Abstract
Immune checkpoint inhibitors (ICI) have shown great promise in a wide spectrum of adult solid and hematological malignancies, achieving objective tumor responses and prolonging survival. However, there is limited clinical success amongst pediatric patients. In this review, we summarize the current understanding of ICI and present an up-to-date overview of recent and ongoing clinical trials of ICI in pediatric malignancies. In addition, we will discuss immunologic and clinical difficulties in this young population, as well as future prospects for combination of ICI with other immune-based and conventional treatments.
Keywords: Immune checkpoint inhibitor, Immunotherapy, Programmed death receptor-1 (PD-1), Programmed death-ligand 1 (PD-L1), Cytotoxic T lymphocyte antigen-4 (CTLA-4)
Introduction
Remarkable advances in cancer immunotherapy in recent years have led to paradigm shifts in oncology. The most noticeable results have been with T-cell-based therapies including immune checkpoint inhibitors (ICI), genetically engineered T-cells and bispecific antibodies (BsAb). T-cells represent a major class of cellular drugs in immunosurveillance and tumor eradication with exquisite specificity and long-term memory. However, during tumor equilibrium or progression, T-cells become exhausted or tolerized to tumor cells [1,2]. A cardinal feature of T-cell exhaustion is the overexpression of inhibitory receptors, including programmed death receptor-1 (PD-1, CD279), cytotoxic T lymphocyte antigen-4 (CTLA-4, CD152), lymphocyte-activation gene-3 (LAG-3), T-cell immunoglobulin domain and mucin domain-3 (TIM-3), IL-10 receptor, killer immunoglobulin receptors, among others. Some of these checkpoint molecules exert their immunosuppressive effects by down-regulating the normal T-cell response and increasing FoxP3+ regulatory T-cells (Tregs) numbers and activity [3,4]. Monoclonal antibody (mAb) based therapies to counteract these checkpoint molecules can remove the brake that restrains tumor-infiltrating T-cells, thereby achieving significant clinical benefits in different malignancies including advanced melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC) [5–8]. However, most of the studies to date are focused on adult cancers and little is known about the role of these ICI in pediatric malignancies. Here, we review the clinical use of these ICI and their limitations regarding toxicity and efficacy in the context of pediatric malignancy. Furthermore, we will discuss the potential for combining ICI with other proven and investigational therapies in children.
Immune checkpoint inhibitors and clinical trials
CTLA-4 antibodies
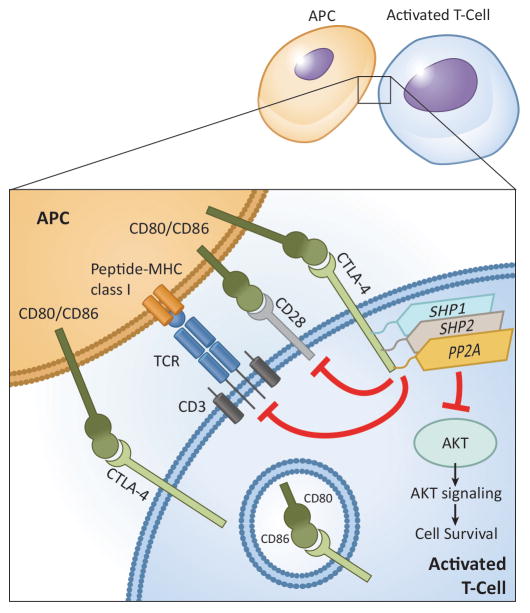
CTLA-4, type I transmembrane glycoprotein that belongs to Ig superfamily, is constitutively expressed on memory T-cells and Tregs, which is critical in preventing self-reactive T-cells from inducing autoimmunity [9]. It is homologous to CD28 and shares the same B7 ligands, B7-1 (CD80) and B7-2 (CD86), but it has a negative effect on T-cell activation. Several suppressive mechanisms for T-cell functions have been attributed to CTLA-4 (Fig. 1). Ipilimumab (IgG4 isotype) was the first CTLA-4 inhibitor to demonstrate overall survival benefit in metastatic melanoma [6,10]. Another CTLA-4 inhibitor, tremelimumab (IgG2 isotype), has also been proven successful in metastatic melanoma and other malignancies [11]. Although the pediatric experience is very limited, a substantial number of clinical trials have extended the age eligibility to patients <=18 years of age (Table 1). In the first report of ipilimumab for advanced solid tumors in pediatric patients, although no major response was noted, some tumor regression was noted to be durable [12]. A comparison of ipilimumab to high-dose interferon (IFN) α-2b among pediatric patients with high-risk melanoma is ongoing, and the combination with IL-2, vaccine, or CD19-chimeric antigen receptor (CAR) expressing T-cell therapy are being tested in patients with metastatic melanoma and advanced malignancies.
Fig. 1.
Resting T-cells rarely express CTLA-4, which is retained inside the secretory granules, but after TCR activation, CTLA-4 is up-regulated and emerges to the plasma membrane of T-cells and binds to B7 ligands (CD80 and CD86) on antigen presenting cells (APCs) with 10–100-fold higher avidity than CD28, resulting in reduced T-cell proliferation and lessened cytokine secretion [185,186]. CTLA-4 exerts TCR inhibitory signal through serine/threonine protein phosphatase 2 (PP2A) and Src-homology 2 domain-containing phosphatase 2 (SHP2), and induces inhibition of serine/threonine kinase AKT on the downstream of phosphatidylinositol-3-kinase (PI3K), resulting altered T-cell metabolism and decreased T-cell proliferation and activity [187,188]. Besides, CTLA-4 shortens the duration of immune synapses as a result of signal attenuation and integrin deactivation and increases the T-cell activation threshold by producing inhibitory signals in the early phase of tumorigenesis.
Table 1.
