Abstract
We examined if apes spontaneously remember one-time, distinctive events across long delays when probed by discriminant cues. Apes witnessed an experimenter hide a cache of food, which they could then retrieve. They retrieved one of two food types; one more distinctive than the other. Two, 10 or 50 weeks later, the apes returned to the same enclosure and found a piece of the previously hidden food on the ground. An experimenter who had not hidden the food was also present. Apes immediately searched the location where the food was previously hidden (no food was here), showing recall of the event. One week later, apes returned to the same enclosure, with the same food on the ground, but now the experimenter that had hidden the food was present. Again, apes immediately searched the hiding location. Apes that had not witnessed the hiding event did not search. There was no significant effect of food type, and retention declined from exposure to the two-week delay, then levelled, consistent with the forgetting curve in humans (Ebbinghaus, H. 1964 Memory: a contribution to experimental psychology (transl. H.A. Ruger & C.E. Bussenvis). New York, NY: Dover. (Original work published 1885.)). This is the first study to show apes can recall a one-time, non-goal-directed event longer than two weeks ago and that apes' recall declines in accordance with a standard retention function.
Keywords: great apes, spontaneous memory, episodic memory, cued recall, distinctiveness, forgetting curve
1. Introduction
Ebbinghaus [1] was the first to divide memory into three distinct types: voluntary, involuntary and unconscious. Involuntary memory refers to the spontaneous recollection of personal past events, often triggered by cues in the present environment (cued recall) [2]. One of the most famous examples of an involuntary memory comes from the French author Marcel Proust [3], who described the taste of a madeleine cookie dipped in lime tea eliciting his childhood memory of visiting his aunt on Sunday mornings. Involuntary memories are a frequent occurrence in our day-to-day lives [2,4,5]. They are often triggered by features of the present situation that match parts of the remembered event [6,7]. Unlike voluntary memories, they are not goal-directed and strategically retrieved, rather they reflect a bottom-up, stimulus-driven associative process, resulting in significantly faster retrieval times for involuntary over voluntary memories [8,9].
Numerous studies have shown that non-human animals (hereafter animals) can recall past events [10–18], however, only recently has it been proposed that animals may also recall past events spontaneously (i.e. involuntarily) [6,19–21]. Because involuntary memories occur spontaneously, with little effort, and are non-goal-directed, they do not rely on executive control processes or recruit pre-frontal brain regions as much as voluntary memories [22,23]. Consequently, they are considered to be the more basic mode of remembering that proceeds the evolutionary development of voluntary memory [6,19]. As such, if animals are capable of recalling past events strategically (voluntarily), then it follows that they should also be able to recall events via the more basic, and evolutionary earlier, involuntary counterpart.
There is some evidence that animals can recall past events spontaneously, when presented with relevant contextual cues [13,15,18,24–27]. For instance, Martin-Ordas et al. [24] tested great apes on their ability to remember two similar tool hiding events. In the first study, apes were presented with a task that required a tool to obtain food. After a 15 min delay, the apes witnessed an experimenter hide two tools in two different locations, only one was useful to solve the task. The apes experienced this four times. Three years later, they were presented with the same task in the same room, and with the same experimenter, however, this time the tools were already hidden in the same locations as before. The apes spontaneously searched the previous locations, and upon finding the appropriate tool, successfully completed the task. A second experiment followed the same procedure, except the apes received a slightly different task and tool, and were only presented with it once. After a two-week retention period, the apes immediately and spontaneously searched the location where the tool was hidden two weeks previously.
These studies show that apes can remember and distinguish between events in their past when features present at the time of encoding are also present at retrieval, and furthermore, that they remember almost instantaneously. This fast cued recall is consistent with the way in which involuntary memories are recalled in humans [2,8]. However, as the apes needed a tool to complete the tasks, it is possible that retrieval was goal-directed, (i.e. they strategically and voluntarily recalled the memory). Many of the studies that show cued recall of a past event in animals incorporate goal-directed tasks [13,18,25,26], and as such direct evidence for involuntary recall of events in animals is limited.
However, a study by Kano & Hirata [15] showed apes ability to recall a past event using a non-goal-directed task. Apes viewed a short movie of a novel event in which an aggressive ‘King Kong’ character entered through one of two doors. An eye-tracker monitored the ape's gaze during this viewing. Twenty-four hours later, while the apes watched the same video again, they made anticipatory looks at the door in which the ‘King Kong’ character had entered the day before. Thus, apes recalled the event 24 h later when cued with the preceding parts of the movie. Critically, the apes were given juice or fruit to eat during viewing of the movie, regardless of their gaze behaviour, thus their recall of the event was not goal-directed. As such, this paradigm is much more in keeping with the way in which involuntary memories are retrieved (i.e. via non-goal-directed cued recall) and provides evidence of the occurrence of involuntary memories in animals.
Although such studies may provide evidence for involuntary recall of events in animals, a number of questions are left unresolved. First, although it has been shown that apes can recall past events that took place as long as 3 years ago [24], this long retention was shown using repeated exposures during learning, meaning that the to-be-remembered event was a repeated (non-specific) event. Involuntary memories are more often of single occurring events rather than repeated ones [8,28–30]. Furthermore, repeated events may be intentionally encoded and recalled as semantic, rule-based knowledge, due to the expectation that they will occur again [31]. That is, if one expects to be asked where a tool was last seen, one may simply learn through repeated associations where the tool item is located, rather than recalling the memory of the hiding event. As such, to specifically test for involuntary recall of an event in animals, the test needs to focus on recall for single exposures. In apes, such recall has only been shown for retention intervals of up to two weeks [24].
