Abstract
A recent study from our group demonstrated that the Ca2+ -sensing receptor (CaSR) was upregulated, and the extracellular Ca2+ -induced increase in cytosolic Ca2+ concentration ([Ca2+]cyt) was enhanced in pulmonary arterial smooth muscle cells from patients with idiopathic pulmonary arterial hypertension and animals with experimental pulmonary hypertension (PH). However, it is unclear whether CaSR antagonists (for example, NPS2143) rescue the development of experimental PH. We tested the rescue effects of NPS2143 in rats with monocrotaline (MCT)-induced PH and mice with chronic hypoxia-induced PH. For the NPS2143 treatment group, rats and mice were i.p. injected with NPS2143 once per day from days 14 to 24. Four weeks after MCT injection or exposure to normobaric hypoxia, the right ventricular (RV) systolic pressure, right heart hypertrophy (RV/LV + S ratio) and RV myocardial fibrosis were rescued or nearly restored to normal levels by NPS2143 treatment. The rescue effects of NPS2143 on experimental PH further support a critical role for the CaSR in the PH mechanism. Therefore, NPS2143 may be a promising potential treatment for pulmonary arterial hypertension.
Keywords: hypoxia, monocrotaline, mouse, NPS2143, rat
Introduction
Pulmonary hypertension (PH) is characterized by a progressive increase in pulmonary vascular resistance and vascular remodeling. Without treatment, this disease leads to right heart failure and death, often within 2 to 3 years of diagnosis.1 Currently, there are limited options available for the prevention and treatment of progressive PH. Increased pulmonary vascular tone and severe structural remodeling of the distal pulmonary arteries are the primary determinants of increased pulmonary vascular resistance. PH subtypes with different etiologies share several common features, for example, increasing severity of pulmonary vasoconstriction, remodeling of pulmonary microvessels and intravascular thrombosis. These changes result in increased medial thickness, microvascular occlusion and the formation of plexiform lesions, all of which contribute to the mechanism of PH.
An increase in the cytosolic Ca2+ concentration ([Ca2+]cyt) in pulmonary arterial smooth muscle cells (PASMCs) is an important stimulus for pulmonary vasoconstriction and vascular remodeling. A recent study from our group indicated that the extracellular Ca2+-sensing receptor (CaSR) is involved in enhanced Ca2+ influx and the proliferation of PASMCs during idiopathic pulmonary arterial hypertension, and the blockade of the CaSR by a specific antagonist (NPS2143) prevents PH in monocrotaline (MCT)-induced PH (MCT-PH) rats and chronic hypoxia-induced PH (HPH) mice.2 The [Ca2+]cyt has an important role in the regulation of the contraction, proliferation and migration of PASMCs. An elevation of the [Ca2+]cyt in PASMCs is caused by Ca2+ release from intracellular stores and Ca2+ influx through plasmalemma Ca2+ channels.1,3–8
The CaSR is a class C GPCR family member of 1085 amino acids.9–11 The CaSR senses extracellular Ca2+, which then triggers intracellular signaling through multiple pathways.9–15 The CaSR also directly interacts with G proteins (Gqα and G11α). The activation of the CaSR by extracellular Ca2+ (or a calcimimetic) induces increases in the [Ca2+]cyt through the Phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol-4,5- bisphosphate to inositol-1,4,5- trisphosphate (IP3) and diacylglycerol. IP3 binds to the IP3 receptor on the SR membrane and induces Ca2+ release from the SR into the cytosol. CaSR ligands and activators include polyvalent cations (for example, Ca2+, Mg2+ and Gd3+), polypeptides (for example, amyloid-peptide), polyamines (for example, spermine, spermidine and putrescine), aminoglycoside antibiotics (for example, neomycin and kanamycin) and amino acids (for example, phenylalanine, tyrosine, tryptophan and glutamate). In addition, there are synthetic CaSR activators and calcimimetics (for example, NPSR-568, NPS-R-467) and CaSR antagonists and calcilytics (for example, NPS2143), which affect CaSR function.9–14 Therefore, NPS2143 was chosen as a CaSR inhibitor in our rescue experiments.
