Abstract
The effect of viewing distance on the perception of visual texture is well known: spatial frequencies higher than the resolution limit of an observer's visual system will be summed and perceived as a single combined colour. In animal defensive colour patterns, distance-dependent pattern blending may allow aposematic patterns, salient at close range, to match the background to distant observers. Indeed, recent research has indicated that reducing the distance from which a salient signal can be detected can increase survival over camouflage or conspicuous aposematism alone. We investigated whether the spatial frequency of conspicuous and cryptically coloured stripes affects the rate of avian predation. Our results are consistent with pattern blending acting to camouflage salient aposematic signals effectively at a distance. Experiments into the relative rate of avian predation on edible model caterpillars found that increasing spatial frequency (thinner stripes) increased survival. Similarly, visual modelling of avian predators showed that pattern blending increased the similarity between caterpillar and background. These results show how a colour pattern can be tuned to reveal or conceal different information at different distances, and produce tangible survival benefits.
Keywords: aposematism, camouflage, defensive colouration, distance, visual ecology, warning signals
1. Background
Camouflage and aposematism are two seemingly contrasting and mutually exclusive forms of antipredator colouration: camouflage reduces the likelihood of detection, whereas aposematic signals communicate directly with predators [1,2]. Aposematism is often associated with high conspicuousness, and increasing conspicuousness has repeatedly been linked to greater speed and accuracy of predator avoidance learning [3,4]. However, rather than developing a complete avoidance of aposematic prey, it is now apparent that predators learn about prey characteristics, actively managing their consumption of defended prey depending on their nutritional requirements, toxin burden and energy expenditure [5–9].
As a consequence, under natural levels of environmental heterogeneity and predator diversity, the costs of increasing conspicuousness can outweigh the benefits of increased signal efficacy [10]. The conspicuousness of an aposematic signal has, therefore, been linked to honest signalling of defence strength, as only more heavily defended individuals can overcome the costs of high detectability [11]. Research into detectability has, however, predominantly focused on colour saturation and the proportions of conspicuous and inconspicuous pattern components [11–15].
An alternative mechanism, which maintains colour saturation, is to manipulate the visual texture of an aposematic pattern. As visual systems are limited in their ability to resolve high spatial frequencies (fine textures), viewing distance can greatly affect the perception of a pattern [16]. It has been suggested that certain patterns can exploit these limitations and appear highly conspicuous at close range while also being camouflaged at longer viewing distances, where fine details can no longer be resolved [12,13,17–21].
Striped aposematic patterns are common in nature, and often combine a bright colour (e.g. yellow) with black to produce a highly contrasting and, therefore, salient pattern [22]. Internal pattern boundaries have been linked to increasing the efficacy of aposematic signalling, with the presence of high contrast patterning being proposed to increase colour contrast above that which is achievable against the background, produce a consistent signal across multiple backgrounds, reduce the impact of partial occlusion, or make the pattern more distinct from palatable species [23–26].
When viewed from sufficient distance, however, a striped pattern cannot be resolved and adjacent stripes will be perceptually summed to produce a combined colour. If this combined colour matches that of the background, a striped pattern may produce effective camouflage to distant observers [17,19,27].
In this study we investigated whether stripe spatial frequency and pattern blending can affect the detectability and survivability of prey which appear conspicuous (yellow-and-black) or cryptic (green-and-black). We predicted that blended colours would be a closer match to the background than their component colours, and that increasing stripe spatial frequency (thinner stripes) would decrease the rate of avian predation due to the effect of pattern blending on detectability. As confirmation of the perceptual effects, in a separate experiment with human observers (electronic supplementary material) we predicted that higher spatial frequency would decrease the distance at which stripes were first visible.