Second, despite previous studies using distinctive events [15,24,27], it has yet to be specifically addressed whether a distinctive event is more likely to be recalled than a less distinctive event. It is known that involuntary memories are often about distinctive events [2,9], and that distinctiveness can improve performance in various memory tasks in rats [32–34] and primates [35,36], but it is unknown whether it improves long-term recall of a one-time event in non-human animals.
Lastly, it is unclear whether different types of cues are equally successful at cueing the recall of a past event. For instance, a study by Mendes & Call [27] incorporated social and non-social cues in a foraging event. They found apes could successfully recall the foraging locations, but acknowledged that they did not disentangle whether the memory for the locations was cued by the social, non-social or a combination of cues. Similarly, Martin-Ordas et al. [24] incorporated the identity of the experimenter as a social cue, but did not test whether it was this cue or other contextual cues (room, apparatus) or combinations thereof that triggered recall of the event. Although there is some direct evidence that apes can recall information about the identity of a person from a past event [37], this was found using a forced recognition paradigm after a fairly short (24 h) delay.
As such, we investigated these aspects within one paradigm. We tested whether three species of great ape could recall a distinctive, one-time hiding event after a minimum of a two-week retention period. During the hiding event, all food was retrieved by the subject, ensuring that there was no expectation or goal of returning to the room to retrieve the food. Furthermore, at retrieval, no task was presented to the apes in which the goal was to obtain food. As such, any recall of the hiding event was likely to be spontaneous rather than a voluntary, goal-directed response. Additionally, we manipulated three variables. First, we included three delay periods: two, 10 and 50 weeks. This enabled us to look at recall over longer time periods, and to assess whether forgetting occurred over time. We used a log-scale, roughly covering a 1-year time period, as this scale best reflects the rate of forgetting in human long-term memory [38].
Second, we tested whether an event that was highly distinctive would be recalled more than a less distinctive (albeit, still distinctive) event. This was achieved by manipulating the type of food hidden during the hiding event. In the less distinctive condition, subjects found a large cache of bread during the hiding event. The bread was familiar to the apes, but was not a regular part of their diet. In the highly distinctive condition, subjects found a large cache of cardamom-flavoured pellets. The apes had never tasted cardamom before; furthermore, the pellets resembled standard flavoured pellets given to the apes daily, and thus were intended to be surprising. We chose to make the taste/odour distinctive, as opposed to the visual appearance, to see whether apes can make use of non-visual distinctive cues, as currently there is only evidence for a distinctiveness effect with visual information [35,36]. Furthermore, odours are often highly successful as cues for retrieving memories in humans [39,40].
Third, we tested whether the addition of a social cue would improve recall relative to when that cue was absent. Specifically, we manipulated whether the presence of the same experimenter that hid food during the hiding event would improve recall performance in comparison to the presence of a different experimenter.
In short, the aim of this study was to see whether apes could recall a one-time, non-goal-directed event when presented with distinctive, diagnostic cues. Previous studies have shown evidence for involuntary recall of events after long time periods, but these have involved repeated exposures or goal-directed tasks (e.g. [1]). Furthermore, the influence of distinctiveness and overlapping of cues at encoding and retrieval has not been directly tested, neither has the forgetting rate of such memories over time. As such, we aimed to address these issues.
2. Material and methods
(a). Subjects
Nineteen chimpanzees (Pan troglodytes; age range 9–50 years), seven orangutans (Pongo abelii; age range 7–36 years) and seven bonobos (Pan paniscus; age range 8–33 years) participated in this study, resulting in a total of 33 apes. All were housed at the Wolfgang Köhler Primate Research Center at Leipzig Zoo (Leipzig, Germany) and had previously participated in cognitive studies. None of the apes were food or water deprived, and all received a healthy and balanced diet during the testing period.
(b). Apparatus
Apes were tested inside their sleeping quarters or observation rooms (here-after testing room). Each testing room consisted of multiple enclosures, connected to each other by hydraulic doors. For this study, two adjacent enclosures were used. Additionally, each testing room contained an area only accessible to the experimenter (experimenter area). The ape always entered the testing room via one enclosure (the right), and the food was always hidden in the adjacent enclosure (the left; see the electronic supplementary material, figure S1).
The hiding location varied between subjects due to constraints of the testing rooms, but was always above the eye-line of an ape from ground level and in an area not normally used for testing. For the majority of subjects, it was located on a ledge above a hydraulic door, accessible to the experimenter only by ladder (see the electronic supplementary material, figure S1), the other locations did not require a ladder.
Two types of food were hidden (exposure food); bread and cardamom-flavoured pellets. The flavoured pellets were very distinctive, as the apes had never tasted cardamom before, additionally, they looked like regular pellets (eaten on a daily basis), thus when eaten were unexpected and (most likely) surprising. The bread was less distinctive, due to being used as an occasional treat, and was not surprising in taste. Still, it was by no means common to the apes. We did not choose a completely familiar food in order to keep the hiding events comparable in nature. Thus, in both cases they would find unexpected food, but with the added element of novelty and surprise when the flavoured pellets were hidden.
(c). Design
We used a mixed design with exposure food (bread; N = 16, flavoured pellet; N = 15) and delay (two weeks; N = 10, 10 weeks; N = 11, 50 weeks; N = 10) between subjects, and condition (experimental, control) and retrieval session (1,2) within subjects. Exposure food referred to the type of food that was hidden during the hiding event. The alternate food type was experienced during the control condition, but was never experienced during the hiding event (see Procedure).