Clinical Trials of immune checkpoint inhibitors for which pediatric patients <=18 are eligible
| Target | Agent | Indication | Age (years) | Phase | Clinical trial (NCT) | Results |
|---|---|---|---|---|---|---|
| CTLA-4 | Ipilimumab | Stage III or IV melanoma | 12–17 | 2 | NCT01696045 | Terminated due to slow accrual |
| Ipilimumab | Treatment resistant cancer (sarcoma, Wilms tumor, lymphoma, neuroblastoma) | 3–21 | 1 | NCT01445379 | Completed | |
| Ipilimumab | Advanced synovial sarcoma | ≥13 | 2 | NCT00140855 | Terminated due to poor accrual | |
| ipilimumab | Metastatic renal cell carcinoma | ≥ 16 | 2 | NCT00057889 | Completed | |
| Ipilimumab plus imatinib | Advanced cancers | ≥15 | 1 | NCT01738139 | Recruiting | |
| Ipilimumab plus paclitaxel | Metastatic melanoma | 12–70 | 2 | NCT01827111 | Active, not recruiting | |
| Ipilimumab or interferon α-2b | Resected stage III or IV melanoma | >12 | 3 | NCT01274338 | Active, not recruiting | |
| Ipilimumab plus peginterferon α-2b | Stage III or IV melanoma | ≥16 | 1 | NCT01496807 | Active, not recruiting | |
| Ipilimumab plus IL-2 | Metastatic melanoma | ≥16 | 1,2 | NCT00058279 | Completed | |
| Ipilimumab plus CD19-CAR T cell | B cell NHL, ALL, CLL | All ages | 1 | NCT00586391 | Active, not recruiting | |
| Ipilimumab plus gp100 peptide vaccine | Stage IV melanoma | ≥ 16 | 2 | NCT00032045 | Completed | |
| Ipilimumab plus gene modified T cells and dendritic cell vaccine | Locally advanced or metastatic malignancies | ≥ 16 | 1 | NCT02070406 | Recruiting | |
| Ipilimumab ± gp100 peptide and montanide ISA-51 vaccine | Stage IV melanoma | ≥ 16 | 2 | NCT00077532 | Completed | |
|
| ||||||
| PD-1 | Nivolumab | Refractory or recurrent hypernutated malignancies in biallelic mismatch repair deficiency positive patients | 1–18 | 1,2 | NCT02992964 | Active, not recruiting |
| Nivolumab | Glioblastoma | ≥ 1 | 2 | NCT02550249 | Recruiting | |
| Nivolumab plus cyclophosphamide | Relapsed pediatric solid tumors | 1–21 | 1,2 | NCT02901145 | Not yet recruiting | |
| Nivolumab plus anti-GD2 antibody (Ch14.18/CHO) | Relapsed or refractory NBL | 1–18 | 1 | NCT02914405 | Not yet recruiting | |
| Nivolumab plus brentuximab vedotin | Hodgkin lymphoma after failure of 1st line therapy | 5–30 | 2 | NCT02927769 | Recruiting | |
| Nivolumab plus brentuximab vedotin | Relapsed/refractory NHL | ≥15 | 1,2 | NCT02581631 | Recruiting | |
| Nivolumab plus EBV specific T cells | Relapsed/refractory EBV positive lymphoma | All ages | 1 | NCT02973113 | Recruiting | |
| Nivolumab ± stereotactic radiosurgery | Recurrent, advanced, or metastatic chordoma | ≥15 | 1 | NCT02989636 | Not yet recruiting | |
| Nivolumab plus ICE chemotherapy (Ifosfamide, carboplatin, and etoposide) | Relapsed/refractory Hodgkin lymphoma | ≥15 | 2 | NCT03016871 | Not yet recruiting | |
| Nivolumab plus gene-modified T cells, and NY-ESO-1 vaccine therapy | Stage IV or locally advanced solid tumors expressing NY-ESO-1 | ≥16 | 1 | NCT02775292 | Not yet recruiting | |
| Nivolumab plus cyclophosphamide ± radiotherapy | Relapsed/refractory malignancies | ≤ 18 | 1,2 | NCT02813135 | Recruiting | |
|
| ||||||
| Pembrolizumab | Recurrent, progressive, or refractory high-grade gliomas, diffuse intrinsic pontine glioma, or hypermutated tumors | 1–29 | NCT02359565 | Recruiting | ||
| Pembrolizumab | Advanced melanoma or advanced, relapsed/refractory PD-L1-positive solid tumors or lymphoma | 0.5–17 | NCT02332668 | Recruiting | ||
| Pembrolizumab | Advanced bone and soft tissue sarcoma | ≥ 12 | 2 | NCT02301039 | Active, not recruiting | |
| Pembrolizumab plus IL-2 | Stage III–IV melanoma | ≥15 | 2 | NCT02748564 | Not yet recruiting | |
| Pembrolizumab plus cyclophosphamide, fludarabine, IL-2, and tumor infiltrating lymphocyte infusion | Metastatic melanoma | 16–70 | 2 | NCT02621021 | Recruiting | |
| Pembrolizumab plus axitinib (anti-VEGF receptor antibody) | Alveolar soft part sarcomas and other soft tissue sarcomas | ≥16 | 2 | NCT02636725 | Recruiting | |
| Pembrolizumab plus 3rd generation GD-2 CAR T cell | Relapsed/refractory NBL | All age | 1 | NCT01822652 | Active, not recruiting | |
| Pembrolizumab plus GSK3359609 (anti-inducible T cell co-stimulator (ICOS) receptor agonist antibody) | Advanced solid tumors | ≤ 18 | 1 | NCT02723955 | Recruiting | |
|
| ||||||
| PD-L1 | Atezolizumab | Solid tumors which failed to primary treatment | ≤ 30 | 1,2 | NCT02541604 | Recruiting |
| Avelumab | Recurrent or progressive osteosarcoma | 12–49 | 2 | NCT03006848 | Recruiting | |
| Durvalumab | Relapsed or refractory solid tumors, lymphoma, and CNS tumors | 1–17 | 1 | NCT02793466 | Recruiting | |
|
| ||||||
| PD-1 and CTLA-4 | Nivolumab± Ipilimumab | Relapsed/refractory solid tumors or sarcoma (metastatic or unresectable solid tumors) | 1–30 | 1,2 | NCT02304458 | Recruiting |
| Nivolumab plus Ipilimumab | Untreated, unresected or metastatic melanoma | ≥15 | 3 | NCT02905266 | Recruiting | |
| Resected stage III and IV melanoma | ≥16 | 2 | NCT02970981 | Not yet recruiting | ||
| Nivolumab plus blinatumomab ± ipilimumab | Poor risk relapsed/refractory CD19+precursor B-lymphoblastic leukemia | ≥16 | 1 | NCT02879695 | Not yet recruiting | |
| Nivolumab plus NY-ESO-1 vaccine ± ipilimumab | Resected stage IIIC/IV melanoma | ≥16 | 1 | NCT01176474 | Active, not recruiting | |
|
| ||||||
| PD-L1 and CTLA-4 | Durvalumab plus tremelimumab | Advanced rare tumors | ≥16 | 2 | NCT02879162 | Recruiting |
|
| ||||||
| B7-H3 | Enoblituzumab (MGA271) | B7-H3 expressing relapsed or refractory solid tumors | 1–30 | 1 | NCT02982941 | Recruiting |
| 131I-8H9 | Desmoplastic small round cell tumors and other solid tumors involving the peritoneum | ≥1 | 1 | NCT01099644 | Recruiting | |
| 131I-8H9 | Relapsed/refractory or advanced CNS or leptomeningeal cancer | All ages | 1 | NCT00089245 | Recruiting | |
| 124I-8H9 | Non-progressive diffuse pontine glioma previously treated with external beam radiation | 3–21 | 1 | NCT01502917 | Recruiting | |
|
| ||||||
| IDO | Indoximod + temozolomide | Progressive primary malignant brain tumors | 3–21 | 1 | NCT02502708 | Recruiting |
PD-1/PD-L1 antibodies
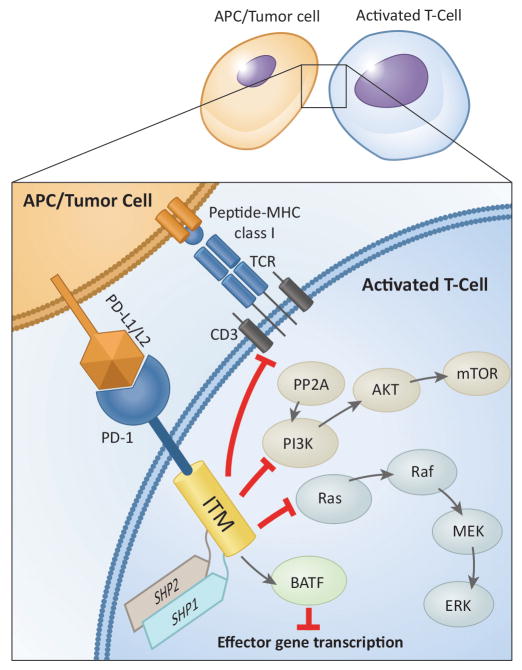
PD-1 is expressed on T-cells following T-cell receptor (TCR) engagement and it declines after resolution of acute inflammation. However, under chronic antigen exposure, PD-1 remains high on chronically activated T-cells that become exhausted [13,14]. Several Inhibitory mechanisms for T-cell functions have been ascribed to PD-1 (Fig. 2). Two PD-1 ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), can engage PD-1 to render T-cell dysfunctional, and to maintain the exhausted T-cell phenotype [15]. While PD-L2 is exclusively expressed on activated dendritic cells and macrophages, PD-L1 has a broad tissue distribution including tumor cells and is rapidly induced by inflammatory mediators (e.g. IFN-γ, lipopolysaccharides, GM-CSF, IL-4 and IL-10). PD-L1 is expressed in many pediatric cancers including leukemia (42–100%), lymphomas (27–80%), glioma (75–100%), Wilms tumor (14%), soft tissue sarcomas (STS) (58%), and metastatic osteosarcoma (75%) [16,17], and upregulation of PD-L1 was consistently associated with poor clinical outcomes [17–25].
