Abstract
The worldwide increase in human outdoor activities raises concerns for wildlife. Human disturbances, even at low levels, are likely to impact species during sensitive periods of the annual cycle. However, experimental studies during the putative sensitive period of territory establishment of birds which not only investigate low disturbance levels, but which also exclude the effect of habitat modification (e.g. walking trails) are lacking. Here, we experimentally disturbed birds in forest plots by walking through twice a day during territory establishment. Later we compared the breeding bird community of experimentally disturbed plots with that of undisturbed control plots. We discovered that the number of territories (−15.0%) and species richness (−15.2%) in disturbed plots were substantially reduced compared with control plots. Species most affected included those sensitive to human presence (assessed by flight-initiation distances), open-cup nesters and above-ground foragers. Long-distance migrants, however, were unaffected due to their arrival after experimental disturbance took place. These findings highlight how territory establishment is a sensitive period for birds, when even low levels of human recreation may be perceived as threatening, and alter settlement decisions. This can have important implications for the conservation of species, which might go unnoticed when focusing only on already established birds.
Keywords: forest birds, nesting guild, foraging guild, flight-initiation distance, nature-based activities, outdoor recreation
1. Introduction
Outdoor recreational activities have increased substantially in past decades [1,2], which has led to repeated encounters between people and wildlife. These encounters may provoke wildlife responses, such as increased vigilance, heightened stress-hormone levels, anti-predator escape responses and, in some cases, a decrease in survival and/or reproduction or even abandonment of an area [3–8]. Wildlife responses to human recreation will not only depend on the characteristics of the animals involved (e.g. species, sex) and on the type of human disturbance (e.g. noise level, number of people), but also on the environmental conditions (e.g. habitat) and on the specific period in an animal's life history in which the encounter with humans occurs [8].
The timing of disturbance events also warrants more attention from researchers [9]. Although a number of studies have been conducted during sensitive periods, such as reproduction or other periods of energetic constraints [7,10–12], territory establishment remains understudied [8,13]. During this phase, even low-intensity and short-lasting disturbance events could prompt animals to perceive habitats as risky and influence their decision on where to breed, thus altering the density and species richness of the breeding community. An increase from no disturbance to low-level disturbance is likely to have a proportionally stronger ecological impact than a change from low- to medium-level disturbance, or from medium- to high-level disturbance [14–17]. This may apply particularly to the sensitive phase of territory establishment.
In the field of avian biology, human outdoor recreation has been linked to lower abundance and reduced species richness [18–20]. However, these studies did not focus on the underlying processes being altered by disturbance (e.g. prevention from settling versus later breeding failure), and often cannot separate direct effects of human presence from indirect effects (e.g. habitat alterations normally associated with recreation). That is, human recreational activities are mostly bound to roads or trails, and thus always occur with habitat alterations [21], which are known to impact species distribution and abundance [22,23]. Therefore, experimental studies are needed to determine the direct effects of human presence on birds and the processes involved [17,24].
The aim of this study was to experimentally investigate whether human recreational activities at relatively low levels altered bird settlement decisions during territory establishment, and thus the resulting breeding-bird community. We expected that experimental disturbance during territory establishment would lead to lower densities of breeding birds. Depending on species-specific tolerances towards disturbance, we also predicted changes in species richness and composition. Notably, we expected a reduction in the abundance and number of bird species sensitive to human disturbance, such as ground-nesting, ground-foraging [20,25–27] and disturbance-sensitive species [28–30].
2. Material and methods
(a). Study site
This study was done in the Forêt domaniale de Chaux, a large (200 km2) oak–hornbeam forest in eastern France (47°05′ N, 05°40′ E) fulfilling all legal and animal welfare regulations (permit number 2014157-0012 of the Direction Régionale de l'Environnement, de l'Aménagement et du Logement de Franche-Comté). This forest is subdivided into approximately rectangular 10 ha plots, where harvest is managed by the Office Nationale des Forêts. The plots were separated from each other by small treeless tracks but otherwise comprised natural vegetation, generated by harvest machines. One side was bordered by a gravel road. The forest was only accessible to the public on foot or by bike, with the exception of two paved roads crossing the forest. Recreational activities were concentrated mainly near the town of Dole (23 000 inhabitants), located at the western border of the forest (figure 1), and near Besançon (117 000 inhabitants), which is 15 km to the east of the forest. In most of the forest, the frequency of human recreational activities was extremely low, and occurred primarily in autumn (i.e. mushroom collection and hunting). During our fieldwork (continuous from March to June of 2014 and 2015, 50 h per week) we rarely saw people off-trail within the plots (less than one person per month) and we saw approximately one person per week on the gravel roads near the study plots.
Figure 1.
Study site Forêt domaniale de Chaux (dark grey area) with the 12 different plots (black rectangles) and Dole as the next town. Shown in detail is a schematic representation of a study plot with the two split-plots (one disturbed, one control). The black-dotted line represents an example of a disturbance walk (the orientation of this transect was turned 90° between disturbance events). A 30 m buffer was left between the disturbance path and the line separating the split-plots (white continuous line), to lower a potential confounding effect into the control split-plot. This distance was selected considering the information available in the literature about flight initiation distances (FID) of bird species found in this forest (FID for more than 80% of the species is below 30 m). The grey dashed line represents the breeding-bird census transect and the individual black dots are vegetation survey points (for simplicity depicted systematically, although stratified random sampling was used).