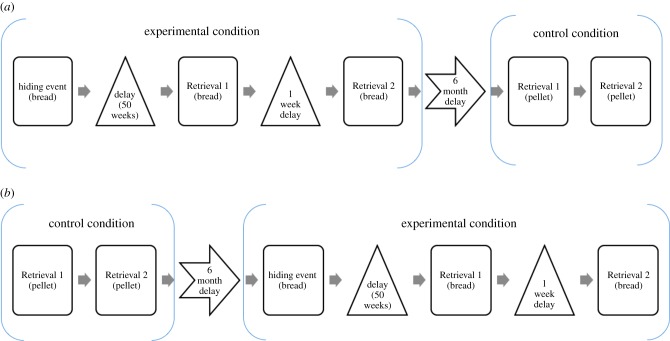
Apes completed both an experimental and a control condition, counterbalanced between subjects. There was a minimum of six months between conditions (range; 182–635 days). In the experimental condition, apes witnessed a hiding event in which the exposure food was hidden. After their allocated delay, they received two retrieval sessions with the exposure food, one-week apart (range; 5–9 days). The control condition differed in that no hiding event occurred before the retrieval sessions, and the alternate food was present during the retrieval sessions. This control condition was used as a baseline for comparison with the experimental retrieval performance. As illustrated in figure 1, two different orders were used. In one (i) participants took part in the experimental condition before the control condition, and in other (ii) the order was reversed, with the control condition preceding the experimental condition.
Figure 1.
Example of the procedure sequence. (a) The experimental condition first, followed by the control condition. The subject is in the bread exposure food and 50-week delay group. (b) The alternative order; the control condition first, followed by the experimental. The subject is in the bread exposure food and 50-week delay group. (Online version in colour.)
The first retrieval session was with an experimenter that was not present during the hiding event, and the second retrieval was with the experimenter that hid the food during the hiding event (figure 1). This enabled us to see if the apes were more successful at recalling the event when social information (i.e. the experimenter identity) overlapped at encoding and retrieval forming a social cue. For the first retrieval, the experimenter was blinded to the condition and delay the subject was participating in (i.e. control or experimental, two, 10 or 50 weeks), so as to avoid any unintentional cueing. It was not possible for the experimenter in the second retrieval to be blinded in this way, as this experimenter was aware of when and who had previously seen (or not seen) a hiding event.
(d). Procedure
During the hiding event, the ape began in one enclosure (the right) and watched the experimenter enter the other enclosure (the left) with a ladder and six food pellets or pieces of bread in her hand (exposure food). The experimenter showed the food to the subject, climbed the ladder and hid the food in the hiding location (see the electronic supplementary material, figure S1 and apparatus). The subject could see that the food had been placed there, but could not see the food itself. The experimenter left the enclosure and entered the experimenter area. The connecting door between the two enclosures was then opened, so that the subject could access both enclosures. Subjects were given a maximum of 5 min to find and eat the food. If the subject failed to do so in this time, the session ended and the subject did not participate any further in the study. Only the experimental condition included this hiding event.
After the allocated delay (two, 10 or 50 weeks), the subject received two retrieval sessions, one-week apart. Retrieval sessions for the experimental and control conditions followed the same procedure. In Retrieval 1, an experimenter that did not hide the food during the hiding event entered the left enclosure and placed a single piece of the exposure food on the ground, directly below the hiding location. The subject was not present to witness this. The experimenter then left the enclosure and stood in the experimenter area, before the subject entered the right enclosure. After a 10 s delay, in which the experimenter was facing the subject and the subject could see the experimenter, the door connecting the two enclosures was opened and the experimenter left the testing room. After 2 min had elapsed, the experimenter re-entered the testing room and stood in the experimenter area, so that the subject could again see the experimenter. The experimenter did not look at the hiding location during this time. After 10 s, the experimenter left the testing room. After 5 min, the session finished. Retrieval 2 followed the same procedure, except now the experimenter was the one who hid the food during the hiding event. No food was present in the hiding location during the retrieval to avoid searching as a result of extraneous cues, such as odour. The control condition differed from the experimental condition in two ways: subjects did not witness the hiding event and the food that was on the enclosure floor was not the exposure food in the experimental condition, but the alternate food. For example, if subjects experienced flavoured pellets as the exposure food, they found bread on the floor. Note that two different orders were used; one in which the control condition preceded the experimental condition and one with the reverse order (figure 1).
(e). Coding and analysis
All sessions were videotaped and later coded as to whether the subject searched or not. A search was defined as the subject climbing to the hiding location and looking/and or searching the location with hands/feet/mouth. For instances of searching, the time taken from picking up the food from the ground to reaching the hiding location was counted (here-after latency). Twenty per cent of the videos were coded by a second coder. Inter-rater reliability for searching was calculated using Cohen's κ, and Pearson's correlation assessed the inter-rater agreement for latencies. Inter-rater reliability for searching was excellent (K = 1, p ≤ 0.001) and for latencies was high (r = 0.88, N = 9, p ≤ 0.01).
Our main question was whether the apes could successfully recall the hiding event, as measured by searching. To test for this, we compared whether searching differed between the experimental and control condition. Furthermore, we tested whether this difference would be influenced by delay, exposure food, retrieval session and the order of condition. As we expected the effect of these predictors to depend on condition (experimental or control), we also included the respective four, two-way interactions.