Fig. 2.
PD-1 is also expressed on T-cells following TCR engagement and activation. PD-1 and PD-L1 ligation exerts inhibitory signals for lymphocyte activation. PD-1 modulates T-cell function through (a) direct antagonism of TCR signaling by recruiting Src-homology 2 domain-containing phosphatase (SHP)-1 and SHP-2 to tyrosine-based inhibitory motifs (ITM; immunoreceptor tyrosine-based motifs) in the PD-1 tail, (b) inhibition of PI3K/AKT/mechanistic target of rapamycin (mTOR) pathway, implicating the role of PD-1 in metabolism, nutrient sensing, survival, and cell growth to cell cycle, (c) modulation of Ras pathway, linking PD-1 to cell cycle and reducing T-cell proliferation, (d) increased expression of basic leucin zipper transcription factor, activating transcription factor (ATF)-like transcription factor (BATF), which can repress expression of effector gene transcription [14,189,190]. Further, these signaling events impair T-cell motility and stability leading to unproductive immune synapses with APCs [170,191].
PD-1/PD-L1 inhibitors enhance anti-tumor immune response by restoring T-cell cytotoxic function, resulting in anti-tumor effect, while facilitating the generation of memory T-cells to provide long term anti-tumor response [26–28]. Nivolumab, a fully human IgG4 mAb, induced objective response in melanoma, NSCLC, and RCC patients (18% to 28%), and most responses were durable for more than 1 year [8,16]. Pembrolizumab, another humanized monoclonal IgG4 anti-PD-1 antibody, also showed high clinical response rates that were durable (37% with median of 11 months) in patients with advanced melanoma [29]. In addition, pidilizumab (CT-011), a humanized IgG1 monoclonal anti-PD-1 antibody, has mainly been investigated in advanced hematologic malignancies [30–32]. Other anti-PD-L1 antibodies (e.g. atezolizumab (MPDL32890A), durvalumab (MEDI4736), and avelumab (MSB0010718C)), inhibit binding of PD-L1 to PD-1 and CD80, without blocking the interaction between PD-L2 and PD-1. They have demonstrated clinical activity with acceptable toxicities, not only against immunogenic cancers, such as melanoma and RCC, but also against less immunogenic epithelial cancers such as NSCLC, colorectal, gastric, and cervical and bladder cancers [33–35]. Most notable was the substantially milder toxicity profile of PD-1/PD-L1 blockades, when compared to anti-CTLA-4-mediated immune related adverse events (irAEs) [36–38].
In contrast to the enthusiasm for the PD-1/PD-L1 inhibitors in adult cancers, few studies have been carried out in pediatric cancers. Blumenthal et al. reported their experience of pembrolizumab in patients with recurrent brain tumors including 5 pediatric patients, but they failed to show a benefit on overall survival [39]. The obvious exceptions are pediatric patients with refractory Hodgkin lymphoma (HL) which have shown durable responses to pembrolizumab [40,41]. Recently, pembrolizumab was approved by FDA for HL in adult and pediatric patients, and this is a first approval of PD-1 inhibitor for pediatric use. Children’s Oncology Group (COG) is conducting a phase I/II study of nivolumab alone or in combination with ipilimumab for relapsed or refractory solid tumors (NCT02304458) and phase I/II study of pembrolizumab for advanced melanoma or PD-L1 positive advanced solid tumors or lymphoma (NCT02332668).
Anti-LAG-3 antibody
Lymphocyte-activation gene-3 (LAG-3, CD223) is another vital immune checkpoint that may have a synergistic interaction with PD-1/PD-L1 [42,43]. LAG-3 is a member of the Ig superfamily and exerts a wide variety of biologic impacts on T-cell function via binding to major histocompatibility complex (MHC) class II with high affinity [44]. It is expressed on activated T-cells, Tregs, NK cells, B-cells, tumor-infiltrating lymphocytes (TILs), and dendritic cells [45–49]. The LAG-3/MHC class II molecule interaction inhibits CD4+ T-cell proliferation and cytokine secretion. Co-expression of LAG-3 and PD-1 correlates with greater T-cell exhaustion, accompanied by impairment of CD8+ effector T-cell function, best demonstrated in chronic viral infection [48,50–52]. They synergistically regulate T-cell function, blunt anti-tumor immune response, and promote tumoral immune escape [43,52].
Although anti-LAG-3 antibody (Immutep, IMP321) failed to demonstrate objective responses, this agent was well tolerated and appeared to correlate with development of CD8+ T-cell effectors [53–55]. When combined with paclitaxel, outcome in breast cancer was improved [56,57]. Although there has been no open clinical trials for pediatric patients so far, and most clinical trials have not yet extended the age eligibility to patients <=18 years of age, clinical trials combining with PD-1 inhibitors are actively recruiting patients with advanced solid tumors (NCT0196819) and recurrent glioblastoma (NCT02658981) to assess the safety, tolerability and efficacy.
Anti-B7-H3 antibody
Human B7-H3 (CD276) is another member of the B7/CD28 Ig superfamily with activating as well as inhibitory roles that regulate T-cell function [2,58,59]. While the receptor for B7-H3 has yet to be discovered, structural studies in the mouse suggested a receptor engagement on T-cell involving the particular segment (FG loop) of the B7-H3 [60,61]. B7-H3 preferentially down-regulates type I helper T-cells (TH1)-mediated immune response and inhibits T-cell proliferation and cytokine production [62–64]. B7-H3 protein showed broad mRNA expression on many tissues and cell types with proven functions on cellular responses, including proliferation, apoptosis, adhesion, and metastasis [65–67]. While its protein expression is restricted in normal tissues, much higher levels are found in human malignancies [68–75], and because of its association with highly aggressive tumor behavior and poor clinical outcome, B7-H3 has utility as a tumor-associated antigen as well [71,74,76].
Anti-B7-H3 antibodies block the inhibitory effects of B7-H3 on T-cells and enhance the efficacy of autologous T-cells against tumors [77,78]. Anti-B7-H3 murine mAb 8H9 has shown promise as a radioimmunoconjugate in xenograft models, and clinically, intrathecal or intra-Ommaya131I-8H9 has demonstrated efficacy in controlling CNS metastasis of neuroblastoma [79]. Currently, intraperitoneal 131I-8H9 is also being tested among adolescent and young adults with desmoplastic small round cells tumor (DSRT), while intra-pontine 124I-8H9 using convention enhanced delivery is applied to diffuse intrinsic pontine glioma (DIPG), with minimal toxicity and encouraging results (Table 1). The humanized and affinity matured forms of 8H9 and its epitope dependence on the integrity of the FG loop were recently reported [75]. Another humanized mAb, enoblituzumab (MGA271) specific for B7-H3, has shown anti-tumor activity in preclinical models of RCC and bladder cancer [77]. Clinical trials of MGA271 as a single agent for refractory cancers that express B7-H3 in pediatric patients (NCT02982941) and advanced prostate cancer in adults (NCT02381314), as well as in combination with ipilimumab (NCT02381314) or pembrolizumab for refractory cancer (NCT02475213) are in progress. Although clinical responses to the standard anti-B7-H3 IgG alone have not been encouraging, its combination with other ICI or even with conventional therapies could offer opportunities. Novel engineered forms such as B7-H3 × CD3 bispecific antibodies may be valuable alternatives [80]. When the receptor(s) and signaling pathways of B7-H3 become better defined, more effective anti-B7-H3 inhibitors could potentially be explored.
