Abstract
Studies on the evolution of cooperative behaviour are typically confined to understanding its adaptive value. It is equally essential, however, to understand its potential to evolve, requiring knowledge about the phenotypic consistency and genetic basis of cooperative behaviour. While previous observational studies reported considerably high heritabilities of helping behaviour in cooperatively breeding vertebrates, experimental studies disentangling the relevant genetic and non-genetic components of cooperative behaviour are lacking. In a half-sibling breeding experiment, we investigated the repeatability and heritability of three major helping behaviours performed by subordinates of the cooperatively breeding fish Neolamprologus pulcher. To experimentally manipulate the amount of help needed in a territory, we raised the fish in two environments differing in egg predation risk. All three helping behaviours were significantly repeatable, but had very low heritabilities. The high within-individual consistencies were predominantly due to maternal and permanent environment effects. The perceived egg predation risk had no effect on helping, but social interactions significantly influenced helping propensities. Our results reveal that developmentally plastic adjustments of provided help to social context shape cooperative phenotypes, whereas heritable genetic variation plays a minor role.
Keywords: cooperation, maternal effects, heritability, repeatability, cichlid
1. Introduction
Cooperation between individuals is considered a crucial step in evolutionary transitions from solitary life to complex social organization [1]. Animals cooperate during hunting, anti-predator behaviours, and territory defence, and particularly also during brood care [2,3]. In eusocial societies, in which assistance with tasks in the colony is obligatory, workers are mostly sterile and rarely disperse and breed on their own. Conversely, in cooperative breeding systems, in which subordinate individuals help raise offspring that have been produced by dominant breeders [4], the degree to which an individual assists the dominants with brood care is variable. This necessitates a decision on the part of the subordinate if and for how long to help and when to disperse. Previous studies on cooperatively breeding vertebrates have shown limited flexibility in this decision, with intrinsic differences among individual helpers in the extent to which they carry out different helping tasks, such as provisioning and protection of young, and territory defence and maintenance [5–8]. This raises questions about the role of genetic and environmental factors in shaping such individual variation in helping effort.
Potential sources of consistent inter-individual variation in helping propensity include genetic and developmental differences as well as differences in state [9], such as body size or hunger level. Furthermore, characteristics of the cooperation partner(s) can influence an individual's helping effort through social effects [10]. Such effects are expected especially for cooperation, because the cooperative behaviours of an individual are likely to be influenced by the behaviours of all interaction partners [11]. While there is evidence that the early social and ecological environment and current state influence the individual's propensity to help [8,12], the question of whether individuals might also differ genetically in their propensity to cooperate has received little experimental scrutiny [13–16]. However, knowledge about among-individual genetic variation is crucial to understand and predict micro-evolutionary scope and pattern of cooperative behaviour [17]. Estimates of the amount of genetic variation underlying cooperative behaviour provide information on the evolutionary potential of a population [18], and estimates of genetic correlations among different helping traits provide insights into whether they have a shared genetic basis and the potential to coevolve [17].
To date, few studies have quantified heritable variation underlying cooperative or helping behaviours in cooperative breeders, and those that did have found remarkably high estimates of heritability. For instance, in a pedigree-based study of a wild population of western bluebirds (Sialia mexicana), heritable differences have been estimated to account for 76% of the phenotypic variance in alloparental helping [19]. Similarly, a study on delayed dispersal in red wolves (Canis rufus), a prerequisite for becoming a helper in the pack, was found to have a strong heritable component (nearly 100%) in males but not in females [20]. The heritabilities for mass of workers, gynes, and males in the ant Temnothorax curvispinosus, which can be understood as the outcome of a cooperative behaviour because workers raise the offspring of a queen, range between 37 and 99%. Finally, studies of human twins suggest significant heritabilities for cooperative behaviour in the trust game (between 10 and 32%) [21]. Importantly, however, observational studies of natural populations are inherently limited in their ability to tease apart genetic inheritance from non-genetic effects of the (maternal) environment [22,23]. Adequately controlling for transgenerational environmental effects might therefore substantially reduce heritability estimates [24]. Carefully planned breeding experiments control confounding sources of variation. For instance, employing a half-sibling breeding design under standardized environmental conditions enables a better separation of additive genetic, maternal, and common environment effects shaping variation in helping behaviour.
Here, we present a quantitative genetic analysis of variation in the three major helping behaviours of the cooperatively breeding cichlid fish Neolamprologus pulcher in an experimental setting. In the wild, groups consist of a dominant breeder pair that monopolizes reproduction and several subordinate helpers that delay dispersal. Helpers assist the dominant pair with direct brood care (egg cleaning), territory maintenance (removal of sand), and with territory defence against fish and egg predators as well as against space competitors [25–27]. We used a paternal half-sibling design and bred fish in the laboratory from wild-caught parents. We quantified our test fish's effort in three major alloparental brood care behaviours: the propensity to clean eggs, the propensity to remove sand from the breeding chamber, and the amount of defence behaviours directed against an egg predator. First, we investigated whether body size, social interactions, previous exposure to egg predators, or clutch size have an impact on helping behaviour. Second, we disentangle (i) the effects of additive genetic variation, (ii) the non-genetic effects of the individual environment that are constant across repeated measurements of this individual, (permanent environment, also includes non-additive genetic effects), (iii) maternal effects, (iv) social effects of the cooperation partner, (v) effects of the shared rearing environment (common environment), and (vi) the effect of individual predators (in the case of defence behaviour) on the phenotypic variation in helping behaviours. This decomposition provides a measure of the fraction of the total phenotypic variance that is attributable to additive genetic effects, i.e. the narrow-sense heritability (h2). Furthermore, we provide a measure of individual consistency, i.e. the repeatability (R).