We fitted a generalized linear mixed model with a Poisson error distribution and log link function [41,42], with condition, delay, exposure food, retrieval session and order of condition as fixed effects, species as a controlled fixed effect and subject as a random effect (N = 33 individuals; total n = 123). As a test of the combined effects of condition, delay, exposure food, retrieval session, order of condition and their interactions, we compared the full model with a null model comprising only species and the random effects using a likelihood ratio test [43,44]. For full details of the statistical model, see the electronic supplementary material, model description.
For every instance of searching, we calculated the average search time (in seconds) from the ape picking up the food on the floor to searching the hiding location. We also conducted a paired samples t-test to see whether search time changed between Retrieval 1 and Retrieval 2 in the experimental condition. This enabled us to see if the subjects that had already searched in Retrieval 1 were slower in Retrieval 2, because of finding no food in the first session. Additionally, we checked for any differences in search times between the two exposure foods in the experimental condition. For subjects that searched in both Retrieval 1 and Retrieval 2, a mean search time was calculated. Search times were then compared between groups using an independent t-test. As Levene's test was significant, the Welch–Satterthwaite calculation was applied. Likewise, search times between the three delay groups were compared (with mean search times calculated as above) using a one-way ANOVA. As Levene's test was significant, we ran the analysis on log-transformed data (which resulted in Levene's test being non-significant).
3. Results
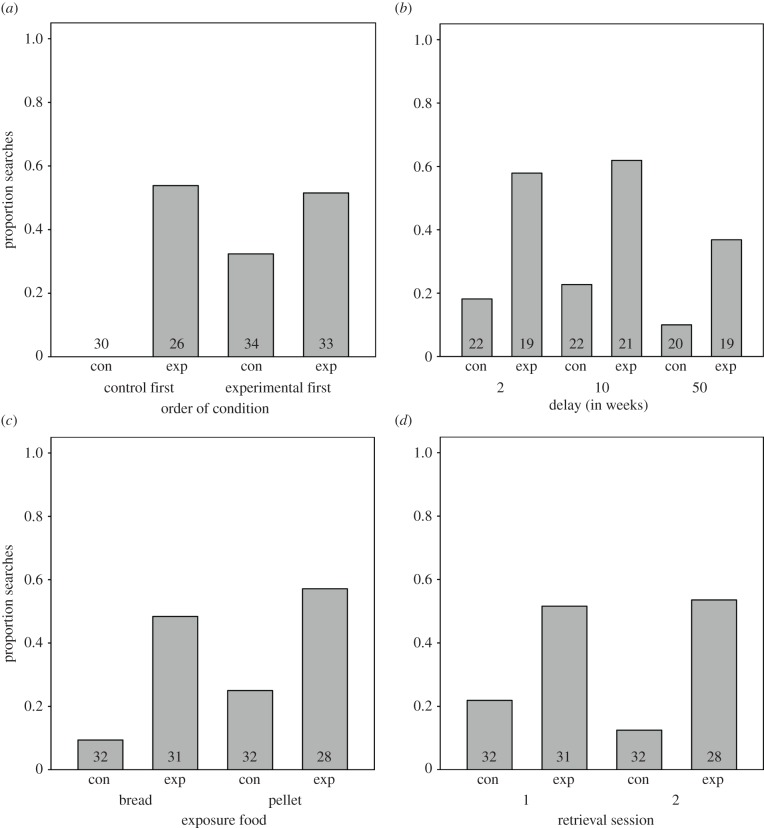
The full model compared to the null model was significant (likelihood ratio test: χ2 = 20.404, d.f. = 9, p = 0.017). More specifically, the interaction between condition and order of condition was significant (χ2 = 6.239, d.f. = 1, p = 0.013; figure 2a). None of the other three interactions were significant (see the electronic supplementary material, table S1; figure 2b-d). The interaction showed that subjects searched significantly more in the experimental condition compared with the control condition when the control condition was completed first (figure 2a). Thus, subjects who had seen the hiding event were searching significantly more than subjects that had yet to see the hiding event (who never searched). Those subjects who searched in the control condition when presented second did so despite experiencing an additional retention period of six months from the initial hiding event and delay period, and crucially, after finding no food in previous experimental retrieval sessions. This included four apes that searched in all four retrieval sessions, one of which received the 50-week delay period, and thus by the fourth retrieval session was still searching, despite the fact that 1 year and five months had passed since this subject had witnessed the hiding events and despite finding no food the previous three times.
Figure 2.
Proportion of searches by condition as a function of: (a) order of condition, (b) delay, (c) exposure food and (d) retrieval session. Numbers inside the bars represent the number of data points (a–c = N × 2 trials, d = N). Con and Exp refer to the control and experimental conditions, respectively.
The lack of any other interaction showed that searching in both conditions did not differ as a result of delay, retrieval session or exposure food (figure 2b–d). This finding indicated that subjects recall did not decline significantly over the three test intervals (i.e. from two to 10 to 50 weeks after exposure), although the performance did decline from initial exposure to retrieval (see below), and that neither the social cue nor the distinctive pellet improved recall performance. Additionally, recall performance between Retrieval 1 and 2 in the experimental condition was nearly identical, with all but three subjects consistently not searching in both sessions, or consistently failing to search in both sessions, further showing that the social cue did not aid performance.