Limitations of immune checkpoint inhibitors
Safety in children
Despite the overall success of ICI in adults, safety data in children have been hard to come by. In adults, ipilimumab-related adverse events were often mild to moderate, but occurred in more than 70% of patients, and these toxicities correlated with its dosage [6,81]. Meta-analysis of 18 clinical trials showed that CTLA-4 inhibitors at higher doses (10 mg/kg) were associated with a higher risk of treatment-related mortality (TRM) [82]. Enterocolitis, hepatitis, and dermatitis were most commonly observed, and these irAEs were associated with tumor responses and favorable outcome [12,81,83–85]. Approximately 30–50% of patients experienced adverse skin reactions including rash and itching, one third enterocolitis, 2–9% hepatotoxicity (some life-threatening), 1.5% hypophysitis whose symptoms included fatigue, headaches, myalgia, loss of appetite, nausea and vomiting [6,81]. Hypophysitis with adrenal insufficiency is potentially fatal and requires urgent attention and treatment [86]. Neurologic toxicities were rare, but life-threatening neuropathies (e.g. Guillan-Barré syndrome, severe motor neuropathy, myasthenia gravis, aseptic meningitis) and optic neuritis have been reported, which required immediate stoppage of the ICI [85,87]. Most irAEs could be controlled with high-dose corticosteroids which did not seem to impair the antitumor effects of ipilimumab [88]. In children the incidence of grade 3 or 4 irAES was 27%, and the spectrum included pancreatitis, pneumonitis, colitis, and hepatitis, similar to those among adult studies [12].
The toxicity profile of PD-1/PD-L1 blockades was less severe than that of CTLA-4, with no significant increase in TRM in a meta-analysis [82], with an overall incidence of 7–14% grade 3 and 4 toxicities [8,89,90]. Most notably, PD-1/PD-L1 inhibitors showed a slightly different toxicity profile including organ-specific inflammatory conditions such as pneumonitis rather than colitis [91]. The most common adverse events were fatigue, with an incidence of 16%–37% with anti-PD-1 and 12–24% with anti-PD-L1, followed by fever, chills, and infusion reactions [91]. Dermatologic toxicities such as rash, pruritis, and vitiligo were frequently observed, and vitiligo was more common than with ipilimumab (10% vs. 2%, respectively) [92]. Colitis, endocrine toxicities, and hepatic toxicities have been described, but most were less extensive than anti-CTLA-4 mAb [91,92]. While pneumonitis was rarely reported in the studies of anti-CTLA-4 mAb alone, up to 10% of patients receiving anti-PD-1/PD-L1 therapy developed this complication, leading to 3 TRM in the early phase of nivolumab [8]. In addition, among patients including children treated with anti-PD-1 mAbs, critical neurologic and endocrinologic adverse reactions have been reported, and some of them being irreversible [82,93–95]. These unpredictable off-target toxicities to critical organs, besides being life-threatening, are particularly concerning for children in whom the organs are less mature and potentially prone to life-long disabilities [91].
Efficacy in children
Another uncertainty in pediatric application of ICI is their uncertain benefit and the lack of appropriate predictive biomarkers. Although clinical trials have shown tumor responses in some patients, few are major responses and most have not been durable [12,39–41]. There is a need to better understand the mechanisms of action of PD-1/PD-L1 in these pediatric tumors, to determine if there is an underlying genetic resistance to ICIs [13,96]. While PD-L1 expression in adult tumors has been proposed as a potential biomarker for response [8,37], PD-L1 expression is often heterogeneous within tumors, and can be even discordant between primary tumor and metastases [17,97]. Furthermore, PD-L1 negative tumors also responded to PD-1 blockade or combination treatment with nivolumab and ipilimumab [38,98,99].
Recent whole genome and exome sequencing of tumors have identified the role of neoantigens and the importance of pre-existing T-cell clones and the mutational threshold for adequate response to ICI. High frequencies of nonsynonymous mutational burden and tumor neoantigens, as well as mutations in DNA repair pathways were strongly associated with therapeutic benefit following anti-CTLA-4 and anti-PD-1 antibodies [96,100]. Neoantigen-reactive CD8-T-cells are responsible for tumor regression after PD1 blockade, suggesting that ICI enhances neoantigen-specific T-cell reactivity [96]. However, given the heterogeneity of most human tumors, neoantigens, if not essential for tumorigenicity, could be downregulated or even lost, leading to tumor escape. An analysis of 27 cancer types showed that the median frequency of non-synonymous mutations varied by more than 1,000-fold across the cancer types [101]. While melanoma and lung cancer exceeded 100/Mb, most pediatric cancers showed frequencies as low as 0.1/Mb (one mutation per exome). If each mutation creates a neoantigen capable to stimulate a T-cell clone, more mutations and more neoantigens should produce a more robust T-cell response. ICI are now known to be more effective in highly mutated cancers, with a mutational threshold estimated at 100 mutations per exome (3.3 mutations/Mb) [100,102], an unfavorable prerequisite for most pediatric solid tumors. The highest mutation frequencies are attributable to extensive exposure to carcinogens, such as UV light in melanoma and tobacco smoking in lung cancers [101], not generally associated with pediatric cancers. Although pediatric tumors express PD-L1, ICI alone may have less or even no effect in pediatric cancers when compared to melanoma and NSCLC [103,104]. However, somatic mutation frequencies can vary widely across patients within a cancer type and even across sites within the same patient [101,105,106]. As present, the role of ICI is being tested for hypermutated malignancies in children with biallelic mismatch repair deficiency (NCT02992964).
Another consideration is the tumor microenvironment as a major component of resistance to immunotherapy. In this context, adult cancers are thought to be preceded by a long-term chronic inflammatory phase such as infections (hepatitis B or C, human papillomavirus, Epstein-Barr virus (EBV), Helicobacter pylori) or repeated exposures to irritants or carcinogens [107]. In contrast, the preneoplastic period in pediatric cancer is much shorter, often only weeks or months, and except in the case of EBV-induced Burkitt lymphoma, an association between preceding inflammation and typical pediatric cancers (i.e., small round blue cell tumors) is not clear. Whereas TILs, a heterogeneous population of lymphocytes growing within a tumor [108], in adult cancers are peritumoral, forming focal inflammatory cell aggregates of diverse cell types, including T-cells and NK cells among macrophages and dendritic cells (DC), TILs in pediatric tumors are scarce and scattered among macrophages. Moreover, these macrophages are commonly CD163/CD68+, the phenotype of M2 tumor-associated macrophage (TAM), which often comprise 60% to 70% of the cellular infiltrate [109]. While M1 TAMs are highly inflammatory and effective killers for microorganisms and tumor cells, M2 TAMs are generally anti-inflammatory, by secreting IL-10 and TGF-β while failing to secrete other proinflammatory cytokines, as well as immunosuppressive, protecting tumor cells from NK cells and T-cells during tumor progression [110–114].