2. Methods
(a). Stimuli
Stimuli were designed to mimic free-living lepidopteran larvae with a variety of antipredator patterns. ‘Caterpillars’ were, approximately 16 mm long by approximately 3 mm diameter, cylinders of coloured dough (see below). The 12 treatments were based on either yellow-and-black (a common aposematic colour) or green-and-black (typical of camouflage in vegetative environments), and were either striped or plain. Striped treatments were designed to differ in spatial frequency while retaining equal ratios of each component colour (figure 1).
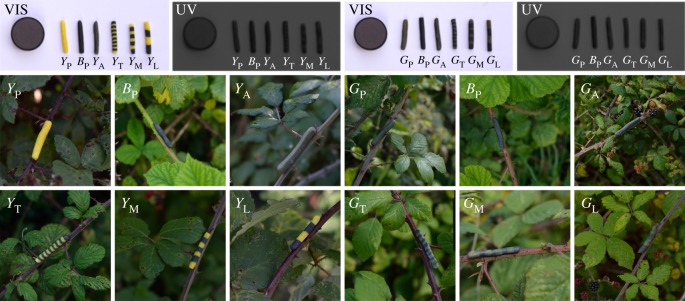
Figure 1.
Yellow-and-black, (left), and green-and-black, (right), dough caterpillars photographed in human visible (VIS) and ultraviolet (UV) light, with a 15% reflectance Spectralon® grey standard (Labsphere, Inc. North Sutton, NH, USA), and photographed in situ on bramble stems (Rubus fruticosus agg. Rosaceae). ‘Caterpillars’ are approximately 16 mm long by 3 mm diameter.
For the yellow-and-black experiment six yellow-and-black treatments were designed: YP—plain yellow; BP—plain black; YA—1 : 1 yellow-black average; YT—16 × 1 mm yellow-and-black stripes (5.00 cycles cm−1); YM—8 × 2 mm yellow-and-black stripes (2.50 cycles cm−1); YL—4 × 4 mm yellow-and-black stripes (1.25 cycles cm−1). These patterns were then recreated based on green-and-black stripes: GP—plain green; BP—plain black; GA—1 : 1 green-black average; GT—16 × 1 mm green-and-black stripes (5.00 cycles cm−1); GM—8 × 2 mm green-and-black stripes (2.50 cycles cm−1); GL—4 × 4 mm green-and-black stripes (1.25 cycles cm−1).
A 3 : 1 mix of flour (British Plain Flour by Sainsbury's, J Sainsbury plc., London, UK) and lard (Sainsbury's Basics Lard) was used to make the dough, which was then coloured yellow (25 ml per 500 g dough; Yellow Food Colouring by Sainsbury's), or black (25 ml per 500 g dough; Black Food Colouring by Sainsbury's). Green was made from a 1 : 1 mix of yellow and black dough, and the average colours were made from a 1 : 1 mix of either yellow and black (YA) or green and black (GA). The stimuli were then built from 16 × 1 mm thick layers of coloured dough (figure 1).
(b). Image analysis
As our experiments used both avian predators (survival experiments) and human participants (detection experiments—electronic supplementary material), assumptions regarding the conspicuousness of each dough colour were checked in relation to models of avian and human visual perception using calibrated photography [28,29].
Dough caterpillars were first photographed using a UV-sensitive Nikon D70 Digital SLR camera, UV-NIKKOR 105 mm lens (Nikon Corporation, Japan), appropriate VIS filters, and a 15% reflectance Spectralon® grey standard (Labsphere Inc., North Sutton, NH, USA). UV photography revealed minimal UV reflectance from all of the dough colours (figure 1 top), and therefore allowed both human and avian vision to be modelled from standard RGB photography.