The average (median) search time in the experimental condition was 9 s (N = 31, Median = 9, Q1 = 6, Q3 = 35), and for the subjects that completed the control condition second, the average search time was 12 s (N = 11, Median = 12, Q1 = 5, Q3 = 26). This means that subjects immediately searched the location. We found no change in search time from Retrieval 1 (M = 38.38, s.e. = 18.23) to Retrieval 2 (M = 21.38, s.e. = 8.43) in the experimental condition; t12 = 1.20, p = 0.25, suggesting that even though the apes found no food in the previous session, they were just as quick to search the hiding location again. Likewise, search times between the two exposure foods (flavoured pellet: M = 49.06, s.e. = 25.83; bread: M = 35.56, s.e. = 19.41) in the experimental condition did not differ; t14.85 = 0.42, p = 0.68. Neither did search times between the delay groups of the experimental condition; F2,15 = 1.96, p = 0.18). Thus, subjects search times in the experimental condition were not influenced by retrieval session, exposure food or delay.
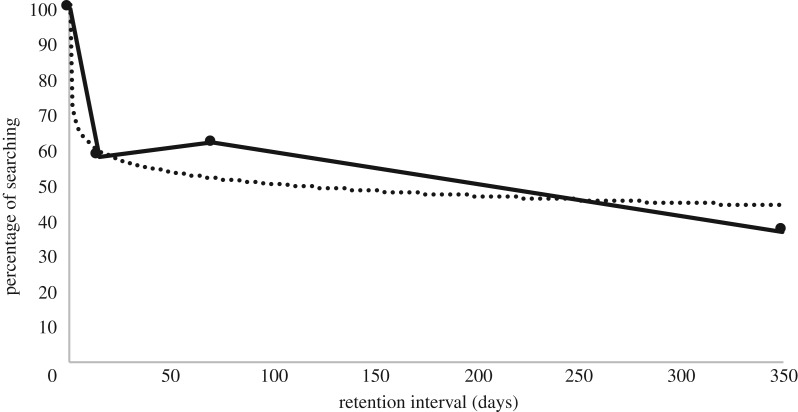
The fact that no significant decline was observed between the three delay intervals (two, 10 and 50 weeks) does not mean that performance showed no decline from the hiding event to retrieval. Compared with the original 100% search rate at the time of exposure, a marked decline was seen at the two-week delay (where 58% searched), after which the decline levelled. When we plotted the rate of searching as a function of days since the observation of the hiding event (estimating the first delay to 5 min = 0.003 days), a standard forgetting curve was observed (figure 3). Retention by time was best described by a logarithmic function (y = −4.853ln(x) + 72.666. R² = 0.92463).
Figure 3.
Percentage of searching as a function of days since exposure to the hiding event (estimating the first delay to 5 min = 0.003 days). Solid black line shows the forgetting rate across the two-, 10- and 50-week delays. Dotted black line shows forgetting as a logarithmic function.
4. Discussion
The purpose of this study was threefold. First, to investigate if apes could recall a one-time, non-goal-directed event. Second, to assess the importance of cue distinctiveness and cue similarity between encoding and retrieval, on recall success. Third, to see whether recall declined over time. Our results showed that apes could successfully recall a one-time, non-goal-directed hiding event upon presentation of cues that matched the memory trace, and did so almost immediately, consistent with involuntary recall of an event. The addition of overlapping social information at encoding and retrieval did not improve recall relative to when the information was absent. Likewise, the highly distinctive cue did not enhance recall relative to the less distinctive cue. Retention showed a marked decline from learning to the two-week delay, then levelled, consistent with the classic forgetting curve observed in human memory [1,38].
The average search time from finding the food on the ground to reaching the hiding location was less than 12 s. As the apes needed to climb to the hiding location, this search time reflects a fast and instantaneous response, indicative of involuntary memory [8,9,45]. This fast response was consistent across the three delay periods, and retrieval sessions, suggesting apes spontaneously recalled the event regardless of how long ago it occurred, and when it was last recalled. Thus, if they recalled it during the first retrieval, they were just as quick to recall it again in the second retrieval. Although fast response times are consistent with involuntary recall, we acknowledge that due to not having a comparison group completing the same action using voluntary recall, we cannot say for certain that the fast response is due to involuntary as opposed to voluntary recall.
However, other support for the involuntary nature of the memory retrieval comes from the lack of a goal-directed task. Involuntary memories frequently spring to mind when one is not doing anything [1], often as a result of features in the environment matching the memory trace [2]. At retrieval, the apes were not presented with a task that needed to be solved by recalling the memory, unlike previous work [24]. Instead, they were simply presented with relevant external cues that matched the hiding event, such as entering the same enclosure, and finding the same exposure food. The apes enter this enclosure daily for testing, and thus, the absence of any obvious testing apparatus and task may have made the context particularly distinctive. This combination of external cues led to a unique overlap between the retrieval situation and the hiding event, and most likely cued the spontaneous recall of the event.
Additionally, we found that subjects who completed the experimental condition first, followed by the control condition, were more likely to search the hiding location during the control trials than those who completed the control condition first (of which none searched). That is, some subjects who witnessed the hiding event and searched in the two retrieval sessions (after two, 10 or 50 weeks), subsequently searched six months later when a different type of food was on the ground. This is despite having not found food in the hiding location the previous two sessions. Owing to the long durations involved and the lack of reinforcement for searching in every retrieval session, as well as providing a cue that did not directly match the food at the hiding event, we did not expect subjects to search. The finding that apes did search, and thus overcame all these difficulties, is a remarkable testament to the robustness of their memory for distinctive events.