2. Material and methods
(a). Study animals, breeding design, and behavioural tests
We used laboratory-bred F1 offspring of parents caught shortly before the start of the experiment in Lake Tanganyika, Zambia. This ensured that our study population captured the full spectrum of genetic variation found in the lake. All fish were kept under similar temperature, feeding, and light conditions. Experimental families were randomly assigned to tanks (for further methodological details, see the electronic supplementary material).
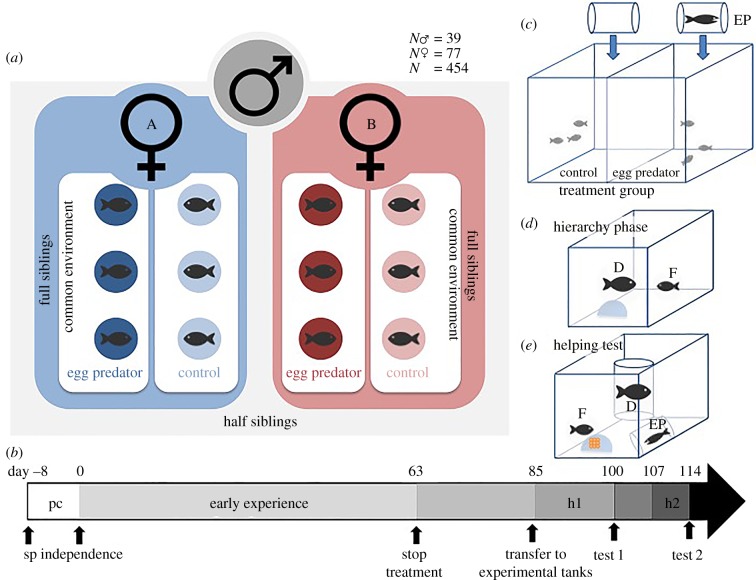
We carried out a nested paternal half-sibling design [28], in which we mated each male (N = 39) to a unique set of two randomly chosen females (N = 77) from the same population, which in total resulted in 3 175 offspring (figure 1a; see the electronic supplementary material for further details). One male died after spawning the first clutch, and hence, no half-sibling group could be produced. The parents were removed 8–10 days after spawning, when they no longer provide direct parental care (defined as ‘day 0’).
Figure 1.
The breeding design, experimental timeline, and illustrations of the breeding and experimental tanks used to obtain phenotypic data on helping behaviours in juvenile helpers. (a) Fish were bred in a paternal nested half-sibling breeding design. (b) From spawning (sp) onwards until independence, the fry were kept with their parents (parental care phase, pc). After the removal of the parents on day 0, each clutch was split into two equal-sized groups that were randomly assigned to one of the egg predator treatments (c) that ended at day 63 (early experience). Between days 85 and 100, test fish were housed in experimental tanks together with a dominant individual to establish a hierarchy (hierarchy phase, h1, see (d), upper panel), when they were tested for their cooperative propensity (test1). To estimate the permanent environment effect, a component of the repeatability of helping behaviours, 86 of the 454 individuals were tested again on day 114 (test2), after a 7-day hierarchy phase (h2). (c) During the early experience phase, a transparent tube with a live egg predator individual or an empty tube (control) was inserted into the breeding tank five times a week for 10 min. (d) Before testing, we confined the dominant (D) in a vertical transparent tube (hierarchy phase, upper panel). (e) At the beginning of the behavioural test, we fixed a portion of a clutch (orange) spawned by unrelated lab-stock pairs on a piece of transparent plastic foil to the inside of the shelter (blue, lower panel). We directly observed all instances of egg cleaning and digging of the focal fish (F), as well as any interactions with the dominant individual. After 10 min, an egg predator (EP) in a transparent plastic container was introduced, after which all the above-described behaviours and all instances of aggression towards the egg predator were recorded for another 10 min. (Online version in colour.)
For the following 63 days, a period when N. pulcher juveniles manifest significant developmental plasticity with respect to social and anti-predator skills [29], one randomly chosen half of each clutch was exposed to the sympatric unspecialized egg predator Telmatochromis vittatus [30,31]. Juveniles in the egg predator treatment group were exposed to an egg predator for 10 min twice a week on randomly chosen days and times. Juveniles in the control group received the same handling but without exposure to an egg predator. Between day 64 and day 85, no treatments took place (neutral phase 1, figure 1b). In the final hierarchy phase, selected test fish were housed in the experimental tanks together with a larger, unrelated territory owner for around 14 days (figure 1d, upper panel) to ensure that the test fish adopted a subordinate status, as only subordinate individuals show alloparental behaviour [32].
In the behavioural test, we recorded egg cleaning, digging, defence and submissive behaviours of the test fish, and the activity of the egg predator according to the ethogram provided in electronic supplementary material, table S2. Further details of the experimental procedures are described in the electronic supplementary material, figure S1.
(b). Statistical analyses
A visual check of the histograms of the raw data of cleaning and digging suggested high zero inflation (electronic supplementary material, figure S2). Thus, we dichotomized the counts of egg cleaning and digging, and modelled these variables as binary responses ‘cleaning propensity’ and ‘digging propensity’ in logit models, where 1 meant that the individual performed the behaviour at least once during the test and 0 that it did not. Defence had a higher incidence (90%) and was thus less zero-inflated, so we modelled the counts of defence behaviours (amount of defence) with a Poisson error family. All models included the full number of tests (N = 454 first tests and N = 86 second tests), but we removed 59 individuals who did not respond to the test stimulus in the behavioural test (see electronic supplementary material for details). Thus, the models included a total of 481 observations (N = 399 first tests and N = 82 second tests). All statistical analyses were carried out in R v. 3.2.0 [33] and calculations were performed on UBELIX, the high-performance computing cluster at the University of Bern.