TILs, particularly those anti-tumor type I T-cells, were associated with better patient survival and seemed to play a critical role in response to ICIs in many adult cancers [115–117]. A general consensus is the absence of clinical response if TILs are absent or insufficient [118]. Clinical scientists have coined the descriptor ‘hot’ for tumors that have high numbers of TILs (T-cell-inflamed) e.g. melanoma, and the opposite end of the spectrum as ‘cold’ (not T-cell-inflamed) e.g. prostate cancer and most pediatric cancers (Fig. 3) [119]. The potential mechanisms for immune evasion in the T-cell-inflamed or ‘hot’ tumors include inhibition by upregulation of ICI (PD-1/PD-L1 and CTLA-4), expression of indoleamine-2,3-dioxygenase (IDO), recruitment of Tregs, loss of antigen expression, and T-cell intrinsic anergy. Increased IDO expression by APCs induces tryptophan depletion, resulting in antigen-specific T-cell anergy, and Tregs recruitment and activation, resulting in T-cell dysfunction. On the other hand, in non-T-cell-inflamed or ‘cold’ tumors, immune escape mechanisms include a lack of innate immune recognition, infiltration by M2 TAMs, paucity of dendritic cell infiltration, lack of chemokines for effective T-cell trafficking, dense sessile stroma with high density of fibroblasts, and a hostile extracellular matrix restricting T-cell access. In addition, altered oncogene pathways could also cause immune escape, e.g. p53 mutation results in decrease of innate immune activation and a lack of T-cell infiltration, inactivation of phosphatase and tension homolog (PTEN) can enhance tumor cell survival and proliferation through increased AKT activity, and activated signal transducer and activator of transcription 3 (STAT3) signaling reduces recruitment of both DC and T-cells and plays inhibitory roles in anti-tumor immunity [119–122]. While hot tumors have a chance to benefit from ICI, cold tumors may require additional strategies to promote T-cell homing into and function within these tumors [121].
Fig. 3.
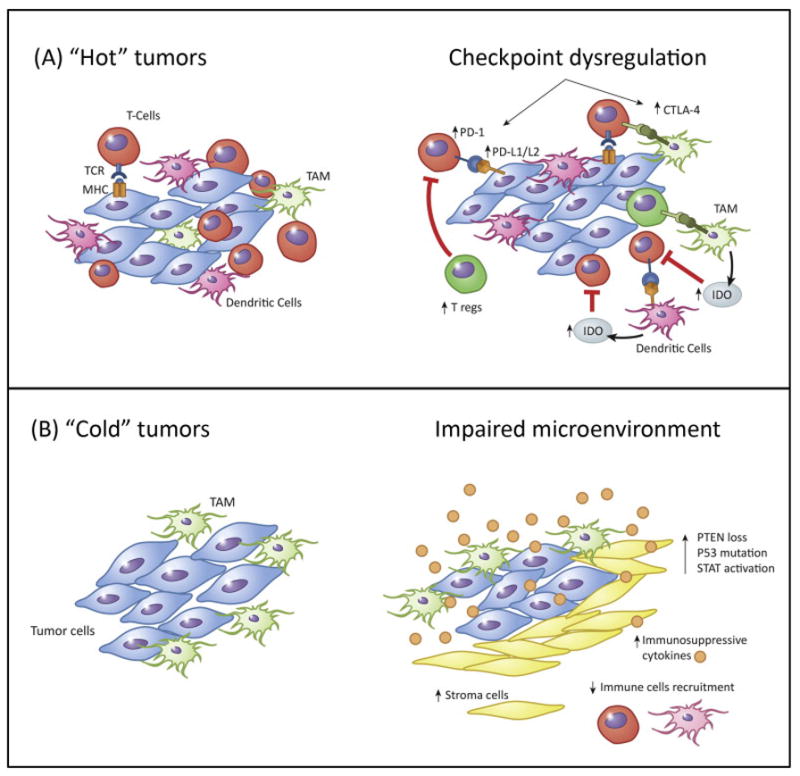
Mechanisms of tumor immune escape. ‘Hot’ tumors (A) may escape through up-regulation of immune checkpoint molecules and Tregs, secretion of immunosuppressive factors, indoleamine-2,3-dioxygenase (IDO), or T-cell anergy. (B) Tumor intrinsic mechanism of escape in “cold tumors” by downregulation of MHC molecules, attraction of M2 tumor-associated macrophages (TAMs), alteration of the tumor microenvironment, discouraging T-cell homing either by subduing inflammation, or suppressing release of T-cell chemokines, or releasing inhibitory cytokines to impair the recruitment of immune cells to the tumor microenvironment, or by dysregulating oncogene pathways including PI3 kinase/PTEN/AKT, p53 and STAT3 signaling.
Other relevant considerations regarding anti-tumor T-cell immunity in children include: (1) most pediatric solid tumors exhibit low or absence of MHC, which presents antigens on tumor cells and could be critical for both the afferent and the efferent arm of the T-cell response, potentially compromising the prospects of an effective T-cell immunity to any neoantigen, (2) immature immune system in young children, (3) dose-intensive chemotherapy in children and hence profound lymphopenia and immunosuppression, (4) immature or altered gut microbiome that could also affect the response to ICI [123–128]. The potential impact of gut microbiome on tumor microenvironment and effectiveness of chemotherapy viewed in the context of drastic changes of gut flora with age and antibiotics is just emerging [129–131]. For these reasons, ICI alone is likely to be insufficient to control pediatric tumors. However, as BsAb or CAR T-cells can retarget polyclonal T-cells to tumor, their combination with ICI may offer novel potentials for some pediatric malignancies (See below) [132,133].
Future directions
The clinical success of ICI in adults has paved the way for evidence-based combinations with conventional therapeutics or additional ICI, with the goal of both additive and synergistic effects to improve the survival of patients.
Combination with other ICI
The combination of ipilimumab and nivolumab was initially studied in advanced melanoma and demonstrated a 40% objective response rate, with 30% of patients exhibiting >80% tumor reduction [134]. Phase II/III trials of this combination also showed impressive response rates (61%) and significantly improved outcomes in melanoma, and even in PD-L1-negative tumors [98,99]. This combination has also been studied in metastatic RCC with acceptable safety and high response rates of 39% (NCT01472081) and in recurrent glioblastoma after standard therapy with surgery, RT, and temozolomide in a phase III trial (NCT02017717). Phase I/II clinical trial for recurrent or refractory solid tumors in children are also recruiting patients (NCT02304458). Similar combinations, pembrolizumab plus ipilimumab, or durvalumab plus tremelimumab are ongoing in prior-treated NSCLC, advanced melanoma and refractory or advanced stage rare tumors. Several other combinations using ICI targeting LAG-3, B7-H3, and TIM3 have shown encouraging results in the pre-clinical models and are currently in clinical trials.
Combination with chemotherapy
Recent studies have also shown that combinations of various chemotherapies with ICI can have synergistic effects. The combination of ipilimumab and dacarbazine has improved the overall survival of patients with metastatic melanoma when compared to dacarbazine monotherapy [10]. Nivolumab in combination with platinum-based chemotherapy for advanced NSCLC also showed an encouraging outcome with a 2-year OS rate of 62%. Although irAEs were greater than those expected with nivolumab monotherapy, most toxicity was manageable [135]. A number of phase I studies are currently underway in NSCLC and other solid tumors including pediatric cancers, aimed at investigating the safety and tolerability of combining ICI with standard chemotherapies [135,136].
Combination with targeted agents
Another strategy is to combine with targeted agents including tyrosine kinase inhibitors (TKI), BRAF inhibitors and anti-angiogenic agents. Despite high response rates with these targeted therapies, most patients progress within 1 year because of acquired resistance through a number of mechanisms, including immune escape via the PD-1/PD-L1 and other immune checkpoint pathways [137,138]. The effective treatment of human gastrointestinal stromal tumors (GIST) with imatinib is associated with an increased intratumoral CD8+effector T-cells (CD8)/Tregs ratio [139]. Increased Tregs could compromise the immune response to tumors, as shown by imatinib-resistant tumors, where the lower CD8/Treg ratios were correlated with increases in immune checkpoint molecules [139–141]. A phase I study of ipilimumab plus imatinib in advanced solid tumors (NCT01738139) and a phase I/II clinical trial of ipilimumab with dasatinib in GIST or STS are ongoing (NCT01643278). The combination of the anti-PD-L1 mAb, durvalumab, and the epidermal growth factor receptor (EGFR)-TKI gefitinib in NSCLC have shown promising clinical activities with mild treatment-related AEs [142].