(b). Experimental design
We used plots (mean size: 9.2 ha; range: 7.5–13 ha) in the centre of the forest (more than 9 km from Dole and Besançon; figure 1) where no timber harvesting occurred during the study period (2014–2015; as agreed with the Office National des Forêts). The composition and structure of the vegetation was homogeneous within plots and similar among plots. The plots were dominated by pedunculate oaks Quercus robur, many older than 100 years (M. Romanski 2016, personal communication), with admixed European beech Fagus sylvatica and European hornbeam Carpinus betulus, as well as Norway spruce Picea abies and Douglas fir Pseudotsuga menziesii in small numbers. The plots were at least 600 m apart from each other to prevent confounding neighbouring effects. We divided all plots in two halves (split-plots, mean 4.7 ha); one half was experimentally disturbed while the other half served as a control. The split-plots receiving the disturbance treatment were chosen randomly with the only constraint that half of them were bordered by the gravel road and the other half not.
Since our objective was to examine the effect of human recreation during bird-territory establishment, we only disturbed birds during the pre-breeding season, from early March until mid-April (7 March–22 April) [31]. Disturbance events consisted of a group of two or three people, carrying a loudspeaker (Hama, smartphone speaker, power 3 W, with a Samsung digital audio player F3) and walking through a pre-established mower-pattern transect with back and forth lines separated by 20 m (figure 1). The loudspeakers were continuously playing human conversations (obtained from several sources and languages, from TV shows to audio books) at an average human-conversation volume level (approx. 60 dB at 1 m distance [32,33]). The orientation of the path was turned 90° from one disturbance event to the next to reduce predictability of the disturbance. These disturbance events lasted around 45 min depending on the split-plot area (mean = 42 min, s.d. = 13 min) and occurred one to three times per day during daytime. The order in which the split-plots were disturbed changed daily to avoid biases in the time of disturbance.
Owing to logistical reasons and man-power limitations, the experimental disturbance in 2014 was restricted to six treatment plots which were disturbed on average 1.6 times per day (each plot at least once each day). In 2015, we were able to extend the experiment to 12 plots which were disturbed 2.3 times per day (each plot at least once per day). In the case of the six plots that were common for both years, we switched the disturbed and control split-plots from one year to the next to exclude possible split-plot-specific effects. In 2014, we therefore disturbed each of the six split-plots 73 times during 45 days, and in 2015 each of the 12 split-plots 105 times during 46 days. The low number of disturbance events allowed us to examine the effects of recreation at intensities much lower than most previous studies in recreation ecology [18,34], thus enabling us to test whether even low levels of disturbance could have an impact when applied during sensitive periods.
(c). Bird-territory mapping
The breeding bird territories were censused in all plots three times per season in both years of the study (first census round 20 April–6 May, second 6 May–22 May, third 28 May–17 June) to include the breeding season of all forest-bird species, from residents to late-arriving long-distance migrants. We did not census earlier, to be sure that birds were already settled and that we did not disturb the control split-plots during the territory establishment period. Censuses started at sunrise and lasted 30–66 min (mean = 42 min) depending on plot size. Censuses followed the Swiss standard breeding-bird survey protocol [35,36] and consisted of recording all birds seen or heard showing territorial behaviour on a map, while following a mower-pattern transect (lines 60 m apart) covering the entire plot (including both disturbed and control split-plots; figure 1). The censuses were performed by the same two observers in both years, each of which always surveyed the same plots. For each round, we determined the number of contacts per species, but counted pairs and families as one to approach the number of territories detected. Long-distance migrants were not considered as breeders, but as transients, if seen or heard before usual arrival dates (according to Schmid et al. [37]; see electronic supplementary material, table S1).
(d). Vegetation surveys
In June 2015, we characterized the vegetation of all 12 plots by using a stratified random sampling (i.e. distributing one survey point per 0.5 ha grid cell, which resulted in 7–13 points per split-plot and 210 survey points in total; figure 1). At each point the following variables were measured: canopy cover (visual estimation of percentage of cover in the observer's visual field when looking straight up; always measured by the same person), ground vegetation cover (in 2 × 2 m quadrats), shrub cover (in 3 × 3 m quadrats), and number of trees per species and standing dead trunks with diameter at breast height (dbh) larger than 5 cm in 8 × 8 m quadrats. Before analyses, we averaged vegetation measures to create mean values per split-plot. There were no significant within-plot differences in any of the vegetation variables between the disturbed and the control split-plots (pairwise t-tests or Wilcoxon tests, depending on data distributions, p > 0.05).