(c). Predictors of helping behaviours
To determine the fixed effects to be included in the quantitative genetic mixed models (see below), we fitted a series of generalized linear mixed effects models (GLMMs) in the ‘lme4’ package v. 1.1–12 in R [34] to test which environmental factors influenced the three helping behaviours (electronic supplementary material, table S3). We subsequently applied a model selection approach and ranked models with all possible combinations of the predictors (mentioned in the electronic supplementary material) according to Akaike information criterion corrected for small sample sizes (AICc) value (‘dredge’ function in R package ‘MuMIn’ v. 1.15.6 [35]). Following the ‘nesting rule’ presented in [36], we selected the final model from a ‘confidence set’ consisting of all models within ΔAICc ≤ 6. The ‘nesting rule’ seeks to avoid the selection of overly complex models by excluding models from the candidate set that contain more parameters than a model with a lower AIC value. In the case of several non-nested models in the confidence set, preference was given to the model with the lowest AICc. We report the confidence set for each model together with their marginal R2 (the variance explained by fixed effects) and the conditional R2 (the variance explained by both fixed and random effects) [37] in the electronic supplementary material, table S4.
(d). Decomposition of variation in helping behaviour
We estimated genetic and environmental effects on the propensities to clean and dig and the amount of defence shown towards an egg predator using a Bayesian animal model (‘MCMCglmm’ package v. 2.22.1 in R [38]). We assumed that the wild-caught parents were unrelated. We specified animal models using a probit-link function to analyse the dichotomous variables cleaning and digging propensity (‘threshold’ model) and a log-link function for the amount of defence (Poisson model). For cleaning and digging propensity, we estimated the variance attributable to additive genetic effects (VA), permanent environment effects (including non-additive genetic effects; VPE), maternal effects (VM), common environment effects (VCE), and social effects (i.e. identity of the dominant individual in the helping trials; VS). In the model for the amount of defence, we furthermore included the effect of the egg predator's identity (VVID). Finally, to control for potential differences among populations, we included the population of parents' origin in the models (VPop). To see whether the inclusion of covariates as fixed effects had an impact on the estimates of the variance components, we additionally fitted conditional models with the set of mean-centred and scaled predictors that were contained in the selected models in the previous step (see electronic supplementary material, tables S5 and S6). Details on model parameterization are given in the electronic supplementary material (Variance components of helping behaviours section). Models were run with parameter-expanded priors and the residual variation was fixed to 1 for the threshold models. Models were run for 2 × 106 iterations and the first 105 iterations of the resulting chain were discarded as burn-in to ensure that the chain had converged. The remaining chain was sampled at an interval of 1 000 iterations, yielding a posterior distribution of 1 900 estimates. The mixing of the chain was evaluated by inspecting the trace plots and by checking the convergence of the chain (Heidelberger diagnostic), the autocorrelation of adjacent samples, and the effective sample size (electronic supplementary material, table S5). To ensure the stability of estimates, we ran three additional models with exactly the same model structure for each helping behaviour (electronic supplementary material, table S7).
Estimates derived from a GLMM framework have to be transformed to the original data scale for inference. Thus, we computed phenotypic means and variances, additive genetic variance (VA,obs) and heritability, on the observed data scale by looping the ‘QGparams’ function in the ‘QGglmm’ package V 0.5 [39] over the posterior distributions of the models. The proportion of variance explained by each random effect was calculated as the posterior distribution of the respective variance component (e.g. VM or VPE) divided by the posterior distributions of the phenotypic variance (VP), defined as the sum of all variance components, including the residual variance. We assumed a distribution-specific residual variance on the expected data scale: 1 for threshold models with a probit-link and log(1/exp(intercept) + 1) for the Poisson model [40]. R2 was calculated following the study of Nakagawa and Schielzeth [37] and using R code for non-Gaussian MCMCglmm models (http://www.i-deel.org/publications.html). Repeatability, the proportion of variance explained by among-individual variation, was calculated as the variance explained by the sum of VA, VPE, VM, and VCE divided by VP [41,42]. The permanent environment effect VPE refers to the variance in helping behaviours that is due to the unique environment experienced by individuals, which might lead, together with additive genetic, maternal, and common environment effects, to a consistency in behaviours through time. We present the modes of the posterior distributions resulting from the models together with the 95% credibility intervals (highest posterior density intervals). Because variance components are constrained to be positive, and credibility intervals will hence never include zero, we inspected the shape of the posterior distributions visually (as described in the electronic supplementary material).
3. Results
We observed egg cleaning, digging, and defence behaviours of 454 fish in the behavioural tests. Approximately half of the test fish did not show egg cleaning and digging behaviours (56 and 46%, respectively), but 90% defended against the egg predator (electronic supplementary material table S1 and figure S2).