Photographs (sample sizes: YA = 9, GA = 9, BP = 8, YP = 8, GP = 10, YT = 10, GT = 9) were taken of each treatment in situ on the stems of mature bramble (Rubus fruticosus agg. Rosaceae) plants, as they were presented to wild avian predators in the survival experiments (figure 1 middle and bottom). Each image was taken with a Nikon D3200 Digital SLR camera and AF-S DX NIKKOR 35 mm prime lens (Nikon Corporation, Tokyo, Japan) and contained a ColorChecker Passport (X-Rite Inc. 2009. Grand Rapids, MI, USA), which allowed size-scaling and linearization of colour values [28] in MATLAB 2015a (The MathWorks Inc., Natick, MA, USA). The locations of the dough caterpillar and the background were labelled by hand in MATLAB and used to generate masks for subsequent selection and analysis.
To represent the avian predators in the survival experiment visual modelling used the tetrachromatic vision of the European starling (Sturnus vulgaris, Sturnidae), typical of many songbirds, with single cone peak absorption (λmax) of 563 nm (Lw), 504 nm (Mw), 449 nm (Sw), and 362 nm (UV), and double cones (D) with a peak absorption (λmax) of 563 nm [29]. In addition, to allow more intuitive comparison between avian and human vision, and to allow interpretation of a detection experiment using human participants (electronic supplementary material), we also used two models of human colour perception: L*a*b* and human LMS. L*a*b* is a perceptually defined colour space produced from discrimination experiments (CIELAB, 1976: http://cie.co.at), however, as there is no avian equivalent of L*a*b*, we also generated a human LMS colour space analogous to the avian cone space, using cone cell absorption distributions: λmax of 564 nm (Lw), 534 nm (Mw), and 420 nm (Sw) [30].
For both cone-based visual systems, colour was measured, as in L*a*b*, in terms of a luminance and two opponent channels, red-green (rg), produced from the relative stimulation of the longwave and mediumwave cones, and yellow-blue (yb), which was produced from the relative response of the combined longwave and mediumwave cones compared to the shortwave cone [31]. Although the opponent mechanisms have not been fully characterized for birds, it is an efficient way to encode the information because, unlike the photoreceptor photon catches themselves, these channels are approximately orthogonal (see discussion of opponent processing in [32] and of this particular representation in [33]).
For avian vision, a pseudo-luminance measure (L) was calculated from the response of the double cone, whereas for human LMS, L was calculated as the mean response of the longwave and mediumwave cones [29,30].
The colours of the background and high spatial frequency striped ‘caterpillars’ (YT and GT) were analysed at two spatial scales: at the resolution of the pixels in the photographs (henceforth ‘High’) and after spatial averaging, where we applied a Gaussian smoother with a standard deviation equal to half the length of the caterpillars (henceforth ‘Low’; function imgaussfilt in MATLAB 2015a). The High condition, therefore, used all of the available information and represented close range viewing. For the Low condition, representing a view from beyond the resolution limit of the pattern, a wavelength equal to half the length of the caterpillar ensured that all pattern components would blend but the caterpillar itself would still technically be resolvable against the background.
(c). Survival protocol
Dough caterpillars were pinned to horizontal stems of bramble bushes (Rubus fruticosus agg., Rosaceae), where they were predated by a variety of small passerine birds (Passeriformes). Caterpillars were pinned along nonlinear transects within suburban areas of green space in the city of Bristol, UK. A randomized block design was used. Fifteen blocks of yellow-and-black caterpillars (YP, BP, YA, YT, YM and YL) were run between June and September 2013 (10 of each treatment per block = 900 caterpillars). In a separate experiment the protocol was repeated with 15 blocks of the green-and-black caterpillars (GP, BP, GA, GT, GM and GL) between November 2013 and June 2014 (n = 900). Each block was conducted in a different location.
The survival of each caterpillar was checked at 24, 48, 72 and 96 h. Avian predation was identified by beak marks in, or complete removal of, the dough caterpillar, whereas Hymenoptera, principally ants, left small pit marks in the dough. For both experiments survival was analysed with a mixed effects Cox model from package coxme [34] and pairwise tests used the false discovery rate from package multcomp [35], to gain a suitable balance between Type I and II errors, in R v. 3.1.3 (The R Foundation for Statistical Computing, Vienna, Austria). Avian predation was included as full events, block as a random factor, and non-avian predation, missing pins, and caterpillars surviving to 96 h were included as censored values. Data are available in Dryad [36].