(e). Data analysis
We tested for the effect of human disturbance during territory establishment on the number of territories and on species richness, both following a Poisson distribution, with two separate generalized linear mixed models (table 1). The factor disturbance treatment (disturbed versus control split-plot) was included as explanatory variable. To differentiate between birds whose territory-establishment period overlapped with the experimental treatment (residents and short-distance migrants) and long-distance migrants arriving afterwards and not being exposed to the disturbance, both number of territories and species richness were calculated separately for these two groups, and a two-level factor migration type (‘long-distance migrants’ versus ‘others’; see electronic supplementary material, table S1) was added to the models. To describe the structure and composition of the vegetation we included the following explanatory variables: ground vegetation cover, shrub cover, canopy cover, amount of deadwood, and tree diversity (Shannon diversity index [38] of the main tree species: pedunculate oak Quercus robur, European beech Fagus sylvatica, European hornbeam Carpinus betulus, and two species of conifers: Picea abies, Pseudotsuga menziesii). Additionally, we incorporated the presence/absence of a gravel road along the split-plot to control for further possible habitat differences between split-plots, the linear and quadratic effect of Julian date to account for bird-breeding phenology, and the year (2014 versus 2015) to control for inter-annual differences in the intensity of disturbance and in climatic conditions. We also added the two and three-way interactions between year, disturbance and migration type. We included the split-plot area (in hectares) into the models to account for unequal plot sizes [39,40]. For the model on the number of territories we had to include the logarithm of the split-plot area as an offset term to model territory densities (after Korner-Nievergelt et al. [39]), while for the model on species richness, we included the quadratic effect of area (split-plot area2).
Table 1.
Results of the GLMMs testing the effect of experimental disturbance (human recreation) on the number of bird territories and species richness. Represented are the estimates of the effect of each variable with the corresponding 95% credible intervals (CrI). Symbols: /, reference categories; —, parameter not tested in the given model. Distribution, Poisson; link function, natural logarithm; random factors, observer, round and split-plot ID nested within plot ID.
| no. territories |
species richness |
|||
|---|---|---|---|---|
| terms | estimate | CrI | estimate | CrI |
| intercept | 0.8810 | 0.5780; 1.1814 | 1.9444 | 1.6828; 2.2046 |
| disturbance | ||||
| disturbed | −0.2232 | −0.4308; −0.0092 | −0.2151 | −0.4790; 0.0584 |
| control | / | / | / | / |
| type | ||||
| others | / | / | / | / |
| long-distance migrant | −3.9117 | −4.8799; −2.9224 | −3.4014 | −4.3907; −2.3873 |
| ground vegetation cover | 0.0253 | −0.0723; 0.1203 | −0.0261 | −0.1163; 0.0664 |
| shrub cover | 0.1084 | −0.0125; 0.2260 | 0.1057 | −0.0151; 0.2272 |
| canopy cover | 0.0294 | −0.0855; 0.1478 | −0.0343 | −0.1736; 0.1071 |
| tree diversity | −0.0235 | −0.1224; 0.0738 | −0.0728 | −0.1704; 0.0261 |
| deadwood | 0.0259 | −0.0806; 0.1336 | 0.0479 | −0.0782; 0.1735 |
| road | ||||
| presence | 0.0190 | −0.1559; 0.1941 | −0.0226 | −0.2317; 0.1800 |
| absence | / | / | / | / |
| Julian date | −0.1231 | −0.2153; −0.0328 | −0.0155 | −0.1327; 0.1072 |
| Julian date2 | −0.0524 | −0.1555; 0.0529 | −0.0111 | −0.1405; 0.1184 |
| year | ||||
| 2014 | / | / | / | / |
| 2015 | 0.1018 | −0.0801; 0.2828 | 0.0519 | −0.1757; 0.2807 |
| split-plot area | −0.0502 | −0.1469; 0.0461 | 0.2374 | −0.7693; 1.2876 |
| split-plot area2 | — | — | 0.9647 | −0.1803; 2.0994 |
| disturbance × type | ||||
| disturbed: long-distance migrant | −0.0873 | −1.5989; 1.3863 | −0.1135 | −1.6684; 1.3940 |
| disturbance × year | ||||
| disturbed: 2015 | 0.1131 | −0.1484; 0.3746 | 0.0966 | −0.2403; 0.4227 |
| type × year | ||||
| long-distance migrant: 2015 | 0.3329 | −0.8118; 1.4819 | 0.3098 | −0.8357; 1.4698 |
| disturbance × type × year | ||||
| disturbed: long-distance migrant: 2015 | 0.2277 | −1.4639; 1.9970 | 0.2847 | −1.4482; 1.9820 |
Additionally, in order to investigate the effects of experimental disturbance on the density and richness of specific types of birds, we classified all bird species according to their nesting guild (ground, open-cup and cavity nesters [41]), their tolerance to human approach (sensitivity; high versus low), and their foraging guild (ground versus above ground [41]) (see electronic supplementary material, table S1). For each of the three classifications, we then applied two models (i.e. for number of territories and species) similar to the ones above, but replacing the factor migration type by the factor corresponding to each classification (i.e. nesting guild, sensitivity or foraging guild). These six models were performed without long-distance migrants (i.e. with only those species whose territory-establishment period overlapped with experimental disturbance). Bird sensitivity classes were approximated by using the mean flight initiation distance (FID) of the given species during the breeding period in non-urban areas, as obtained from Díaz et al. [42]. Low-sensitivity species had an average FID lower or equal to the overall median value of FID for all species observed breeding in our plots (median FID = 13.13 m), whereas high-sensitivity species had FID larger than the overall median FID. We acknowledge that FID might not always truly represent species sensitivity, as modulating factors (e.g. vegetation) may influence birds' antipredator behaviour [8]; however, FID is an acceptable and widely available measure for approximating sensitivity towards human disturbance [29].