(a). Predictors of helping behaviours
Model selection (see ‘Material and methods’ and electronic supplementary material, table S4 for potential predictors and procedures) yielded a final model that contained two predictors of ‘cleaning propensity’: the size of the clutch a test fish was exposed to in the helping test and the amount of submission it displayed towards the dominant individual (model 18 in the electronic supplementary material, table S4a): larger clutches were more likely to be cleaned, whereas test fish that showed more submissive behaviours were less likely to clean. Growing up with or without egg predators and the size of the test fish relative to its siblings (relative size) did not influence cleaning propensity. Random and fixed effects explained 24% of the variance (conditional R2), whereas the fixed effects alone explained only 7% (marginal R2). The final model for ‘digging propensity’ contained ‘clutch size’, ‘relative size’, ‘submission’, and ‘acceptance status’ as predictor variables (model 16 in the electronic supplementary material, table S4b). The probability to dig increased with clutch size and the test fish's relative body size, and it decreased with the amount of submissive behaviours. Test fish with acceptance status ‘not determined’ and ‘tolerated’ dug less than those with status ‘accepted’ (Tukey post hoc comparisons: ‘not determined’ versus ‘accepted’: estimate = −1.13, s.e. = 0.39, z = −2.9, p = 0.035; ‘tolerated’ versus ‘accepted’: estimate = −1.57, s.e. = 0.50, z = −3.15, p = 0.018). This model explained 26% of the variance, of which the fixed effects explained only 7%. The final model for ‘amount of defence’ contained ‘egg predator activity’, ‘submission’, and ‘acceptance status’ as predictors (model 53 in the electronic supplementary material, table S4c). As in the two previous models, submission had an attenuating effect. Furthermore, active egg predator individuals were attacked more than inactive ones, and ‘fully accepted’ fish defended more than ‘accepted’ (‘fully accepted’ versus ‘accepted’: estimate = 0.58, s.e. = 0.18, z = 3.24, p = 0.013) and ‘not determined’ (‘not determined’ versus ‘fully accepted’: estimate = −0.12, s.e. = 0.19, z = −3.16, p = 0.017). The other pairwise comparisons for digging propensity and the amount of defence were not significant. Most notably, the ‘evicted’ fish in our data set were not less likely to clean eggs, dig, or defend. The selected model explained 56% of the variance, and the fixed effects alone explained 4%.
(b). Variance components of helping behaviours
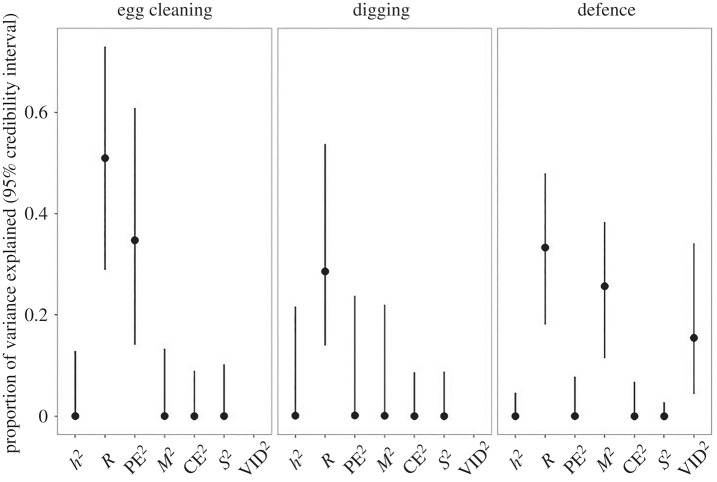
The variance decomposition of helping behaviours using the animal model showed that repeatability, i.e. the variance that can be attributed to differences between individuals, was substantial for cleaning propensity, and moderate for digging propensity and the amount of defence (table 1 and figure 1). However, the additive genetic variance, heritability (the proportion of the phenotypic variance that is explained by additive genetic variation), was very low for all helping behaviours (table 1). Growing up in the same social group (common environment effect, VCE) and the identity of the dominant fish (social effect, VS) both explained very little to none of the observed variation. For the number of defence actions towards the egg predator, there was a moderate maternal effect (VM), and the egg predator's identity (egg predator effect, VVID) had a small effect (figure 2). These results were reflected in the large amount of overlap between the real posterior distributions with null distributions derived from random draws of VA, VCE, and VS (see electronic supplementary material, table S8). By contrast, the variance component of individual identity (VPE) for egg cleaning and the maternal and egg predator identity (VM and VVID) for defence had less than 2% overlap with the null distributions, and hence, these effects can be considered as statistically significant. The maternal identity variance component (VM) of digging overlapped only 6% with the null distribution, even though the posterior mode of the estimate was rather low (VM = 0.0014 (2.9 × 10−6, 0.246)). Interestingly, despite the posterior modes of VA, VPE, VM, and VCE all being close to zero for digging, the repeatability estimate, obtained by adding up the posterior distributions of these variance components, was significant. This is attributable to the posterior distributions of VA and VM being negatively correlated (Pearson's correlation: r = −0.28, CI = (−0.32, −0.24)), suggesting that the model had difficulties in partitioning the variance between these two components: for each single model of the posterior distribution, the variance was either attributed to VA or VM. This indicates that, even though the analysis produced a reliable and significant estimate of repeatability, the pattern in the data for digging did not allow disentangling the effects beyond that level, i.e. at the level of single variance components.
Table 1.
Quantitative genetic parameters for models including only random effects computed with the ‘QGglmm’ package in R [39], except for repeatability that was calculated as (VA + VPE + VM + VCE)/VP (see the Material and methods section for a detailed description). Estimates are the modes of the posterior distributions, which are presented together with their 95% credibility intervals.
| egg cleaning | digging | defence | |
|---|---|---|---|
| trait mean | 0.42 (0.36, 0.51) | 0.54 (0.45, 0.61) | 34.94 (17.3, 65.6) |
| phenotypic variance (VP) | 0.25 (0.23, 0.25) | 0.25 (0.24, 0.25) | 13 144 (1287, 39611) |
| add. genet. variance (VA) | 1 × 10−4 (8 × 10−10, 3 × 10−2) | 3 × 10−4 (1 × 10−9, 0.05) | 7.0 (1 × 10−4, 542.0) |
| heritability (h2) | 6 × 10−4 (3 × 10−9, 0.13) | 1 × 10−3 (1 × 10−9, 0.22) | 4 × 10−4 (6 × 10−9, 0.05) |
| permanent environment (VPE) | 0.40 (0.13, 0.60) | 0.001 (8.1 × 10−8, 0.26) | 5.6 × 10−4 (1.9 × 10−8, 0.08) |
| repeatability (R) | 0.51 (0.29, 0.73) | 0.29 (0.14, 0.54) | 0.33 (0.18, 0.48) |
| coefficient of determination (R2) | 0.81 | 0.70 | 0.96 |
Figure 2.