BRAF inhibitors which target driver mutations in the tumor cells can promote adaptive immunity, but, can concurrently upregulate T-cell exhaustion markers including PD-1 and TIM-3 and PD-L1 on tumor cells, consistent with a potential immune resistance [143]. Although a phase I study of vemurafenib with ipilimumab was terminated prematurely due to hepatotoxicity [144], BRAFV600 inhibitors plus ICI should produce synergy given the high response rates from BRAF inhibitors and the durable remissions induced by ICI [145,146]. Currently, there are several open or pending clinical trials in lymphoma, advanced melanoma, RCC and other refractory solid tumors studying ICI in combination with targeted agents (NCT02465060, NCT02224781, NCT02027961 and NCT02858921).
Anti-angiogenic mAbs against vascular endothelial growth factor (VEGF), such as bevacizumab, and multi-targeted TKIs, such as sunitinib and pazopanib, have also been tested in combination with anti-PD-1/PD-L1 mAbs. Blockade of VEGF produced immunomodulatory effects, which included promoting dendritic cell maturation and effector T-cell trafficking, while decreasing myeloid-derived suppressor cells (MDSCs), Tregs and suppressive cytokines at the tumor microenvironment [147–149]. Combination of bevacizumab and ipilimumab has been studied in glioblastoma and advanced melanoma, showing promising activity with manageable toxicity profile [150,151]. Bevacizumab with anti-PD-L1 inhibitor, atezolizumab, also showed clinical activity without exacerbation of irAEs, and phase III clinical trial of this combination is ongoing in advanced RCC (NCT02420821).
Combination with radiotherapy (RT)
Tumor irradiation has immunologic effects, such as increased tumor antigen presentation, increased chemokine release, and recruitment of effector T-cells to the tumor microenvironment, although potentially deleterious effects can also be induced, such as upregulation of PD-L1, secretion of TGF-β, and induction of Tregs [152–155]. Localized RT has an abscopal effect on nonirradiated tumor sites through immunostimulation, which could be exploited and combined with immunotherapy [156–158]. While radiation shapes the TCR repertoire of the expanded peripheral clones, anti-CTLA-4 mAb promotes expansion of T-cells and contraction of Tregs; hence, their combination may have synergistic benefit [159–162]. Studies in prostate cancer and melanoma combining RT with ipilimumab showed clinical antitumor activity and manageable irAEs [158,163]. Although another study in advanced melanoma failed to demonstrate significant benefits of anti-CTLA-4 inhibitor, it did show persistent T-cell exhaustion in melanoma with high PD-L1 could be reversed by PD-L1 blockade. The authors suggested that the combination of radiation, anti-CTLA-4 and anti-PD-L1 mAbs might promote more potent anti-tumor immune response [164]. Clinical studies to determine the safety and efficacy of RT with various ICI are currently underway to identify the optimal radiation dose, radiation fractionation, and dose and timing of ICI.
Combination with T-cell based therapies
Adoptive T-cell therapy using CAR T-cells or BsAb (blinatumomab) specific for CD19 has been major breakthroughs in the treatment of acute lymphoblastic leukemia (ALL) [165,166]. When non-clonal T-cells are gene-modified with CAR or armed with bispecific antibodies [132,167], they mediate potent anti-tumor cytotoxicity, leading to strong T-cell activation and production of proinflammatory cytokines. However, despite promising clinical responses (e.g. CD19-directed T-cell based immunotherapy), tumor recurrence was observed, partly because of genomic instability and the effects of cancer immune editing [168]. Additional resistance mechanisms include downregulation or loss of target antigen expression, tumor-associated dendritic cell dysfunction, increased Tregs, immunosuppressive cytokines, activation of alternative signaling pathways, and anti-antibody formation [66,93,168–170].
T-cells driven by CAR or BsAb can trigger tumor cells to develop various immunosuppressive strategies, resulting in the release of inhibitory factors and a hostile tumor microenvironment, leading to T-cell exhaustion and tumor escape [168]. Upregulation of checkpoint molecules has been suggested as one of the main mechanisms of adaptive resistance in adoptive T-cell therapies [171], and evidence has continued to accumulate to support a key role of the PD-1/PD-L1 axis in attenuating anti-tumor immune responses [172,173]. Although PD-1/PD-L1 expression may not be robust at the time of diagnosis, they can be rapidly induced following blinatumomab treatment and is associated with disease relapse and resistance [174,175]. Cytokine-release syndrome (CRS), one of the major side effects of both CAR T-cells and BsAbs, results from massive cytokine secretion (IFN-γ, IL-6 and IL-10) associated with T-cell engagement and proliferation [176], leading to upregulation of PD-1/PD-L1 expression and immune resistance [174,177]. Blockade of PD-1/PD-L1 signaling could significantly increase anti-tumor cytotoxicity and T-cell proliferation and activity [171]. Given the significant acute (CRS) and chronic (B cell aplasia) toxicities from CD19-directed immune therapies, addition of ICI could intensify these side effects.
Combination of blinatumomab and pembrolizumab was administered in a pediatric patient with ALL. She was refractory to blinatumomab, and her blasts showed high PD-L1 expression. She was treated with blinatumomab and pembrolizumab after transplant and attained a remission without significant toxicities or exacerbation of CRS [174]. A phase I study of blinatumomab in combination with nivolumab or both nivolumab and ipilimumab in patients with relapsed or refractory CD19+ precursor B-acute leukemia has started (NCT02879695). Combined treatment with BsAbs [178–180] and various ICI including anti-PD-L1, anti-CTLA-4, anti-LAG-3, and anti-B7-H3 could be alternative therapeutic strategies in refractory or relapsed cancers in children, although toxicities could become prohibitive.
CAR T-cell therapy is another highly promising immunotherapy for children and adults with B-cell leukemia. However, the clinical results in solid tumors have not been encouraging. For optimal tumor eradication, CAR T-cells must have proper target antigen selection, co-stimulatory signaling, and the ability to move into the tumor, as well as persistence or proliferation, while avoiding T-cell exhaustion and T-cell death, now believed to be a major limiting factor [181]. Recent clinical trials have shown that tumor burden and chemotherapy conditioning before CAR T-cell therapy are critical, and it is likely that CAR T-cell therapy alone will be insufficient for cure [182]. Combination with ICI could be a future direction. Blocking PD-1/PD-L1 can unleash the cytotoxic functions of adoptively transferred T-cells, and potentially promote the development of endogenous T-cells that target neoantigens [168,183].
The antitumor effect of combinational therapy with CAR T-cells and PD-1 inhibitor was investigated preclinically using transgenic Her2 mice treated with Her2-specific CAR T-cells. Tumor Her2 antigen triggered PD-1 upregulation in CAR T-cells, and PD-1 blockade enhanced Her2-specific T-cell functions and decreased MDSC in the tumor microenvironment, leading to enhanced anti-tumor effect [36]. A clinical trial to study the combination of CD19-CAR T-cells and ipilimumab in patients with B-cell lymphoid malignancies including pediatric patients has been initiated (NCT00586391).