The willingness to continue searching despite finding no food goes strongly against any potential critique that the apes used associative learning to encode and recall the hiding location. The apes experienced no food in the hiding location more often than they experienced food (which occurred only once), thus any association with this location and food would have been weakened. This was further supported by the lack of difference in recall between retrieval sessions 1 and 2 of the experimental condition (i.e. subjects that searched in Retrieval 1 continued to search in Retrieval 2) despite finding no food, indeed, only one subject who searched in Retrieval 1 of the experimental condition subsequently failed to search in Retrieval 2. What is perhaps more surprising is that the apes who recalled the event in the control condition did so even when the cues did not directly overlap. Here, the food on the ground was not the same as the food that had been hidden during the event. However, this finding is not completely at odds with involuntary memory. Although the overlapping of features at encoding and retrieval is often found to trigger involuntary memories, it is not the extent of the overlap that is important, rather it is the uniqueness of the overlap [46]. Thus, the uniqueness of the location of the food (directly below the hiding location) and the set up (or lack of set up) of the room may have been sufficient to cue recall of the event.
This finding is consistent with the finding that the addition of a social cue made no difference to recall, as evidenced by no difference between Retrieval 1 and Retrieval 2 in which the experimenter identity differed. This is further reinforced by the fact that only two subjects who did not search in Retrieval 1 went on to search in Retrieval 2. As previously discussed, the uniqueness of the cues rather than the sheer number of overlapping cues could explain this result. In this case, the experimenter's identity was not a unique or diagnostic cue; both experimenters in this study have tested the apes on other tasks, and thus, their identity may be associated with other memories of past experiences. The more memory traces a cue is associated with, the less likely that cue will trigger a specific episode, referred to as cue overload [47]. As the identity of the experimenter was not specific to the hiding event, it was not effective as a retrieval cue.
Alternately, it may be that experimenter identity was confounded with the order of retrieval sessions. As the experimenter that hid the food was always in Retrieval 2, which occurred one week after Retrieval 1, it could be performance was enhanced by the matching identity but hindered by the additional retention period, resulting in no difference in performance overall. However, due to not finding a significant decline in recall from the two-week to the 50-week delay, this is unlikely. Another possibility is that the apes simply did not pay attention to the experimenter. The apes participate in many studies with many experimenters, and more often than not, the experimenter's identity is not important to the task. As such, the apes may have paid more attention to other aspects of the hiding event, resulting in the identity of the experimenter being overshadowed [48]. This could potentially explain why the addition of a social cue did not improve recall performance. As such, our results suggest that using experimenter identity as a social cue may be of limited effect at retrieval, especially in the presence of other more unique and diagnostic cues, something that is consistent with other work ([49], MJ Beran 2016, personal communication).
With regard to the two exposure foods, we found no difference in memory recall. This was unexpected, as we predicted the novelty of the flavoured pellet to enhance memory recall. Although it is unclear why this was not the case, we propose two potential explanations. Firstly, the hiding event was very distinctive regardless of which food type was hidden, in that a human entering the enclosure and hiding a large cache of food in an unusual location is a unique event to all the apes. Additionally, the bread was fairly distinctive in itself; it was not a common food type and thus finding a large cache of it was a rather rare occurrence. Consequently, the memorability of the event may not have been dependent upon which food was hidden. Secondly, cardamom was a completely novel flavour for the apes, and so it was possible that not all the apes liked it. During the hiding event, two of the apes (Frodo and Luiza) did not eat the flavoured pellets, with Frodo returning the pellets to the experimenter by pushing them through the enclosure meshing. As such, the reason why more apes did not search in this condition could be that the apes simply did not like the food, and thus were not motivated to search for it.
In conclusion, we show that apes can spontaneously recall a distinctive one-time, non-goal-directed event after delays as long as 50 weeks, with their rate of recall across time following a standard retention function. Furthermore, apes continue to recall this event after failing to find food in that location repeatedly. These results are consistent with involuntary memory in humans, and thus provide compelling evidence for the existence of involuntary memory in apes.
Supplementary Material
Supplementary Material
Acknowledgements
The authors wish to thank the staff and ape keepers at Leipzig Zoo. Special thanks are given to Colleen Stevens and Roger Mundry for their help with the statistical analyses, and to David C. Rubin for comments regarding retention functions.
Ethics
The study was ethically approved by an internal committee at the Max Planck Institute for Evolutionary Anthropology and Zoo Leipzig. Animal husbandry and research comply with the ‘EAZA Minimum Standards for the Accommodation and Care of Animals in Zoos and Aquaria’, the ‘EEP Bonobo Husbandry Manual’, the ‘WAZA Ethical Guidelines for the Conduct of Research on Animals by Zoos and Aquariums’ and the ‘Guidelines for the Treatment of Animals in Behavioral Research and Teaching’ of the Association for the Study of Animal Behavior (ASAB).
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
A.L. carried out the testing, the data and statistical analysis, participated in the design of the study and drafted the manuscript; J.C. contributed to testing, helped with the data analysis, conceived of the study, participated in the design of the study and helped draft the manuscript. D.B. helped with the data analysis, conceived of the study, participated in the design of the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Danish National Research Foundation (DNRF89).