The proportion of variance of helping behaviours explained by additive genetic effects (heritability, h2), by consistent between-individual differences (repeatability, R), by the permanent individual environment (PE2, estimated from the variance component of individual identity of those individuals tested twice), by maternal identity (maternal effects, M2), by the shared environment during growing up (common environment, CE2), by the identity of the territory owner (social effects, S2), and by the effect of the egg predator's identity (VID2). Results are presented as point estimates together with their 95% credible interval.
4. Discussion
(a). Low additive genetic variance of helping behaviours
Here, we present a measure of the evolutionary potential of vertebrate cooperative behaviour, variance-standardized heritability (h2), in an experiment controlling for confounding non-genetic sources of resemblance among relatives. Our results demonstrate that albeit repeatable, heritabilities of the three major forms of helping behaviours performed by subordinate N. pulcher—egg cleaning, keeping the breeding chamber clear from sand (digging), and defence of the brood against egg predators—were close to zero. Thus, the standing genetic variation in these behaviours was very low, and hence, the response to selection is predicted to be small. The low heritabilities observed here are in line with the relatively low heritabilities that have been reported for a range of traits closely associated with fitness (i.e. behaviours and life-history traits) [43,44]. Although this pattern can arise if fitness-related traits show a disproportionally large amount of variance attributable to non-additive genetic variance (e.g. dominance or epistasis [45]), indirect genetic effects [46], or residual variance [47], this is an unlikely explanation in our case, given the posterior modes of zero for VA. In this study, low heritabilities are likely to reflect low absolute levels of additive genetic variance.
Our results contrast the few other studies investigating the genetic basis of helping behaviours in cooperative breeders, which have reported high heritabilities. For example, heritable genetic variation was reported to have a strong influence on the probability of being a helper and receiving help in western bluebirds [19], and the age at dispersal in the cooperatively breeding red wolf [20]. This variation in heritability might reflect differences in the relative importance of environmental variance and thus the degree of flexibility in helping behaviours that different populations experience. In both studies, however, heritability estimates were obtained from observations of field populations and non-genetic transgenerational effects were not accounted for. For instance, the effects of inheriting a territory of a certain quality (which potentially entails differences in the ability to attract helpers or to influence the delay of dispersal) and other parental effects could not be separated from heritable genetic effects. This could also have potentially caused the substantially higher heritability estimates in those studies compared to our study [23,48].
(b). Flexible adjustment of helping strategies
As cooperative behaviours need to be fine-tuned to a specific situation, they require a certain degree of flexibility in response to environmental cues during development. This includes the permanent environment [12] and information from mothers to offspring about her environment (maternal effects [49]), as well as any short-term changes in the costs and benefits of helping versus dispersing in the current environment [50,51]. Hence, we expect low to moderate heritability, allowing for selection for flexible rules to adjust helping propensity to the environment.
Although the lack of additive genetic variation underlying helping behaviour implies a certain inter-generational plasticity, our finding that helping behaviours in N. pulcher are repeatable shows that individuals are consistent in their behaviour throughout their helper stage, and hence show little flexibility on a short timescale. This consistency can arise during ontogeny when juveniles integrate information on their social and ecological environment and their own condition to decide whether to follow a breeder or a helper strategy. It has been hypothesized that the existence of ‘alternative cooperative phenotypes' promotes the evolution and maintenance of cooperative breeding because individuals reliably signal their commitment to helping [52]. The existence of such stable helper personalities has been shown in banded mongooses, Mungos mungo [8]. In N. pulcher, individuals who delay dispersal and stay in their natal territory have to act submissively towards the dominant breeders to remain accepted in their natal group. The dispersing strategy, on the other hand, is characterized by higher alloparental effort (egg cleaning) but lower investment in submissive displays [12]. This is consistent with our finding that submissive behaviour is negatively correlated with helping behaviours in all three cases, and helping behaviour varied consistently between individuals. We also found that early experience with an egg predator had no effect on the propensity to help. Thus, one may speculate that social experiences during ontogeny affecting submissive behaviour may ultimately influence helping, rather than the direct cues indicating the need of help (e.g. threat of egg predation).
Nevertheless, unlike social insects that diverge very early and irreversibly into different developmental trajectories, cooperatively breeding vertebrates are thought to maintain a certain degree of flexibility throughout their development because subordinates potentially become breeders themselves later in life. The degree of this plasticity that enables individuals to switch between strategies is likely to vary among species [53]. Even though the repeatability estimates of all helping behaviours were considerable in our study, there was scope for individuals to react to the challenges posed by their current situation. For instance, the identity of the egg predator in the behavioural test explained approximately 15% of the phenotypic variation of defence (figure 2), meaning that fish could flexibly adjust their defence effort to the particular egg predator individual they were confronted with.
(c). Decomposing individual variation
Our repeatability estimate included three different effects apart from additive genetic variance, each reflecting a different source of information that an individual can base a developmental decision on. First, the permanent environment effect, which we found to be relatively important, includes experiences that occur during individual ontogeny. This effect is specific to each individual and might last throughout its life. For instance, the social niche [54,55] or, more generally, social interaction dynamics in a family [56] may influence the specialization into ‘cleaner types’ that might persist into adulthood [12]. Second, the common environment of siblings exposed to the same egg predator treatment group could have resulted in a higher similarity of group members compared with others, including full siblings in the other treatment group. As all fish were kept under standardized conditions, it is unlikely that abiotic factors contributed to these between-group differences. More likely, social dynamics might result in differences of helping propensity between groups. However, the common environment effect was not significant for any of the helping behaviours, and hence, we did not find evidence for a group-level helping propensity. Instead, maternal full siblings resembled each other in terms of their defence effort (and probably digging propensity), even if they were in different treatment groups. This suggests that females influenced levels of helping in their offspring, possibly either through egg effects or through their behaviour towards the fry. Although we removed the parents a week after their eggs hatched, mothers could have influenced their offspring in the period before this removal. For example, mothers can transmit information either via the egg by adjusting the provisioning of the egg with nutrients or hormones [57], or via epigenetic modifications in the offspring induced by maternal behaviour [58]. The type of information transmitted from the mother to the offspring could be based on the predation risk and sand load in the mother's current or previous environment. However, although the mothers of our test fish were caught in the field before the start of the breeding experiment and were kept under standardized, predator-free environmental conditions upon arrival in our facility, we did not find evidence for an adaptive maternal effect concerning fish or egg predation risk or sand load in the populations of origin of our test fish's parents in Lake Tanganyika (see electronic supplementary material). Furthermore, we found a maternal correlation between egg cleaning and defence behaviours (electronic supplementary material, table S9). A potential mechanism that causes this correlation could be a maternal effect on the expression of a prolactin-like hormone (tiPRLII), which has been suggested to be implicated in parental behaviour in cichlid fish [59] and other teleosts [60].