In all models, to account for observer effect we included observer (two-level factor) as a random factor. To account for variations among rounds and plots, census round (first, second, third) and split-plot-ID were nested within plot-ID. All analyses were performed in R v. 3.3.0 [43] with the function glmer from package lme4 [44]. All numeric explanatory variables were standardized (mean = 0 and s.d. = 1) to facilitate model convergence. We used a Bayesian framework to calculate the 95% credible intervals (CrI) of the parameter estimates and model predictions. To do so we simulated random samples (n = 10 000) from the joint posterior distribution of the model parameters using the function sim from the R-package arm [45] (electronic supplementary material, figures S3 and S4), from which we used the 2.5% and 97.5% quantiles as the lower and upper limit of the 95% CrI. To assess how split-plot type (disturbed and control) interacted with year and bird characteristics, we calculated the posterior probability (between 0.5 and 1; using Monte Carlo simulation) of the hypothesis that the mean number of territories or of species at disturbed sites was lower than at control sites (figures 2 and 3). Using this approach, higher probabilities represent a stronger difference between treatments. Goodness-of-fit was assessed through visual examination of plotted residuals, and we confirmed that there was no overdispersion (values of the R-function dispersion_glmer always below 1, after Korner-Nievergelt et al. [39]). Note that the numbers of territories and species obtained are not absolute numbers, because we did not account for imperfect detection. Accounting for imperfect detection would have added a layer of complexity to our models, which would have been incompatible with sample size (over-parameterization) and caused problems of convergence.
Figure 2.
Effects of experimental human disturbance on (a,b) the number of territories and (c,d) species richness per split-plot (4.7 ha) according to year and migration type: the graphs (a) and (c) include only the resident and short-distance migrant species, while the graphs (b) and (d) include only the long-distance migrants. Note the different y-axes. Represented are mean fitted values with 95% credible intervals (table 1) and the posterior probability (PP, from 0.5 to 1) that the difference between disturbed and control split-plots is different from zero. The larger the PP, the more likely it is that disturbed and control split-plots are different.
Figure 3.
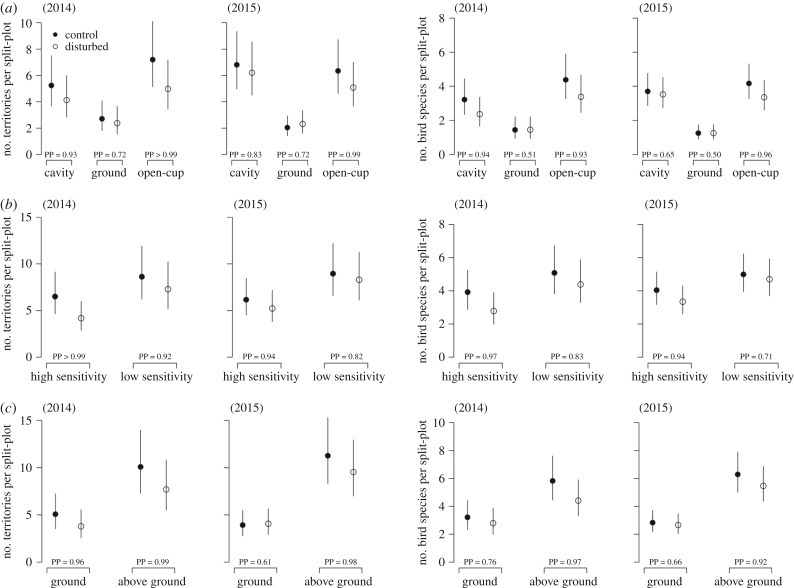
Effect of experimental human disturbance on the number of territories and species richness per split-plot (4.7 ha) according to (a) nesting-guild (cavity, ground and open-cup nesters), (b) sensitivity of the species (high = FID > median FID, low = FID ≤ median FID), and (c) foraging guild (ground and aboveground) in 2014 and 2015, respectively (only for residents and short distance migrants). Represented are mean fitted values with 95% credible intervals and the corresponding posterior probabilities (PP) that the differences between disturbed and control split-plots are different from zero.
3. Results
Both the number of territories and species richness were substantially lower in disturbed split-plots than in the control ones (table 1, figure 2). Moreover, this effect was only observed for resident and short-distance migrant species which experienced the experimental disturbance (figure 2a,c). It was not observed for species arriving later in the season (long-distance migrants; figure 2b,d). We found a reduction of about 15% in the number of territories for residents and short-distance migrants per mean disturbed split-plot compared with the control split-plot over both years (19.8% in 2014 and 10.2% in 2015). Species richness of resident and short-distance migrants also dropped by 15% in the disturbed split-plots compared with control split-plots (19.4% in 2014 and 10.9% in 2015). Independent of the disturbance treatment, the number of territories detected decreased with Julian date.
The response to experimental disturbance varied depending on the characteristics of the species (electronic supplementary material, figure S1 and figure S2). The effect of disturbance appeared to be largest on open-cup nesters compared with cavity or ground nesters (figure 3a; electronic supplementary material, table S2). High-sensitivity species showed a stronger negative response to disturbance than low-sensitivity species (figure 3b; electronic supplementary material, table S3). Finally, above-ground foragers appeared more affected by disturbance than ground foragers (figure 3c; electronic supplementary material, table S4). Overall, there seemed to be a stronger effect of disturbance on the number of territories and species richness in the first year (2014) compared with the second year (2015).