3. Results
(a). Image analysis
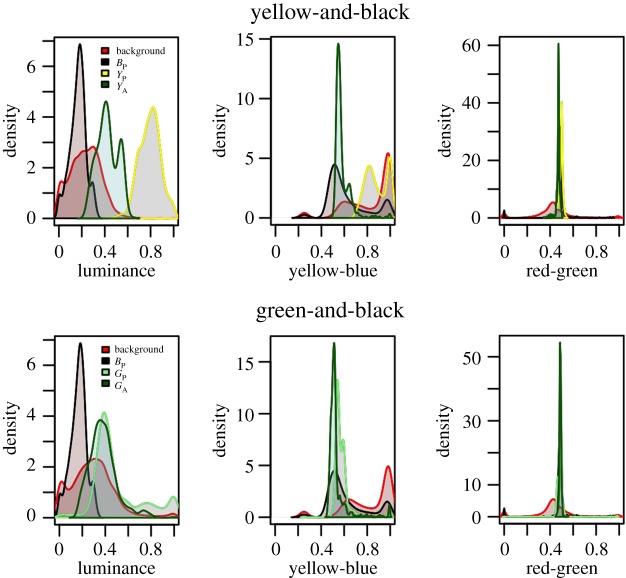
We found a high correlation between the response of human LMS and the avian visual model for each visual channel (L = 0.997, rg = 0.826, yb = 0.996). There was a weaker correlation between human LMS and L*a*b* colour space (L* − L = 0.991, a* − rg = 0.489, b* − yb = 0.524) due to the nonlinear relationship between the two visual models (although the same perceptual trends are conserved, see electronic supplementary material). Plotting the avian visual model response for each treatment indicates that the majority of variation is found in the luminance (L) and yb channels, and that the ‘cryptic’ treatments (BP, YA, GP, and GA) are well represented in the background. Yellow dough (YP), in contrast, differs in both the luminance and yb channels (figure 2).
Figure 2.
Dough caterpillar and bramble colours as viewed by a model of avian visual perception (top—yellow-and-black; bottom—green-and-black). All colours are well represented in the background apart from YP which forms an obvious outlier in luminance. (Online version in colour.)
These data suggest that for the yellow-and-black dough experiments the combined colour (YA) is a closer match to the background than the plain yellow (YP). Similarly, in the green-and-black experiment, although both constituent colours (GP and BP) are represented in the background, the combined colour (GA) does not contain the high luminance components found in the plain green (GP).
Plotting avian model response at different spatial resolutions shows that at high spatial resolution (representing close viewing conditions; figure 3 top) both treatments can be distinguished from the background, whereas at low spatial resolution (representative of far viewing conditions; figure 3 bottom) caterpillar colours converge with those of the background.
Figure 3.
High spatial frequency striped dough caterpillar treatments (yellow—YT; green—GT) viewed by the avian visual model in relation to the bramble background at high, (top), and low, (bottom), spatial resolutions. At low spatial resolutions the colours of both striped targets blend together and converge with the colours of the background across all three channels. (Online version in colour.)
These data, therefore, support the hypothesis that the combined colours (YA and GA) were better matches to the background than their constituent colours (YP, and GP respectively), and that for striped patterns pattern blending at greater viewing distances can produce more effective camouflage.