(d). Social effects on helper phenotypes
Social interactions are likely to influence cooperative behaviours [16]. If those social effects have a genetic basis (indirect genetic effects [61]), they can alter the cooperative trait's response to selection, also in the absence of direct genetic effects. These indirect genetic effects are expected to arise when carers are sensitive to the helping behaviour of others, for example, when parents negotiate the amount of care they provide [62], when helpers enable breeders to reduce their effort [63] or when breeders coerce subordinates into helping [64]. Both reduction of maternal effort and coercion have been demonstrated in N. pulcher [65,66]. In this study, we investigated phenotypic social effects by including the identity of the dominant territory owner in the models, but the dominant's identity did not explain any of the variation in egg cleaning, digging, or defence behaviours. Still, we found indirect evidence that cooperative behaviours were influenced by the dominant's phenotype, because the test fish's submissive behaviour, which was highly correlated with the dominant's aggression (Spearman's ρ = 0.69, p < 0.0001), was an important predictor for all helping behaviours.
5. Conclusion
This study showed that N. pulcher helpers exhibit both long-term plasticity and short-term flexibility when adjusting their amount of alloparental brood care to environmental conditions. Hence, the developmental and genetic architecture of cooperative behaviours in this species might be more complex than previously thought. Likewise, approaches that do not take into account parental and other indirect genetic effects are unlikely to reflect the actual response to selection for a number of reasons [67], in particular because of its strong focus on additive genetic variance [68]. These effects have the potential to alter the rate of evolution, especially when their influence spans more than one generation (i.e. maternal effects, [49]).
Supplementary Material
Acknowledgements
We thank Jana Keller, Ahana Fernandez, Dario Bayani, Jonas Richner, Evi Zwygart, and Tanja Schreier for help with data collection and animal caretaking. Pierre de Villemereuil provided support and R code for the multivariate analyses, and Yimen Araya Ajoy helped with the null distributions. Dario Josi and Joachim Frommen provided data on sand load and predation risk in field populations of N. pulcher. Bernhard Voelkl, Leif Engqvist, Christina Riehl, and two anonymous reviewers provided comments that improved the manuscript significantly.
Ethics
All procedures were conducted under the license 52/12 of the Veterinäramt Bern and adhered to the guidelines of the Association for the Study of Animal Behaviour.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4b45d [69].
Authors' contributions
C.K., M.K., and B.T. conceived and designed experiments. C.K. performed experiments, C.K. and E.P. analysed data and C.K. wrote the first draft of the manuscript. All authors contributed to revisions.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the ‘ProDoc’ program of the Swiss National Science Foundation (SNF, projects PDFMP3_137196 and 31003A_156881 to B.T.), and the ‘120% support grant’ to C.K. of the University of Bern.
References
- 1.Bourke AFG. 2011. Principles of social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Wilson EO. 1975. Sociobiology: the new synthesis. Harvard, UK: Harvard University Press. [Google Scholar]
- 3.Dugatkin LA. 1997. Cooperation among animals: an evolutionary perspective. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Bergmüller R, Johnstone RA, Russell AF, Bshary R. 2007. Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Processes 76, 61–72. ( 10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 5.English S, Nakagawa S, Clutton-Brock TH. 2010. Consistent individual differences in cooperative behaviour in meerkats (Suricata suricatta). J. Evol. Biol. 23, 1597–1604. ( 10.1111/j.1420-9101.2010.02025.x) [DOI] [PubMed] [Google Scholar]
- 6.Le Vin AL, Mable BK, Taborsky M, Heg D, Arnold KE. 2011. Individual variation in helping in a cooperative breeder: relatedness versus behavioural type. Anim. Behav. 82, 467–477. ( 10.1016/j.anbehav.2011.05.021) [DOI] [Google Scholar]
- 7.Carter AJ, English S, Clutton-Brock TH. 2014. Cooperative personalities and social niche specialization in female meerkats. J. Evol. Biol. 27, 815–825. ( 10.1111/jeb.12358) [DOI] [PubMed] [Google Scholar]
- 8.Sanderson JL, Stott I, Young AJ, Vitikainen EIK, Hodge SJ, Cant MA. 2015. The origins of consistent individual differences in cooperation in wild banded mongooses, Mungos mungo. Anim. Behav. 107, 193–200. ( 10.1016/j.anbehav.2015.06.022) [DOI] [Google Scholar]
- 9.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams MJ, Robinson MR, Mannarelli M, Hatchwell BJ. 2015. Social genetic and social environment effects on parental and helper care in a cooperatively breeding bird. Proc. R. Soc. B 282, 20150689 ( 10.1098/rspb.2015.0689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGlothlin JW, Wolf JB, Brodie ED, Moore AJ. 2014. Quantitative genetic versions of Hamilton's rule with empirical applications. Phil. Trans. R. Soc. B 369, 20130358 ( 10.1098/rstb.2013.0358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer S. 2014. The influences of early and current environment on social and antipredator behaviour in a cooperatively breeding cichlid. PhD thesis at the University of Bern, Switzerland, Institute of Ecology and Evolution, Dept of Behavioural Ecology.