4. Discussion
Our findings confirm our hypothesis that even low levels of disturbance during territory establishment, with no concomitant habitat alteration, can have a negative effect on both density of breeding birds and species richness. Such an effect was not apparent in long-distance migrants, as they arrived after the end of the experimental disturbance, and thus were not exposed to it. These findings are in agreement with Steven et al. [15] and Monz et al. [14], which state that even low levels of disturbance (such as ours) can have significant importance. Contrary to other experimental studies, which also show a negative link between human disturbance and bird density and/or diversity [46–49], we restricted disturbance to the territory establishment period. Thus, our results suggest that territory establishment may be a sensitive period, in which human disturbances could greatly affect the density and diversity of breeding birds.
During the territory establishment period birds select breeding sites, and the presence of humans might ‘invisibly' lower the quality of the habitat [50]. A possible explanation for the observed effects could be that birds perceive recreationists as predators [34]. Indeed, the presence of predators has been shown to strongly affect breeding site selection [51,52]. Birds are therefore anticipated to select against habitats with more recreational activity, resulting in altered breeding-bird communities as shown in this study. These results emphasize the important role played by human disturbance on species abundance and diversity.
As predicted, we found that the effect of experimental disturbance varied according to species characteristics. Open-cup nesters were more affected than cavity nesters. This finding is in accordance with Kangas et al. [20] and Martin & Li [53], suggesting that cavities confer extra protection, which lowers the effect of disturbance and predation. Surprisingly, we did not observe an effect of experimental recreation on ground-nesting birds, as has been previously suggested by Kangas et al. [20]. Similarly, there was no effect of experimental disturbance on ground-foragers. This was probably due to the low number of ground-nesting and ground-foraging species in our study sites (see electronic supplementary material, table S1), making an effect of disturbance hard to detect. As expected, we demonstrated a stronger impact of experimental disturbance on the more sensitive species (i.e. with larger FID) than on the less sensitive species. Species with larger FID are generally larger-bodied species [54] and therefore human disturbance is expected to affect these species the most.
The negative response of birds to the experimental disturbance in the first study year was stronger than in the second year, despite increased disturbance intensity in the second year. This finding could be a consequence of the greater total number of territories found in the second year, which could have forced birds to also accept non-preferred (i.e. disturbed) habitats [55–58], diminishing the differences between treatments in the second study year. Habituation effects, on the other hand, can be ruled out due to the treatment switching from the first to the second study year. Carryover effects from the first to the second year in interaction with treatment switching could also partially explain this inter-annual difference in impact. That is, if birds experiencing the disturbance split-plot in the first year tried to avoid it in the second year, this could lead to lower starting numbers in this split-plot, which became the control split-plot in the second year. This uneven starting number could have partially obscured the effect of disturbance the second year. However, the overall increased numbers of territories in both split-plots in the second year suggest that these carryover effects, if at all present, would play only a marginal role. Another partial explanation of this decreased effect in the second year could be differences in weather conditions or that food availability was better in the second year, thus increasing the perceived quality of disturbed split-plots. Unfortunately, we did not measure food resources.
Our findings highlight how the impacts of disturbance can go unnoticed when examined later in the season. Indeed, individuals and species establish breeding territories early in the season. A pre-selection in favour of bolder personalities and species might have already occurred during the pre-breeding phase of territory establishment (by tourists or researchers visiting and area). We should therefore be careful when planning and interpreting the results of studies occurring during the breeding season sensu stricto. Future studies should investigate the consequences of these recreation-driven reductions in number of territories and species for subsequent breeding parameters, survival and overall population dynamics.
In conclusion, this study emphasizes that negative effects of human recreational disturbance can already occur after low-intensity disturbance events, even when occurring over a short time period. This is especially relevant during territory establishment in early spring, when improving weather conditions entail an increase in outdoor recreation (at least in temperate regions; R. Schmidt 2015, unpublished data). Given the potential conservation implications of these results, we suggest that conservationists and park managers should not only manage disturbance during the main breeding season, but also during territory establishment. Disturbance management could include limiting human access to certain areas that are likely to be used by vulnerable species to establish breeding territories. Additionally, the network of trails open to the public could be reduced temporarily to increase the size of the undisturbed patches. Furthermore, appropriate information should be provided to visitors about the importance of staying on trails to minimize their impacts on wildlife. These measures could help protect sensitive birds (species or individuals of certain personalities) that would settle in an area if there were no human activities during the pre-breeding season.
Ethics
This study was carried out in accordance with permit 2014157-0012 from the Direction Régionale de l'Environnement, de l'Aménagement et du Logement de Franche-Comté.