References
- 1.Ebbinghaus H. 1964. Memory: a contribution to experimental psychology (transl. H.A. Ruger & C.E. Bussenvis). New York, NY: Dover; (Original work published 1885.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berntsen D. 1996. Involuntary autobiographical memories. Appl. Cogn. Psychol. 10, 435–454. ( 10.1002/(SICI)1099-0720(199610)10:5%3C435::AID-ACP408%3E3.0.CO;2-L) [DOI] [Google Scholar]
- 3.Proust M. 1981. Remembrance of things past, vol. 3. New York, NY: Random House. [Google Scholar]
- 4.Rubin DC, Berntsen D. 2009. The frequency of voluntary and involuntary autobiographical memories across the life span. Mem. Cognit. 37, 679–688. ( 10.3758/37.5.679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen AS, Berntsen D. 2011. The unpredictable past: spontaneous autobiographical memories outnumber autobiographical memories retrieved strategically. Conscious Cogn. 20, 1842–1846. ( 10.1016/j.concog.2011.07.010) [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen AS, Berntsen D. 2009. The possible functions of involuntary autobiographical memories. Appl. Cogn. Psychol. 23, 1137–1152. ( 10.1002/acp.1615) [DOI] [Google Scholar]
- 7.Berntsen D. 2009. Involuntary autobiographical memories: an introduction to the unbidden past. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Schlagman S, Kvavilashvili L. 2008. Involuntary autobiographical memories in and outside the laboratory: how different are they from voluntary autobiographical memories? Mem. Cognit. 36, 920–932. ( 10.3758/mc.36.5.920) [DOI] [PubMed] [Google Scholar]
- 9.Berntsen D, Staugaard SR, Sorensen LM. 2013. Why am I remembering this now? Predicting the occurrence of involuntary (spontaneous) episodic memories. J. Exp. Psychol. Gen. 142, 426–444. ( 10.1037/a0029128) [DOI] [PubMed] [Google Scholar]
- 10.Clayton NS, Dickinson A. 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395, 272–274. ( 10.1038/26216) [DOI] [PubMed] [Google Scholar]
- 11.Menzel CR. 1999. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. J. Comp. Psychol. 113, 426–434. ( 10.1037/0735-7036.113.4.426) [DOI] [PubMed] [Google Scholar]
- 12.Martin-Ordas G, Haun D, Colmenares F, Call J. 2010. Keeping track of time: evidence for episodic-like memory in great apes. Anim. Cogn. 13, 331–340. ( 10.1007/s10071-009-0282-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crystal JD, Smith AE. 2014. Binding of episodic memories in the rat. Curr. Biol. 24, 2957–2961. ( 10.1016/j.cub.2014.10.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman ML, Beran MJ, Washburn DA. 2009. Memory for ‘what’, ‘where’, and ‘when’ information in rhesus monkeys (Macaca mulatta). J. Exp. Psychol. Anim. Behav. Process. 35, 143–152. ( 10.1037/a0013295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano F, Hirata S. 2015. Great apes make anticipatory looks based on long-term memory of single events. Curr. Biol. 25, 2513–2517. ( 10.1016/j.cub.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 16.Dere E, Huston JP, De Souza Silva MA. 2005. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiol. Learn. Mem. 84, 214–221. ( 10.1016/j.nlm.2005.07.002) [DOI] [PubMed] [Google Scholar]
- 17.Fugazza C, Pogány Á, Miklósi Á. 2016. Recall of others’ actions after incidental encoding reveals episodic-like memory in dogs. Curr. Biol. 26, 3209–3213. ( 10.1016/j.cub.2016.09.057) [DOI] [PubMed] [Google Scholar]
- 18.Panoz-Brown D, Corbin HE, Dalecki SJ, Gentry M, Brotheridge S, Sluka CM, Wu JE, Crystal JD. 2016. Rats remember items in context using episodic memory. Curr. Biol. 26, 2821–2826. ( 10.1016/j.cub.2016.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berntsen D. 2010. The unbidden past: involuntary autobiographical memories as a basic mode of remembering. Curr. Dir. Psychol. Sci. 19, 138–142. ( 10.1177/0963721410370301) [DOI] [Google Scholar]
- 20.Clayton NS, Bussey TJ, Emery NJ, Dickinson A. 2003. Prometheus to Proust: the case for behavioural criteria for ‘mental time travel’. Trends Cogn. Sci. 7, 436–437. ( 10.1016/j.tics.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 21.Berntsen D, Jacobsen AS. 2008. Involuntary (spontaneous) mental time travel into the past and future. Conscious Cogn. 17, 1093–1104. ( 10.1016/j.concog.2008.03.001) [DOI] [PubMed] [Google Scholar]
- 22.Hall SA, Rubin DC, Miles A, Davis SW, Wing EA, Cabeza R, Berntsen D. 2014. The neural basis of involuntary episodic memories. J. Cogn. Neurosci. 26, 2385–2399. ( 10.1162/jocn_a_00633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall NM, Gjedde A, Kupers R. 2008. Neural mechanisms of voluntary and involuntary recall: a PET study. Behav. Brain Res. 186, 261–272. ( 10.1016/j.bbr.2007.08.026). [DOI] [PubMed] [Google Scholar]
- 24.Martin-Ordas G, Berntsen D, Call J. 2013. Memory for distant past events in chimpanzees and orangutans. Curr. Biol. 23, 1438–1441. ( 10.1016/j.cub.2013.06.017) [DOI] [PubMed] [Google Scholar]