- 13.Komdeur J. 2006. Variation in individual investment strategies among social animals. Ethology 112, 729–747. ( 10.1111/j.1439-0310.2006.01243.x) [DOI] [Google Scholar]
- 14.Aubin-Horth N, Renn SCP. 2009. Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol. Ecol. 18, 3763–3780. ( 10.1111/j.1365-294X.2009.04313.x) [DOI] [PubMed] [Google Scholar]
- 15.Bell AM, Aubin-Horth N. 2010. What can whole genome expression data tell us about the ecology and evolution of personality? Phil. Trans. R. Soc. B 365, 4001–4012. ( 10.1098/rstb.2010.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleakley BH, Wolf JB, Moore AJ. 2010. The quantitative genetics of social behaviour. In Social behaviour: genes, ecology and evolution (eds Székely T, Moore AJ, Komdeur J), pp. 29–54. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Kruuk L, Slate J, Wilson A. 2008. New answers for old questions: the evolutionary quantitative genetics of wild animal populations. Annu. Rev. Ecol. Evol. Syst. 39, 525–548. ( 10.1146/annurev.ecolsys.39.110707.173542) [DOI] [Google Scholar]
- 18.Hansen TF, Pélabon C, Houle D. 2011. Heritability is not evolvability. Evol. Biol. 38, 258–277. ( 10.1007/s11692-011-9127-6) [DOI] [Google Scholar]
- 19.Charmantier A, Keyser AJ, Promislow DEL. 2007. First evidence for heritable variation in cooperative breeding behaviour. Proc. R. Soc. B 274, 1757–1761. ( 10.1098/rspb.2007.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparkman AM, Adams JR, Steury TD, Waits LP, Murray DL. 2012. Evidence for a genetic basis for delayed dispersal in a cooperatively breeding canid. Anim. Behav. 83, 1091–1098. ( 10.1016/j.anbehav.2012.01.041) [DOI] [Google Scholar]
- 21.Cesarini D, Dawes CT, Fowler JH, Johannesson M, Lichtenstein P, Wallace B. 2008. Heritability of cooperative behavior in the trust game. Proc. Natl Acad. Sci. USA 105, 3721–3726. ( 10.1073/pnas.0710069105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruuk LE. B. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Villemereuil P, Gimenez O, Doligez B. 2013. Comparing parent-offspring regression with frequentist and Bayesian animal models to estimate heritability in wild populations: a simulation study for Gaussian and binary traits. Methods Ecol. Evol. 4, 260–275. ( 10.1111/2041-210X.12011) [DOI] [Google Scholar]
- 24.Hadfield JD, Heap EA, Bayer F, Mittell EA, Crouch NM. A. 2013. Disentangling genetic and prenatal sources of familial resemblance across ontogeny in a wild passerine. Evolution 67, 2701–2713. ( 10.1111/evo.12144) [DOI] [PubMed] [Google Scholar]
- 25.Taborsky M. 1984. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252. ( 10.1016/S0003-3472(84)80241-9) [DOI] [Google Scholar]
- 26.Balshine S, Leach B, Neat F, Reid H, Taborsky M. 2001. Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140. ( 10.1007/s002650100343) [DOI] [Google Scholar]
- 27.Bergmüller R, Heg D, Taborsky M. 2005. Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B 272, 325–331. ( 10.1098/rspb.2004.2960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conner JK, Hartl DL. 2004. A primer of ecological genetics. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 29.Arnold C, Taborsky B. 2010. Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim. Behav. 79, 621–630. ( 10.1016/j.anbehav.2009.12.008) [DOI] [Google Scholar]
- 30.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394. ( 10.1016/j.anbehav.2010.10.004) [DOI] [Google Scholar]
- 31.Ochi H, Yanagisawa Y. 1998. Commensalism between cichlid fishes through differential tolerance of guarding parents towards intruders. J. Fish Biol. 52, 985–996. ( 10.1111/j.1095-8649.1998.tb00598.x) [DOI] [Google Scholar]
- 32.von Siemens M. 1990. Broodcare or egg cannibalism by parents and helpers in Neolamprologus brichardi (Poll 1986) (Pisces:Cichlidae): a study on behavioural mechanisms. Ethology 84, 60–80. ( 10.1111/j.1439-0310.1990.tb00785.x) [DOI] [Google Scholar]
- 33.R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/. [Google Scholar]
- 34.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 35.Bartoń K. 2016. MuMIn: Multi-Model Inference. R package version1.15.6. https://CRAN.R-project.org/package=MuMIn.