(b). Survival: yellow-and-black
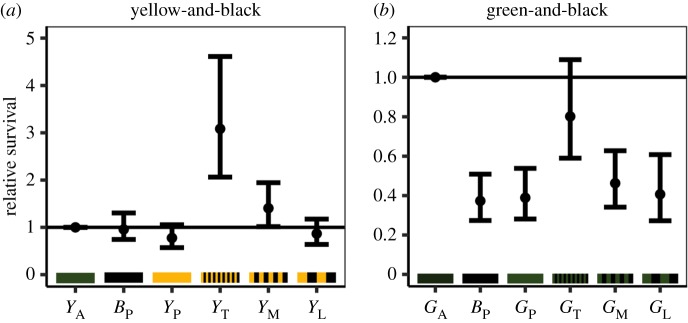
There was a significant effect of treatment on the survival of the yellow-and-black caterpillars (χ2 = 70.43, d.f. = 5, p < 0.001; figure 4 left). Pairwise tests show that there was no significant difference in survival between plain treatments (YA − BP: z = −0.28, p = 1.00; YA − YP: z = −1.62, p = 0.581; BP − YP: z = −1.33, p = 0.765), or between the plain treatments and the lowest spatial frequency stripes (YL − YA: z = 0.92, p = 0.940; YL − BP: z = −0.63, p = 0.988; YL − YP: z = −0.72, p = 0.980). The medium stripes survived equally to the plain average and plain black (YM − YA: z = −2.05, p = 0.308; YM − BP: z = −2.32, p = 0.185), but survival was higher than the plain yellow (YM − YP: z = −3.61, p = 0.004). The thinnest stripes had higher survival than all of plain treatments (YT − YA: z = −5.49, p < 0.001; YT − BP: z = −5.70, p < 0.001; YT − YP: z = −6.74, p < 0.001).
Figure 4.
Relative survival of dough caterpillars (odds ratios compared to the average colour treatment with 95% CI from the model). For both the yellow-and-black, (a), and the green-and-black, (b), stripes, increasing spatial frequency increases survival. For the yellow-and-black stripes survival increases beyond that of the average colour (YA); whereas, for the green-and-black stripes, as spatial frequency increases, the survival of striped patterns moves towards than of the more cryptic average (GA). (Online version in colour.)
There was a stepwise decrease in survival as spatial frequency decreased, with the thinnest stripes having higher survival than the medium and lowest spatial frequencies (YT − YM: z = −3.73, p = 0.003; YT − YL: z = −6.24, p < 0.001), and the medium having higher survival than the lowest spatial frequency (YM − YL: z = −2.96, p = 0.036).
(c). Survival: green-and-black
For the green-and-black caterpillars there was a significant effect of treatment on survival (χ2 = 90.22, d.f. = 5, p < 0.001; figure 4 right).
There was no significant difference between the plain black and plain green (BP − GP: z = 0.33, p = 0.999). There was no significant difference between the medium and low spatial frequency stripes (GM − GL: z = 1.00, p = 0.918), and no difference between the medium or low spatial frequency stripes and the plain black or plain green (z < 1.68, p > 0.546).
There was no significant difference in survival between the highest spatial frequency stripes and the average colour (GT − GA: z = 1.48, p = 0.674), but both had significantly higher survival than the medium and low spatial frequency stripes (GT − GM: z = −3.98, p < 0.001; GT − GL: z = −4.94, p < 0.001; GA − GM: z = −5.38, p < 0.001; GA − GL: z = −6.28, p < 0.001), as well as the plain black and plain green caterpillars (GT − BP: z = −5.57, p < 0.001; GT − GP: z = −5.29, p < 0.001; GA − BP: z = −6.86, p < 0.001; GA − GP: z = −6.60, p < 0.001).
4. Discussion
Aposematic signals are often associated with high contrast patterns [22], which are thought to increase the saliency, ease of learning, and memorability of the warning signal [23–26]. It has also been suggested that these pattern components might provide camouflage when viewed from a distance [12,13,17–21,27]. The latter effect has potentially been underappreciated, as many studies have been conducted in the laboratory or on unnatural backgrounds.