Supplementary Material
Supplementary Material
Acknowledgements
We greatly thank all field assistants and trainees who helped during the disturbance phase, which laid the basis for the entire experiment. In alphabetical order: C. Arnace, R. Baffoin, M. Crespo Ballester, S. Bühler, B. Garde, L. Gillespie, M. Hitt, F. Le Bagousse, H. Lemke, F. Leugger, N. Mortier, J. Nouri, M. Quetstroey, A. Rouméas, J. Vasseur. We thank H. Lemke, for doing half of the bird censuses, M. Romanski and J. L. Dessolin from the Office National des Forêts (ONF), as well as B. Dorbani from the DREAL (Direction Régionale de l'Environnement, de l'Aménagement et du Logement de Franche-Comté) for allowing us to do this study in the Forêt domaniale de Chaux. We thank R. Scheifler from the University Franche-Comté, Besançon, who advised us on local affairs and permits during the preparation of this study. We thank three anonymous reviewers for their helpful comments, Kiran L. Dhanjal-Adams for improving the English, and B. Almasi, M. Kéry and F. Korner-Nievergelt for support with the statistics.
Data accessibility
The dataset used for this manuscript is available from Dryad Digital Repository [59].
Authors' contributions
Y.B. participated in the study design, collected field data, analysed the data and wrote the manuscript. Z.T. helped with designing the study and analysing the data, and worked on manuscript drafts. L.J. participated in the study design and coordination, and worked on manuscript drafts.
Competing interests
All authors gave final approval for publication and have no competing interests.
Funding
Funded by the Swiss Ornithological Institute.
References
- 1.Boyle SA, Samson FB. 1985. Effects of nonconsumptive recreation on wildlife: a review. Wildl. Soc. Bull. 13, 110–116. [Google Scholar]
- 2.Balmford A, Beresford J, Green J, Naidoo R, Walpole M, Manica A. 2009. A global perspective on trends in nature-based tourism. PLoS Biol. 7, 1–6. ( 10.1371/journal.pbio.1000144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiel D, Jenni-Eiermann S, Braunisch V, Palme R, Jenni L. 2008. Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: a new methodological approach. J. Appl. Ecol. 45, 845–853. ( 10.1111/j.1365-2664.2008.01465.x) [DOI] [Google Scholar]
- 4.Mathot KJ, Wright J, Kempenaers B, Dingemanse NJ. 2012. Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 121, 1009–1020. ( 10.1111/j.1600-0706.2012.20339.x) [DOI] [Google Scholar]
- 5.Tarjuelo R, Barja I, Morales MB, Traba J, Benítez-López A, Casas F, Arroyo B, Delgado MP, Mougeot F. 2015. Effects of human activity on physiological and behavioral responses of an endangered steppe bird. Behav. Ecol. 26, 828–838. ( 10.1093/beheco/arv016) [DOI] [Google Scholar]
- 6.Ikuta LA, Blumstein DT. 2003. Do fences protect birds from human disturbance? Biol. Conserv. 112, 447–452. ( 10.1016/S0006-3207(02)00324-5) [DOI] [Google Scholar]
- 7.Arlettaz R, Nusslé S, Baltic M, Vogel P, Palme R, Jenni-Eiermann S, Patthey P, Genoud M. 2015. Disturbance of wildlife by outdoor winter recreation: allostatic stress response and altered activity-energy budgets. Ecol. Appl. 25, 1197–1212. [DOI] [PubMed] [Google Scholar]
- 8.Tablado Z, Jenni L. 2017. Determinants of uncertainty in wildlife responses to human disturbance. Biol. Rev. 92, 216–233. ( 10.1111/brv.12224) [DOI] [PubMed] [Google Scholar]
- 9.Buckley R. 2013. Next steps in recreation ecology. Front. Ecol. Environ. 11, 399 ( 10.1890/1540-9295-11.8.399) [DOI] [Google Scholar]
- 10.Strasser EH, Heath JA. 2013. Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J. Appl. Ecol. 50, 912–919. ( 10.1111/1365-2664.12103) [DOI] [Google Scholar]
- 11.Arroyo B, Razin M. 2006. Effect of human activities on bearded vulture behaviour and breeding success in the French Pyrenees. Biol. Conserv. 128, 276–284. ( 10.1016/j.biocon.2005.09.035) [DOI] [Google Scholar]
- 12.Martín B, Delgado S, de la Cruz A, Tirado S, Ferrer M. 2015. Effects of human presence on the long-term trends of migrant and resident shorebirds: evidence of local population declines. Anim. Conserv. 18, 73–81. ( 10.1111/acv.12139) [DOI] [Google Scholar]
- 13.Götmark F. 1992. The effects of investigator disturbance on nesting birds. In Current ornithology (ed. Power DM.), pp. 63–104. Boston, MA: Springer US. [Google Scholar]
- 14.Monz CA, Pickering CM, Hadwen WL. 2013. Recent advances in recreation ecology and the implications of different relationships between recreation use and ecological impacts. Front. Ecol. Environ. 11, 441–446. ( 10.1890/120358) [DOI] [Google Scholar]