- 36.Richards SA, Whittingham MJ, Stephens PA. 2011. Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav. Ecol. Sociobiol. 65, 77–89. ( 10.1007/s00265-010-1035-8) [DOI] [Google Scholar]
- 37.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 38.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.1002/ana.22635)20808728 [DOI] [Google Scholar]
- 39.de Villemereuil P, Schielzeth H, Nakagawa S, Morrissey M. 2016. General methods for evolutionary quantitative genetic inference from generalised mixed models. Genetics 204, 1281–1294. ( 10.1534/genetics.115.186536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 41.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 42.Taylor RW, Boon AK, Dantzer B, Réale D, Humphries MM, Boutin S, Gorrell JC, Coltman DW, McAdam AG. 2012. Low heritabilities, but genetic and maternal correlations between red squirrel behaviours. J. Evol. Biol. 25, 614–624. ( 10.1111/j.1420-9101.2012.02456.x) [DOI] [PubMed] [Google Scholar]
- 43.Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity (Edinb.) 59, 181–197. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 44.Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289. ( 10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 45.Meffert LM, Hicks SK, Regan JL. 2002. Nonadditive genetic effects in animal behavior. Am. Nat. 160, S198–S213. ( 10.1086/342896) [DOI] [PubMed] [Google Scholar]
- 46.Wolf JB, Brodie ED, Cheverud JM, Moore AJ, Wade MJ. 1998. Indirect genetic effects. Trends Ecol. Evol. 13, 64–69. ( 10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 47.Merilä J, Sheldon BC. 2000. Lifetime reproductive success and heritability in nature. Am. Nat. 155, 301–310. ( 10.1086/303330) [DOI] [PubMed] [Google Scholar]
- 48.Kruuk LEB, Hadfield JD. 2007. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903. ( 10.1111/j.1420-9101.2007.01377.x) [DOI] [PubMed] [Google Scholar]
- 49.Räsänen K, Kruuk LEB. 2007. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421. ( 10.1111/j.1365-2435.2007.01246.x) [DOI] [Google Scholar]
- 50.Koenig WD, Pitelka FA, Carmen WJ, Mumme RL, Stanback MT. 1992. The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 67, 111–150. ( 10.1086/417552) [DOI] [PubMed] [Google Scholar]
- 51.Stiver KA, Dierkes P, Taborsky M, Balshine S. 2004. Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 65, 91–105. ( 10.1111/j.0022-1112.2004.00427.x) [DOI] [Google Scholar]
- 52.Bergmüller R, Schürch R, Hamilton IM. 2010. Evolutionary causes and consequences of consistent individual variation in cooperative behaviour. Phil. Trans. R. Soc. B 365, 2751–2764. ( 10.1098/rstb.2010.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.English S, Browning LE, Raihani NJ. 2015. Developmental plasticity and social specialization in cooperative societies. Anim. Behav. 106, 37–42. ( 10.1016/j.anbehav.2015.05.006) [DOI] [Google Scholar]
- 54.Bergmüller R, Taborsky M. 2010. Animal personality due to social niche specialisation. Trends Ecol. Evol. 25, 504–511. ( 10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 55.Edwards HA, Dugdale HL, Richardson DS, Komdeur J, Burke T. 2016. Exploration is dependent on reproductive state, not social state, in a cooperatively breeding bird. Behav. Ecol. 27, 1889–1896. ( 10.1093/beheco/arw119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kölliker M. 2005. Ontogeny in the family. Behav. Genet. 35, 7–18. ( 10.1007/s10519-004-0852-9) [DOI] [PubMed] [Google Scholar]
- 57.Bernardo J. 1996. Maternal effects in animal ecology. Am. Sci. 36, 83–105. [Google Scholar]
- 58.Weaver ICG, Meaney MJ, Szyf M. 2006. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl Acad. Sci. USA 103, 3480–3485. ( 10.1073/pnas.0507526103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tacon P, Baroiller J, Le Bail P, Prunet P, Jalabert B. 2000. Effect of egg deprivation on sex steroids, gonadotropin, prolactin, and growth hormone profiles during the reproductive cycle of the Mouthbrooding cichlid fish Oreochromis niloticus. Gen. Comp. Endocrinol. 117, 54–65. ( 10.1006/gcen.1999.7388) [DOI] [PubMed] [Google Scholar]
- 60.de Ruiter AJ, Wendelaar Bonga SE, Slijkhuis H, Baggerman B. 1986. The effect of prolactin on fanning behavior in the male three-spined stickleback, Gasterosteus aculeatus L. Gen. Comp. Endocrinol. 64, 273–83. ( 10.1016/0016-6480(86)90014-6) [DOI] [PubMed] [Google Scholar]
- 61.Moore AJ, Brodie ED, Wolf JB. 1997. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution. 51, 1352–1362. ( 10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- 62.McNamara JM, Houston AI. 2005. Conflict between parents over care. Trends Ecol. Evol. 20, 33–38. ( 10.1016/j.tree.2004.10.008) [DOI] [PubMed] [Google Scholar]
- 63.Johnstone RA. 2011. Load lightening and negotiation over offspring care in cooperative breeders. Behav. Ecol. 22, 436–444. ( 10.1093/beheco/arq190) [DOI] [Google Scholar]
- 64.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209–216. ( 10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 65.Taborsky B, Skubic E, Bruintjes R. 2007. Mothers adjust egg size to helper number in a cooperatively breeding cichlid. Behav. Ecol. 18, 652–657. ( 10.1093/beheco/arm026) [DOI] [Google Scholar]
- 66.Fischer S, Zottl M, Groenewoud F, Taborsky B. 2014. Group-size-dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Proc. R. Soc. B 281, 20140184 ( 10.1098/rspb.2014.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrissey MB, Kruuk LE. B, Wilson AJ. 2010. The danger of applying the breeder's equation in observational studies of natural populations. J. Evol. Biol. 23, 2277–2288. ( 10.1111/j.1420-9101.2010.02084.x) [DOI] [PubMed] [Google Scholar]
- 68.Bijma P. 2011. A general definition of the heritable variation that determines the potential of a population to respond to selection. Genetics 189, 1347–1359. ( 10.1534/genetics.111.130617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasper C, Kölliker M, Postma E, Taborsky B. 2017. Data from: Consistent cooperation in a cichlid fish is caused by maternal and developmental effects rather than heritable genetic variation. Dryad Digital Repository. ( 10.5061/dryad.4b45d) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kasper C, Kölliker M, Postma E, Taborsky B. 2017. Data from: Consistent cooperation in a cichlid fish is caused by maternal and developmental effects rather than heritable genetic variation. Dryad Digital Repository. ( 10.5061/dryad.4b45d) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4b45d [69].