At greater viewing distances, adjacent patches of colour can no longer be resolved and will be summed by the visual system and thus perceived as a single combined colour. The distance at which this summation occurs will depend on the spatial frequency of the pattern and the visual acuity of the observer. We found that for both yellow-and-black and green-and-black stripes, the spatially averaged colours were a closer match to the background than their more conspicuous elements (YP and GP respectively) for both human and avian vision, and increasing spatial frequency (thinner stripes) decreased the rate of predation by wild avian predators. Furthermore, we found that increasing spatial frequency also decreased the distance at which human observers could resolve the stripes (electronic supplementary material).
Increasing spatial frequency, therefore, decreased the distance at which stripes would blend to form a more cryptic colour. For our green-and-black striped caterpillars we found that as spatial frequency increased, survival increased towards that of the average colour (GA = GT > GM = GL). In contrast, for the yellow-and-black caterpillars the survival of higher spatial frequencies surpasses that of the average colour (YT > YM > YL = YA). We suggest that for the green-and-black stripes, pattern blending leads to a closer match to the background and better camouflage, whereas for the yellow-and-black stripes the combination of camouflage and aversive signalling produces a combined strategy which is more effective than either in isolation [12,13,18–21,27].
It has also been suggested that aposematic pattern components could provide disruptive camouflage (breaking up the organism's outline into incongruent patches) [37], however, the regular geometric structure of these stimuli are unlike the irregular patterns normally associated with disruptive camouflage [38]. This possibility, however, does deserve further research.
These data suggest that detection distance can be reduced without necessarily compromising the effectiveness of salient defensive colouration. For an aposematic pattern, this is influenced by the internal colour contrasts, the colours themselves, and, perhaps the ratio of colour components [1,3,4,10,25–27,31]. Striped patterns may therefore enable an animal to combine highly salient aposematic signalling with effective background matching camouflage. Varying stripe spatial frequency can create a stable and highly salient pattern, while also controlling the distance at which a pattern is detectable. These mechanisms may be exploited in order to balance different selection pressures, to alter detectability during ontogeny as pattern size and defence strength develop together, to minimize the long-range detectability of other conspicuous signals (i.e. sexual signals where mate attraction and predation work over different spatial scales), or as a mechanism for Batesian mimic species to reduce the risk of detection while retaining a pattern which is perceptually grouped with that of their model [38].
Internal pattern boundaries may, therefore, provide a wide range of different benefits to the aposematic organism, including increased saliency at close range and reduced detection distance. Viewing distance is likely to be an underappreciated aspect of visual ecology, and a more inclusive study of animal colouration may reveal new insights into how different functions interact within a single phenotype.
Supplementary Material
Acknowledgements
We thank all members of the CamoLab at the University of Bristol. I.C.C. thanks the Wissenschaftskolleg zu Berlin for support during part of the study.
Ethics
Experiments were approved by the University of Bristol Animal Welfare & Ethical Review Body (birds) and the Faculty of Science Ethics Research Committee (humans: electronic supplementary material). All human participants gave informed consent in accordance with the Declaration of Helsinki.
Data accessibility
Raw data can be accessed from the Dryad data repository: http://dx.doi.org/10.5061/dryad.2h6nf [36].
Authors' contributions
J.B.B. collected the data, and all authors participated in experimental design, analysis and writing of the manuscript.
Competing interests
We have no competing interests.
Funding
J.B.B. was supported by a Postgraduate Research Scholarship from the University of Bristol.