Conclusion
Although overall survival rates for pediatric malignancies approached upwards of 80% [184], further improvements have slowed down in the past decades, despite the use of dose-intensive genotoxic therapies approaching their toxicity limits. New approaches in pediatric patients with advanced stage, relapsed or refractory cancers are desperately needed. Several novel therapeutic agents including small molecular targeted agents, monoclonal antibodies, and T-cell based immunotherapies have shown promise. To fully exploit these powerful modalities against tumors with low mutational load and weak TIL content (‘cold’ tumors), emphasis should be placed on proven and novel strategies to drive T-cells selectively and quantitatively into cold tumors, to activate them to proliferate inside a hostile tumor microenvironment, and to avoid exhaustion or activation induced cell death. Once T-cells can infiltrate, persist, proliferate and survive, the addition of ICI should vastly enhance their potential in any of these pediatric malignancies. However, there remain significant hurdles with regard to both acute and late toxicities. A concerted effort should be made not to run redundant studies, but to systematically confront these limitations for a more streamlined pediatric integration. Combination treatment should be the framework and no single approach, whether cell therapy or antibody will likely be curative.
Highlights.
Immune checkpoint inhibitors (ICI) have achieved a great success in adult cancers.
There is growing interest in ICI for pediatric malignancies.
There are significant barriers for successful pediatric integration of ICI.
Combination with other treatment modalities could be a promising option.
Acknowledgments
We thank Dr. Irene Y. Cheung for reviewing and editing the manuscript.
Footnotes
Conflicts of interest: none
References
- 1.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–65. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 2.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012;61:1327–41. doi: 10.1007/s00262-012-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 11.Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Onco Targets Ther. 2016;9:1767–76. doi: 10.2147/OTT.S65802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin Cancer Res. 2016;22:1364–70. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–14. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 16.van Dam LS, de Zwart VM, Meyer-Wentrup FA. The role of programmed cell death-1 (PD-1) and its ligands in pediatric cancer. Pediatr Blood Cancer. 2015;62:190–7. doi: 10.1002/pbc.25284. [DOI] [PubMed] [Google Scholar]
- 17.Lussier DM, O’Neill L, Nieves LM, McAfee MS, Holechek SA, Collins AW, et al. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother. 2015;38:96–106. doi: 10.1097/CJI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014;7:1–17. doi: 10.1016/j.hemonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Liu S, Wang L, Zhang W, Ji Y, Ma X. Clinical significance of B7-H1 (PD-L1) expression in human acute leukemia. Cancer Biol Ther. 2008;7:622–7. doi: 10.4161/cbt.7.5.5689. [DOI] [PubMed] [Google Scholar]
- 20.Kronig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol. 2014;92:195–203. doi: 10.1111/ejh.12228. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11:757–66. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Routh JC, Ashley RA, Sebo TJ, Lohse CM, Husmann DA, Kramer SA, et al. B7-H1 expression in Wilms tumor: correlation with tumor biology and disease recurrence. J Urol. 2008;179:1954–9. doi: 10.1016/j.juro.2008.01.056. discussion 9–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–44. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 24.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, et al. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100:2093–100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryan LJ, Gordon LI. Pidilizumab in the treatment of diffuse large B-cell lymphoma. Expert Opin Biol Ther. 2014;14:1361–8. doi: 10.1517/14712598.2014.942637. [DOI] [PubMed] [Google Scholar]
- 33.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421–7. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha E, Wallin J, Kowanetz M. PD-L1 inhibition with MPDL3280A for solid tumors. Semin Oncol. 2015;42:484–7. doi: 10.1053/j.seminoncol.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 36.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 37.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34:2980–7. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal DT, Yalon M, Vainer GW, Lossos A, Yust S, Tzach L, et al. Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors. J Neurooncol. 2016;129:453–60. doi: 10.1007/s11060-016-2190-1. [DOI] [PubMed] [Google Scholar]
- 40.Foran AE, Nadel HR, Lee AF, Savage KJ, Deyell RJ. Nivolumab in the Treatment of Refractory Pediatric Hodgkin Lymphoma. J Pediatr Hematol Oncol. 2016 doi: 10.1097/MPH.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 41.Shad AT, Huo JS, Darcy C, Abu-Ghosh A, Esposito G, Holuba MJ, et al. Tolerance and effectiveness of nivolumab after pediatric T-cell replete, haploidentical, bone marrow transplantation: A case report. Pediatr Blood Cancer. 2016 doi: 10.1002/pbc.26257. [DOI] [PubMed] [Google Scholar]
- 42.Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huard B, Prigent P, Pages F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26:1180–6. doi: 10.1002/eji.1830260533. [DOI] [PubMed] [Google Scholar]
- 45.Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994;39:213–7. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- 46.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081–8. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- 48.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–91. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–69. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okagawa T, Konnai S, Deringer JR, Ueti MW, Scoles GA, Murata S, et al. Cooperation of PD-1 and LAG-3 Contributes to T-Cell Exhaustion in Anaplasma marginale-Infected Cattle. Infect Immun. 2016;84:2779–90. doi: 10.1128/IAI.00278-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prigent P, El Mir S, Dreano M, Triebel F. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. Eur J Immunol. 1999;29:3867–76. doi: 10.1002/(SICI)1521-4141(199912)29:12<3867::AID-IMMU3867>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Brignone C, Grygar C, Marcu M, Schakel K, Triebel F. A soluble form of lymphocyte activation gene-3 (IMP321) induces activation of a large range of human effector cytotoxic cells. J Immunol. 2007;179:4202–11. doi: 10.4049/jimmunol.179.6.4202. [DOI] [PubMed] [Google Scholar]
- 55.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15:6225–31. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 56.Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010;8:71. doi: 10.1186/1479-5876-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–78. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 59.Zhuang X, Shen J, Jia Z, Wu A, Xu T, Shi Y, et al. Anti-B7-H3 monoclonal antibody ameliorates the damage of acute experimental pancreatitis by attenuating the inflammatory response. Int Immunopharmacol. 2016;35:1–6. doi: 10.1016/j.intimp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21:707–17. doi: 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–9. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 62.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764–71. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 64.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125:3335–46. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z, Zhao H, et al. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett. 2013;5:805–12. doi: 10.3892/ol.2013.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong F, et al. B7-H3 overexpression in pancreatic cancer promotes tumor progression. Int J Mol Med. 2013;31:283–91. doi: 10.3892/ijmm.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–51. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–7. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S, Zhao X, Du R, Zhu Q, Fang H, Zhang X, et al. Overexpression of B7-H3 correlates with aggressive clinicopathological characteristics in non-small cell lung cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bin Z, Guangbo Z, Yan G, Huan Z, Desheng L, Xueguang Z. Overexpression of B7-H3 in CD133+ colorectal cancer cells is associated with cancer progression and survival in human patients. J Surg Res. 2014;188:396–403. doi: 10.1016/j.jss.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 72.Guery T, Roumier C, Berthon C, Renneville A, Preudhomme C, Quesnel B. B7-H3 protein expression in acute myeloid leukemia. Cancer Med. 2015;4:1879–83. doi: 10.1002/cam4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–5. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8:e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J Biol Chem. 2015;290:30018–29. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benzon B, Zhao SG, Haffner MC, Takhar M, Erho N, Yousefi K, et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. 2016 doi: 10.1038/pcan.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–45. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 78.Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22:3425–31. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–18. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma J, Ma P, Zhao C, Xue X, Han H, Liu C, et al. B7-H3 as a promising target for cytotoxicity T cell in human cancer therapy. Oncotarget. 2016;7:29480–91. doi: 10.18632/oncotarget.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 82.Abdel-Rahman O, Helbling D, Schmidt J, Petrausch U, Giryes A, Mehrabi A, et al. Treatment-related Death in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. Clin Oncol (R Coll Radiol) 2016 doi: 10.1016/j.clon.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–43. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 87.Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol. 2011;22:991–3. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 88.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol. 2011;28:1140–4. doi: 10.1007/s12032-010-9606-0. [DOI] [PubMed] [Google Scholar]
