014;12:265. doi: 10.1186/s12967-014-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lim JYH, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother CII. 2014;63:259–71. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schuler PJ, Harasymczuk M, Visus C, Deleo A, Trivedi S, Lei Y, et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:2433–44. doi: 10.1158/1078-0432.CCR-13-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, et al. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28:468–75. doi: 10.7555/JBR.28.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer—review of current data. Oral Oncol. 2015;51:12–5. doi: 10.1016/j.oraloncology.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:312–21. doi: 10.1158/1078-0432.CCR-14-0202. [DOI] [PubMed] [Google Scholar]
- 138.Shah JP. Cervical lymph node metastases–diagnostic, therapeutic, and prognostic implications. Oncol Williston Park N. 1990;4:61–9. discussion 72, 76. [PubMed] [Google Scholar]
- 139.Park J, Kim J-M, Park JK, Huang S, Kwak SY, Ryu KA, et al. Association of p21-activated kinase-1 activity with aggressive tumor behavior and poor prognosis of head and neck cancer. Head Neck. 2015;37:953–63. doi: 10.1002/hed.23695. [DOI] [PubMed] [Google Scholar]
- 140.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 141.Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koppikar P, Choi S-H, Egloff AM, Cai Q, Suzuki S, Freilino M, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:4284–91. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nozawa H, Howell G, Suzuki S, Zhang Q, Qi Y, Klein-Seetharaman J, et al. Combined inhibition of PLC{gamma}-1 and c-Src abrogates epidermal growth factor receptor-mediated head and neck squamous cell carcinoma invasion. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:4336–44. doi: 10.1158/1078-0432.CCR-07-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fury MG, Baxi S, Shen R, Kelly KW, Lipson BL, Carlson D, et al. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) Anticancer Res. 2011;31:249–53. [PMC free article] [PubMed] [Google Scholar]
- 145.Rosenthal EL, Matrisian LM. Matrix metalloproteases in head and neck cancer. Head Neck. 2006;28:639–48. doi: 10.1002/hed.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 147.Albini A, Tosetti F, Benelli R, Noonan DM. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–41. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 148.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:1434–40. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 149.Cohen EEW, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–57. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Argiris A, Karamouzis MV, Gooding WE, Branstetter BF, Zhong S, Raez LE, et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:1140–5. doi: 10.1200/JCO.2010.33.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Argiris A, Kotsakis AP, Hoang T, Worden FP, Savvides P, Gibson MK, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2013;24:220–5. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dorsey K, Agulnik M. Promising new molecular targeted therapies in head and neck cancer. Drugs. 2013;73:315–25. doi: 10.1007/s40265-013-0025-3. [DOI] [PubMed] [Google Scholar]