- 15.Steven R, Pickering C, Castley JG. 2011. A review of the impacts of nature based recreation on birds. J. Environ. Manage. 92, 2287–2294. ( 10.1016/j.jenvman.2011.05.005) [DOI] [PubMed] [Google Scholar]
- 16.Steidl RJ, Powell BF. 2006. Assessing the effects of human activities on wildlife. The George Wright Forum 23, 50–58. [Google Scholar]
- 17.Hill D, Hockin D, Price D, Tucker G, Morris R, Treweek J. 1997. Bird disturbance: improving the quality and utility of disturbance research. J. Appl. Ecol. 34, 275–288. ( 10.2307/2404876) [DOI] [Google Scholar]
- 18.Van der Zande AN, Berkhuizen JC, Van Latesteijn HC, ter Keurs WJ, Poppelaars AJ. 1984. Impact of outdoor recreation on the density of a number of breeding bird species in woods adjacent to urban residential areas. Biol. Conserv. 30, 1–39. ( 10.1016/0006-3207(84)90018-1) [DOI] [Google Scholar]
- 19.Patthey P, Wirthner S, Signorell N, Arlettaz R. 2008. Impact of outdoor winter sports on the abundance of a key indicator species of alpine ecosystems. J. Appl. Ecol. 45, 1704–1711. ( 10.1111/j.1365-2664.2008.01547.x) [DOI] [Google Scholar]
- 20.Kangas K, Luoto M, Ihantola A, Tomppo E, Siikamäki P. 2010. Recreation-induced changes in boreal bird communities in protected areas. Ecol. Appl. 20, 1775–1786. ( 10.1890/09-0399.1) [DOI] [PubMed] [Google Scholar]
- 21.Forman RTT, Alexander LE. 1998. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 29, 207–231. ( 10.1146/annurev.ecolsys.29.1.207) [DOI] [Google Scholar]
- 22.Butler LK, Ries L, Bisson I.-A, Hayden TJ, Wikelski MM, Romero LM. 2012. Opposite but analogous effects of road density on songbirds with contrasting habitat preferences. Anim. Conserv. 16, 77–85. ( 10.1111/j.1469-1795.2012.00576.x) [DOI] [Google Scholar]
- 23.Fernández-Juricic E. 2000. Local and regional effects of pedestrians on forest birds in a fragmented landscape. Condor 102, 247–255. ( 10.1650/0010-5422(2000)102%5B0247:LAREOP%5D2.0.CO;2) [DOI] [Google Scholar]
- 24.Gill JA. 2007. Approaches to measuring the effects of human disturbance on birds. Ibis (Lond. 1859). 149, 9–14. ( 10.1111/j.1474-919X.2007.00642.x) [DOI] [Google Scholar]
- 25.Langston RHW, Liley D, Murison G, Woodfield E, Clarke RT. 2007. What effects do walkers and dogs have on the distribution and productivity of breeding European nightjar Caprimulgus europaeus? Ibis (Lond. 1859) 149, 27–36. ( 10.1111/j.1474-919X.2007.00643.x) [DOI] [Google Scholar]
- 26.Mallord JW, Dolman PM, Brown AF, Sutherland WJ. 2007. Linking recreational disturbance to population size in a ground-nesting passerine. J. Appl. Ecol. 44, 185–195. ( 10.1111/j.1365-2664.2006.01242.x) [DOI] [Google Scholar]
- 27.Thompson B. 2015. Recreational trails reduce the density of ground-dwelling birds in protected areas. Environ. Manag. 55, 1181–1190. ( 10.1007/s00267-015-0458-4) [DOI] [PubMed] [Google Scholar]
- 28.Livezey KB, Fernández-Juricic E, Blumstein DT. 2016. Database and metadata of bird flight initiation distances worldwide to assist in estimating human disturbance effects and delineating buffer areas. J. Fish Wildl. Manag. 7, 1–11. ( 10.3996/082015-JFWM-078) [DOI] [Google Scholar]
- 29.Blumstein DT, Anthony LL, Harcourt R, Ross G. 2003. Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Conserv. 110, 97–100. ( 10.1016/S0006-3207(02)00180-5) [DOI] [Google Scholar]
- 30.Weston MA, McLeod EM, Blumstein DT, Guay PJ. 2012. A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu 112, 269–286. ( 10.1071/MU12026) [DOI] [Google Scholar]
- 31.von Blotzheim UNG, Bauer K, Bezzel E. 1993. Handbuch der Vögel Mitteleuropas. Frankfurt aM, Germany: Akademische Verlagsgesellschaft. [Google Scholar]
- 32.Hacki T. 1996. Comparative speaking, shouting and singing voice range profile measurement: physiological and pathological aspects. Logop. Phoniatr. Vocology 21, 123–129. ( 10.3109/14015439609098879) [DOI] [PubMed] [Google Scholar]
- 33.Byrne D., et al. 1994. An international comparison of long-term average speech spectra. J. Acoust. Soc. Am. 96, 2108–2120. ( 10.1121/1.410152) [DOI] [Google Scholar]
- 34.Beale CM, Monaghan P. 2004. Human disturbance: people as predation-free predators? J. Appl. Ecol. 41, 335–343. ( 10.1111/j.0021-8901.2004.00900.x) [DOI] [Google Scholar]
- 35.Schmid H, Spiess M.. 2008. Brutvogelaufnahmen bei BDM-Z7 und MHB: Anleitung zur Entscheidfindung bei Grenzfällen und zur Revierausscheidung. Sempach, Switzerland: Schweizerische Vogelwarte [Google Scholar]
- 36.Bibby CJ, Burgess ND, Hill DA, Mustoe SH. 2000. Bird census techniques, 2nd edn London: Academic Press. [Google Scholar]
- 37.Schmid H, Luder R, Naef-Daener B, Graf R, Zbinden N.. 1998. Schweizer Brutvogelatlas: Verbreitung der Brutvögel in der Schweiz und im Fürstentum Liechtenstein 1993–1996. Sempach, Switzerland: Schweizerische Vogelwarte. [Google Scholar]