- 89.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 90.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 93.Zhu X, McDowell MM, Newman WC, Mason GE, Greene S, Tamber MS. Severe cerebral edema following nivolumab treatment for pediatric glioblastoma: case report. J Neurosurg Pediatr. 2016:1–5. doi: 10.3171/2016.8.PEDS16326. [DOI] [PubMed] [Google Scholar]
- 94.Mandel JJ, Olar A, Aldape KD, Tremont-Lukats IW. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci. 2014;344:229–31. doi: 10.1016/j.jns.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iglesias P, Soria A, Diez JJ. Autoimmune endocrinopathies induced by immunomodulating antibodies in the treatment of cancer. Medicina clinica. 2015;145:264–8. doi: 10.1016/j.medcli.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 96.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015;3:1158–64. doi: 10.1158/2326-6066.CIR-15-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts SS, Chou AJ, Cheung NK. Immunotherapy of Childhood Sarcomas. Front Oncol. 2015;5:181. doi: 10.3389/fonc.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14:703–18. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 108.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14:468–74. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vakkila J, Jaffe R, Michelow M, Lotze MT. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: a major nosologic difference with adult tumors. Clin Cancer Res. 2006;12:2049–54. doi: 10.1158/1078-0432.CCR-05-1824. [DOI] [PubMed] [Google Scholar]
- 110.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 113.Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 114.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schatton T, Scolyer RA, Thompson JF, Mihm MC., Jr Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol. 2014;1102:287–324. doi: 10.1007/978-1-62703-727-3_16. [DOI] [PubMed] [Google Scholar]
- 116.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for immunotherapy of cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 118.Gajewski TF. Molecular profiling of melanoma and the evolution of patient-specific therapy. Semin Oncol. 2011;38:236–42. doi: 10.1053/j.seminoncol.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. International immunology. 2016;28:383–91. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery. 2016;6:202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–76. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Ihara S, Kida H, Arase H, Tripathi LP, Chen YA, Kimura T, et al. Inhibitory roles of signal transducer and activator of transcription 3 in antitumor immunity during carcinogen-induced lung tumorigenesis. Cancer Res. 2012;72:2990–9. doi: 10.1158/0008-5472.CAN-11-4062. [DOI] [PubMed] [Google Scholar]
- 123.Haworth KB, Leddon JL, Chen CY, Horwitz EM, Mackall CL, Cripe TP. Going back to class I: MHC and immunotherapies for childhood cancer. Pediatr Blood Cancer. 2015;62:571–6. doi: 10.1002/pbc.25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–6. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berghuis D, de Hooge AS, Santos SJ, Horst D, Wiertz EJ, van Eggermond MC, et al. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol. 2009;218:222–31. doi: 10.1002/path.2537. [DOI] [PubMed] [Google Scholar]
- 126.Mechtersheimer G, Staudter M, Majdic O, Dorken B, Moldenhauer G, Moller P. Expression of HLA-A,B,C, beta 2-microglobulin (beta 2m), HLA-DR, -DP, -DQ and of HLA-D-associated invariant chain (Ii) in soft-tissue tumors. Int J Cancer. 1990;46:813–23. doi: 10.1002/ijc.2910460512. [DOI] [PubMed] [Google Scholar]
- 127.Haworth KB, Arnold MA, Pierson CR, Leddon JL, Kurmashev DK, Swain HM, et al. Characterization of MHC Class I and beta-2-Microglobulin Expression in Pediatric Solid Malignancies to Guide Selection of Immune-Based Therapeutic Trials. Pediatr Blood Cancer. 2016;63:618–26. doi: 10.1002/pbc.25842. [DOI] [PubMed] [Google Scholar]
- 128.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yankelevich M, Kondadasula SV, Thakur A, Buck S, Cheung NK, Lum LG. Anti-CD3 × anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer. 2012;59:1198–205. doi: 10.1002/pbc.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Houot R, Schultz LM, Marabelle A, Kohrt H. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res. 2015;3:1115–22. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 134.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34:2969–79. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol. 2016 doi: 10.1093/annonc/mdw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sierra JR, Cepero V, Giordano S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol Cancer. 2010;9:75. doi: 10.1186/1476-4598-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garrido G, Rabasa A, Garrido C, Lopez A, Chao L, Garcia-Lora AM, et al. Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene. 2014;33:3129–39. doi: 10.1038/onc.2013.288. [DOI] [PubMed] [Google Scholar]
- 139.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]
- 141.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–11. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 142.Gibbons DL, Chow LQ, Kim DW, Kim SW, Yeh T, Song X, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11:S79. [Google Scholar]
- 143.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–31. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 145.Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32:2248–54. doi: 10.1200/JCO.2013.52.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burgess M, Gorantla V, Weiss K, Tawbi H. Immunotherapy in Sarcoma: Future Horizons. Curr Oncol Rep. 2015;17:52. doi: 10.1007/s11912-015-0476-7. [DOI] [PubMed] [Google Scholar]
- 147.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 148.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carter T, Shaw H, Cohn-Brown D, Chester K, Mulholland P. Ipilimumab and Bevacizumab in Glioblastoma. Clin Oncol (R Coll Radiol) 2016;28:622–6. doi: 10.1016/j.clon.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 151.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–42. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sharma A, Bode B, Studer G, Moch H, Okoniewski M, Knuth A, et al. Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clin Cancer Res. 2013;19:4843–53. doi: 10.1158/1078-0432.CCR-13-0352. [DOI] [PubMed] [Google Scholar]
- 155.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126. doi: 10.1155/2013/658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Merrick A, Errington F, Milward K, O’Donnell D, Harrington K, Bateman A, et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br J Cancer. 2005;92:1450–8. doi: 10.1038/sj.bjc.6602518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–9. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 161.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 162.Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2:831–8. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 167.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370–83. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sun C, Dotti G, Savoldo B. Utilizing cell-based therapeutics to overcome immune evasion in hematologic malignancies. Blood. 2016;127:3350–9. doi: 10.1182/blood-2015-12-629089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 170.Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–74. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Kohnke T, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30:484–91. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 172.Madorsky Rowdo FP, Baron A, Urrutia M, Mordoh J. Immunotherapy in Cancer: A Combat between Tumors and the Immune System; You Win Some, You Lose Some. Front Immunol. 2015;6:127. doi: 10.3389/fimmu.2015.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feucht J, Kayser S, Gorodezki D, Hamieh M, Doring M, Blaeschke F, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016 doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kohnke T, Krupka C, Tischer J, Knosel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111. doi: 10.1186/s13045-015-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dondero A, Pastorino F, Della Chiesa M, Corrias MV, Morandi F, Pistoia V, et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology. 2016;5:e1064578. doi: 10.1080/2162402X.2015.1064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu H, Cheng M, Guo H, Chen Y, Huse M, Cheung NK. Retargeting T cells to GD2 pentasaccharide on human tumors using Bispecific humanized antibody. Cancer immunology research. 2015;3:266–77. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lopez-Albaitero A, Xu H, Guo HF, Wang LL, Wu Z, Tran H, et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. OncoImmunology. 2017 doi: 10.1080/2162402X.2016.1267891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spiess C, Zhai QT, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Molecular Immunology. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 181.Newick K, O’Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2016 doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 182.Zhang T, Cao L, Xie J, Shi N, Zhang Z, Luo Z, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6:33961–71. doi: 10.18632/oncotarget.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, et al. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19:6151–62. doi: 10.1158/1078-0432.CCR-13-1189. [DOI] [PubMed] [Google Scholar]
- 184.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 185.Leung HT, Bradshaw J, Cleaveland JS, Linsley PS. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J Biol Chem. 1995;270:25107–14. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- 186.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 188.Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–3. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 189.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Honda T, Egen JG, Lammermann T, Kastenmuller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40:235–47. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]