References
- 1.Mappes J, Marples N, Endler JA. 2005. The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603. ( 10.1016/j.tree.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 2.Stevens M, Merilaita S. 2009. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B. 364, 423–427. ( 10.1098/rstb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamberale-Stille G. 2001. Benefit by contrast: an experiment with live aposematic prey. Behav. Ecol. 12, 768–772. ( 10.1093/beheco/12.6.768) [DOI] [Google Scholar]
- 4.Prudic KL, Skemp AK, Papaj DR. 2007. Aposematic coloration, luminance contrast, and the benefits of conspicuousness. Behav. Ecol. 18, 41–46. ( 10.1093/beheco/arl046) [DOI] [Google Scholar]
- 5.Barnett C, Bateson M, Rowe C. 2007. State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav. Ecol. 18, 645–651. ( 10.1093/beheco/arm027) [DOI] [Google Scholar]
- 6.Barnett CA, Skelhorn J, Bateson M, Rowe C. 2012. Educated predators make strategic decisions to eat defended prey according to their toxin content. Behav. Ecol. 23, 418–424. ( 10.1093/beheco/arr206) [DOI] [Google Scholar]
- 7.Skelhorn J, Rowe C. 2007. Predators’ toxin burdens influence their strategic decisions to eat toxic prey. Curr. Biol. 17, 1479–1483. ( 10.1016/j.cub.2007.07.064) [DOI] [PubMed] [Google Scholar]
- 8.Chatelain M, Halpin CG, Rowe C. 2013. Ambient temperature influences birds’ decisions to eat toxic prey. Anim. Behav. 86, 733–740. ( 10.1016/j.anbehav.2013.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpin CG, Skelhorn J, Rowe C. 2014. Increased predation of nutrient-enriched aposematic prey. Proc. R. Soc. B. 281, 20133255 ( 10.1098/rspb.2013.3255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endler JA, Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547. ( 10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 11.Summers K, Speed MP, Blount JD, Stuckert AMM. 2015. Are aposematic signals honest? A review. J. Evol. Biol. 28, 1583–1599. ( 10.1111/jeb.12676) [DOI] [PubMed] [Google Scholar]
- 12.Tullberg BS, Merilaita S, Wiklund C. 2005. Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc. R. Soc. B 272, 1315–1321. ( 10.1098/rspb.2005.3079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohlin T, Tullberg BS, Merilaita S. 2008. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Anim. Behav. 76, 577–584. ( 10.1016/j.anbehav.2008.02.012) [DOI] [Google Scholar]
- 14.Blount JD, Rowland HM, Drijfhout FP, Endler JA, Inger R, Sloggett JJ, Hurst GDD, Hodgson DJ, Speed MP. 2012. How the ladybird got its spots: effects of resource limitation on the honesty of aposematic signals. Funct. Ecol. 26, 334–342. ( 10.1111/j.1365-2435.2012.01961.x) [DOI] [Google Scholar]
- 15.Bohlin T, Gamberale-Stille G, Merilaita S, Exnerová A, Štys P, Tullberg BS. 2012. The detectability of the colour pattern in the aposematic firebug, Pyrrhocoris apterus: an image-based experiment with human ‘predators’. Biol. J. Linn. Soc. 105, 806–816. ( 10.1111/j.1095-8312.2011.01834.x) [DOI] [Google Scholar]
- 16.Campbell FW, Green DG. 1965. Optical and retinal factors affecting visual resolution. J. Physiol. 181, 576–593. ( 10.1113/jphysiol.1965.sp007784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottram JC. 1915. Some observations on pattern-blending with reference to obliterative shading and concealment of outline. Proc. Zool. Soc. Lond. 85, 679–692. ( 10.1111/j.1469-7998.1915.00679.x) [DOI] [Google Scholar]
- 18.Endler JA. 1978. A predator's view of animal color patterns. Evol. Biol. 11, 319–364. ( 10.1007/978-1-4615-6956-5_5) [DOI] [Google Scholar]
- 19.Marshall NJ. 2000. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. Lond. B 355, 1243–1248. ( 10.1098/rstb.2000.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caro T, Stankowich T, Kiffner C, Hunter J. 2013. Are spotted skunks conspicuous or cryptic? Ethol. Ecol. Evol. 25, 144–160. ( 10.1080/03949370.2012.744359) [DOI] [Google Scholar]