- 25.Clayton NS, Yu KS, Dickinson A. 2001. Scrub jays (Aphelocoma coerulescens) form integrated memories of the multiple features of caching episodes. J. Exp. Psychol. Anim. Behav. Process. 27, 17–29. ( 10.1037/0097-7403.27.1.17) [DOI] [PubMed] [Google Scholar]
- 26.Crystal JD, Alford WT, Zhou W, Hohmann A. G. 2013. Source memory in the rat. Curr. Biol. 23, 387–391. ( 10.1016/j.cub.2013.01.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes N, Call J. 2014. Chimpanzees form long-term memories for food locations after limited exposure. Am. J. Primatol. 76, 485–495. ( 10.1002/ajp.22248) [DOI] [PubMed] [Google Scholar]
- 28.Berntsen D, Hall NM. 2004. The episodic nature of involuntary autobiographical memories. Mem. Cogn. 32, 789–803. ( 10.3758/bf03195869) [DOI] [PubMed] [Google Scholar]
- 29.Berntsen D. 2001. Involuntary memories of emotional events: do memories of traumas and extremely happy events differ? Appl. Cogn. Psychol. 15, S135–S158. ( 10.1002/acp.838) [DOI] [Google Scholar]
- 30.Rasmussen AS, Johannessen KB, Berntsen D. 2014. Ways of sampling voluntary and involuntary autobiographical memories in daily life. Conscious Cogn. 30, 156–168. ( 10.1016/j.concog.2014.09.008) [DOI] [PubMed] [Google Scholar]
- 31.Zentall TR. 2006. Mental time travel in animals: a challenging question. Behav. Processes 72, 173–183. ( 10.1016/j.beproc.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 32.Reed P, Richards A. 1996. The von Restorff effect in rats (Rattus norvegicus). J. Comp. Psychol. 110, 193–198. ( 10.1037/0735-7036.110.2.193) [DOI] [PubMed] [Google Scholar]
- 33.Reed P, Chih-Ta T, Aggleton JP, Rawlins JNP. 1991. Primacy, recency, and the von Restorff effect in rats’ nonspatial recognition memory. J. Exp. Psychol. Anim. Behav. Process. 17, 36–44. ( 10.1037/0097-7403.17.1.36) [DOI] [Google Scholar]
- 34.Izquierdo LA, Barros DM, Medina JH, Izquierdo I. 2003. Exposure to novelty enhances retrieval of very remote memory in rats. Neurobiol. Learn. Mem. 79, 51–56. ( 10.1016/S1074-7427(02)00006-0) [DOI] [PubMed] [Google Scholar]
- 35.Parker A, Wilding E, Akerman C. 1998. The Von Restorff effect in visual object recognition memory in humans and monkeys. The role of frontal/perirhinal interaction. J. Cogn. Neurosci. 10, 691–703. ( 10.1162/089892998563103) [DOI] [PubMed] [Google Scholar]
- 36.Beran MJ. 2011. Chimpanzees (Pan troglodytes) show the isolation effect during serial list recognition memory tests. Anim. Cogn. 14, 637–645. ( 10.1007/s10071-011-0398-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz BL, Colon MR, Sanchez IC, Rodriguez IA, Evans S. 2002. Single-trial learning of ‘what’ and ‘who’ information in a gorilla (Gorilla gorilla gorilla): implications for episodic memory. Anim. Cogn. 5, 85–90. ( 10.1007/s10071-002-0132-0) [DOI] [PubMed] [Google Scholar]
- 38.Rubin DC, Wenzel A. 1996. One hundred years of forgetting: a quantitative description of retention. Psychol. Rev. 103, 734–760. ( 10.1037/0033-295X.103.4.734) [DOI] [Google Scholar]
- 39.Saive A-L, Ravel N, Thévenet M, Royet J-P, Plailly J. 2013. A novel experimental approach to episodic memory in humans based on the privileged access of odors to memories. J. Neurosci. Methods 213, 22–31. ( 10.1016/j.jneumeth.2012.11.010) [DOI] [PubMed] [Google Scholar]
- 40.Aggleton JP, Waskett L. 1999. The ability of odours to serve as state-dependent cues for real-world memories: can Viking smells aid the recall of Viking experiences? Br. J. Psychol. 90, 1–7. ( 10.1348/000712699161170) [DOI] [PubMed] [Google Scholar]
- 41.Baayen RH. 2008. Analyzing linguistic data: a practical introduction to statistics using R. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.McCullagh P, Nelder JA. 1989. Generalized linear models. London, UK: Chapman and Hall. [Google Scholar]
- 43.Dobson AJ, Barnett A. 2008. An introduction to generalized linear models. Boca Raton, FL: Chapman and Hall/CRC Press. [Google Scholar]
- 44.Forstmeier W, Schielzeth H. 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 66, 47–55. ( 10.1007/s00265-010-1038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Field A. 2005. Discovering statistics using SPSS. London, UK: Sage Publications. [Google Scholar]
- 46.Nairne JS. 2002. The myth of the encoding–retrieval match. Memory 10, 389–395. ( 10.1080/09658210244000216) [DOI] [PubMed] [Google Scholar]
- 47.Watkins OC, Watkins MJ. 1975. Buildup of proactive inhibition as a cue-overload effect. J. Exp. Psychol. Hum. Learn. Mem. 1, 1–15. ( 10.1037/0278-7393.1.4.442) [DOI] [Google Scholar]
- 48.Smith SM. 1994. Theoretical principles of context-dependent memory. In Theoretical aspects of memory (eds Gruneberg MM, Morris PE), pp. 168–195. London, UK: Taylor & Francis [Google Scholar]
- 49.Lewis A, Call J, Berntsen D. 2017. Distinctiveness enhances long-term event memory in non-human primates, irrespective of reinforcement. Am. J. Primatol. 22665 ( 10.1002/ajp.22665) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material.