- 38.Spellerberg IF, Fedor PJ. 2003. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon-Wiener’ index. Glob. Ecol. Biogeogr. 12, 177–179. ( 10.1046/j.1466-822X.2003.00015.x) [DOI] [Google Scholar]
- 39.Korner-Nievergelt F, Roth T, von Felten S, Guélat J, Almasi B, Korner-Nievergelt P. 2015. Bayesian data analysis in ecology using linear models with R, BUGS, and Stan. London, UK: Academic Press. [Google Scholar]
- 40.Connor EF, McCoy ED. 1979. The statistics and biology of the species-area relationship. Am. Nat. 113, 791–833. [Google Scholar]
- 41.Perrins C, Cramp S. 1998. The complete birds of the Western Palearctic on CD-ROM. Oxford, UK: Oxford University Press. [Google Scholar]
- 42.Díaz M, Møller AP, Flensted-Jensen E, Grim T, Ibáñez-Álamo JD, Jokimäki J, Markó G, Tryjanowski P. 2013. The geography of fear: a latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE 8, e64634 ( 10.1371/journal.pone.0064634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 44.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 45.Gelman A, Su Y-S. 2015. Arm: data analysis using regression and multilevel/hierarchical models. R package version 1.8-6. See https://CRAN.R-project.org/package=arm. [Google Scholar]
- 46.Holm TE, Laursen K. 2009. Experimental disturbance by walkers affects behaviour and territory density of nesting black-tailed godwit Limosa limosa. Ibis (Lond. 1859). 151, 77–87. ( 10.1111/j.1474-919X.2008.00889.x) [DOI] [Google Scholar]
- 47.Gutzwiller KJ, Anderson SH. 1999. Spatial extent of human-intrusion effects on subalpine bird distributions. Condor 101, 378–389. ( 10.2307/1370001) [DOI] [Google Scholar]
- 48.Baines D, Richardson M. 2007. An experimental assessment of the potential effects of human disturbance on black grouse Tetrao tetrix in the North Pennines, England. Ibis (Lond. 1859). 149, 56–64. ( 10.1111/j.1474-919X.2007.00638.x) [DOI] [Google Scholar]
- 49.Riffell SK, Gutzwiller KJ, Anderson SH. 1996. Does repeated human intrusion cause cumulative declines in avian richness and abundance? Ecol. Appl. 6, 492–505. [Google Scholar]
- 50.Reed SE, Merenlender AM. 2008. Quiet, nonconsumptive recreation reduces protected area effectiveness. Conserv. Lett. 1, 146–154. ( 10.1111/j.1755-263X.2008.00019.x) [DOI] [Google Scholar]
- 51.Norrdahl K, Korpimäki E. 1998. Fear in farmlands: how much does predator avoidance affect bird community structure? J. Avian Biol. 29, 79–85. ( 10.2307/3677344) [DOI] [Google Scholar]
- 52.Fontaine JJ, Martin TE. 2006. Habitat selection responses of parents to offspring predation risk: an experimental test. Am. Nat. 168, 811–818. ( 10.1086/508297) [DOI] [PubMed] [Google Scholar]
- 53.Martin TE, Li P. 1992. Life history traits of open- vs. cavity-nesting birds. Ecology 73, 579–592. [Google Scholar]
- 54.Blumstein DT, Fernández-Juricic E, Zollner PA, Garity SC. 2005. Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 42, 943–953. ( 10.1111/j.1365-2664.2005.01071.x) [DOI] [Google Scholar]
- 55.Ferrer M, Donazar JA. 1996. Density-dependent fecundity by habitat heterogeneity in an increasing population of Spanish imperial eagles. Ecology 77, 69–74. ( 10.2307/2265655) [DOI] [Google Scholar]
- 56.Rodenhouse NL, Sillett TS, Doran PJ, Holmes RT. 2003. Multiple density-dependence mechanisms regulate a migratory bird population during the breeding season. Proc. R. Soc. Lond. B 270, 2105–2110. ( 10.1098/rspb.2003.2438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reijnen R, Foppen R, Ter Braak C, Thissen J. 1995. The effects of car traffic on breeding bird populations in woodland. III. Reduction of density in relation to the proximity of main roads. J. Appl. Ecol. 32, 187–202. [Google Scholar]
- 58.Komdeur J, Huffstadt A, Prast W, Castle G, Mileto R, Wattel J. 1995. Transfer experiments of Seychelles warblers to new islands: Changes in dispersal and helping behaviour. Anim. Behav. 49, 695–708. ( 10.1016/0003-3472(95)80202-9) [DOI] [Google Scholar]
- 59.Bötsch Y, Tablado Z, Jenni L. 2017. Data from: Experimental evidence of human recreational disturbance effects on bird-territory establishment. Dryad Digital Repository. ( 10.5061/dryad.nd951) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bötsch Y, Tablado Z, Jenni L. 2017. Data from: Experimental evidence of human recreational disturbance effects on bird-territory establishment. Dryad Digital Repository. ( 10.5061/dryad.nd951) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset used for this manuscript is available from Dryad Digital Repository [59].