- 21.Barnett JB, Cuthill IC. 2014. Distance-dependent defensive coloration. Curr. Biol. 24, R1157–R1158. ( 10.1016/j.cub.2014.11.015) [DOI] [PubMed] [Google Scholar]
- 22.Stevens M, Ruxton GD. 2012. Linking the evolution and form of warning coloration in nature. Proc. R. Soc. B 279, 417–426. ( 10.1098/rspb.2011.1932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenward B, Wachtmeister CA, Ghirlanda S, Enquist M. 2004. Spots and stripes: the evolution of repetition in visual signal form. J. Theor. Biol. 230, 407–419. ( 10.1016/j.jtbi.2004.06.008) [DOI] [PubMed] [Google Scholar]
- 24.Hauglund K, Hagen SB, Lampe HM. 2006. Responses of domestic chicks (Gallus gallus domesticus) to multimodal aposematic signals. Behav. Ecol. 17, 392–398. ( 10.1093/beheco/arj038) [DOI] [Google Scholar]
- 25.Aronsson M, Gamberale-Stille G. 2013. Evidence of signaling benefits to contrasting internal color boundaries in warning coloration. Behav. Ecol. 24, 349–354. ( 10.1093/beheco/ars170) [DOI] [Google Scholar]
- 26.Aronsson M, Gamberale-Stille G. 2009. Importance of internal pattern contrast and contrast against the background in aposematic signals. Behav. Ecol. 20, 1356–1362. ( 10.1093/beheco/arp141) [DOI] [Google Scholar]
- 27.Barnett JB Redfern AS, Bhattacharyya-Dickson R, Clifton O, Courty T, Ho T, Hopes A, McPhee T, Merrison K, Owen R, Scott-Samuel NE, Cuthill IC. 2016. Stripes for warning and stripes for hiding: spatial frequency and detection distance. Behav. Ecol. 28, 373–381. ( 10.1093/beheco/arw168) [DOI] [Google Scholar]
- 28.Stevens M, Parraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237. ( 10.1111/j.1095-8312.2007.00725.x) [DOI] [Google Scholar]
- 29.Hart NS, Partridge JC, Cuthill IC. 1998. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J. Exp. Biol. 201, 1433–1446. [DOI] [PubMed] [Google Scholar]
- 30.Merbs SL, Nathans J. 1992. Absorption spectra of human cone pigments. Nature 356, 433–435. ( 10.1038/356433a0) [DOI] [PubMed] [Google Scholar]
- 31.Barnett JB, Scott-Samuel NE, Cuthill IC. 2016. Aposematism: balancing salience and camouflage. Biol. Letts. 12, 20160335 ( 10.1098/rsbl.2016.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelber A, Osorio D. 2010. From spectral information to animal colour vision: experiments and concepts. Proc. R. Soc. B 277, 1617–1625. ( 10.1098/rspb.2009.2118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao F, Cuthill IC. 2016. Background complexity and the detectability of camouflaged targets by birds and humans. Proc. R. Soc. B 283, 20161527 ( 10.1098/rspb.2016.1527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Therneau TM.2015. coxme: mixed effects cox models. R package version 2.2-5. See https://CRAN.R-project.org/package=coxme .
- 35.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 36.Barnett JB, Cuthill IC, Scott-Samuel NE. 2017. Data from: Distance-dependent pattern blending can camouflage salient aposematic signals. Dryad Digital Repository. ( 10.5061/dryad.2h6nf) [DOI] [PMC free article] [PubMed]
- 37.Homna A, Mappes J, Valkonen JK. 2015. Warning coloration can be disruptive: aposematic marginal wing patterning in the wood tiger moth. Ecol. Evol. 5, 4863–4874. ( 10.1002/ece3.1736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens M. 2007. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B 274, 1457–1464. ( 10.1098/rspb.2007.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Barnett JB, Cuthill IC, Scott-Samuel NE. 2017. Data from: Distance-dependent pattern blending can camouflage salient aposematic signals. Dryad Digital Repository. ( 10.5061/dryad.2h6nf) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data can be accessed from the Dryad data repository: http://dx.doi.org/10.5061/dryad.2h6nf [36].