NPS2143(2-chloro-6-[(2R)-3-[[1,1-dimethyl-2-(2-naphthaleny-l)ethyl]amino-2-hydroxypropoxy]benzonitrile hydrochloride) is a selective CaSR antagonist. The block of the CaSR increases the cytoplasmic Ca2+ concentrations elicited by human Ca2+ receptors expressed in HEK293 cells (IC50 = 43 nM), and it stimulates parathyroid hormone (PTH) secretion from bovine parathyroid cells (EC50 = 41 nM).16–18
To assess the protective effect of NPS2143 in PH, both preventative and rescue experiments should be performed in PH animal models. Therefore, in this study, rescue experiments were performed in rats with MCT-induced PH (MCT-PH) and mice with chronic HPH to elucidate the pharmacological effects of the calcilytic NPS2143 on PH.
Methods
Preparation of animal PASMCs
Approval to use animal lung tissues and cells was granted by the University of Illinois at Chicago (UIC) Institutional Review Board (Chicago, IL, USA). In some experiments, we used freshly dissociated primary PASMCs from rats19 and mice.20
[Ca2+]cyt measurement
The [Ca2+]cyt measurements for the composition of solutions used for in vitro experiments were performed as previously described.2 The [Ca2+]cyt was measured in PASMCs using fura-2 and a Nikon digital fluorescence imaging system (Chicago, IL, USA).3 Cells were loaded with 4 μmol l–1 fura-2 acetoxymethyl ester for 60min at 25°C, and the [Ca2+]cyt was measured using a ratiometric method at 32°C. NPS2143 (Tocris, Ellisville, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) at 10 mmoll–1 as a stock solution.
Preparation of MCT-PH rats and HPH mice
All experiments were approved by the Ethics/Animal Care Committee of the UIC. For MCT-PH rat experiments, male Sprague–Dawley rats (190–200 g) were treated with a single s.c. injection of vehicle (DMSO) or 60 mg kg–1 MCT. NPS2143-treated rats were i.p. injected with NPS2143 (Tocris) at a dose of 4.5 mg kg–1 per day from days 14 to 24. At 28 days after injection, the rats were anesthetized with ketamine/xylazine, and right ventricular systolic pressure (RVSP) was then measured using an MPVS Ultra system (Millar Instruments, Chicago, IL, USA). For HPH mouse experiments, 8-week-old male mice (C57BL/6) were exposed to hypoxia (10% O2) in a ventilated chamber to allow the development of PH. NPS2143-treated mice were i.p. injected with NPS2143 (1.0 mg kg–1 per day on days 14–24). Four weeks after exposure to normobaric hypoxia, mice were anesthetized with ketamine/xylazine, and RVSP was then measured by right heart catheterization.
Acute application of NPS2143 in MCT-PH rats and HPH mice
For acute MCT-PH rat experiments, male Sprague–Dawley rats (14 days after a single s.c. injection of DMSO or 60 mg kg–1 MCT) were treated with a single i.p. injection of vehicle (DMSO) or 4.5 mg kg–1 NPS2143. For HPH mouse experiments, male mice (C57BL/6) were treated with a single i.p. injection of vehicle (DMSO) or 1 mg kg–1 NPS2143 14 days after normoxia or chronic hypoxia. At 0, 30, 60 and 80 min after injection, the rats and mice were anesthetized with ketamine/xylazine, and RVSP and heart rate were measured using an MPVS Ultra system (Millar Instruments).
Hemodynamic measurements
Animals were initially i.p. anesthetized with ketamine (100 mg kg–1) and xylazine (26 mg kg–1). The RVSP, ±dp/dtmax and heart rate were determined with a pressure transducer catheter (Millar Instruments) inserted through the right jugular vein using the MPVS Ultra data acquisition system (Chicago, IL, USA). The systemic blood pressure (SBP) was determined with a pressure transducer catheter (Millar Instruments) inserted through the jugular artery using the MPVS Ultra data acquisition system. Data were then recorded and analyzed with AD Instruments Lab Chart Pro 7.0 software (AD Instruments company, Chicago, IL, USA).
Western blot
Solubilized protein isolated from endothelium-denuded pulmonary arteries and lung tissue was separated in an 8% polyacrylamide gel, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA), and immunoblotted with an anti-CaSR monoclonal antibody (MA1–934,1:200; Thermo Scientific, Rockford, IL, USA). Signals were detected using a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). The protein levels were normalized to β-tubulin (sc-9104, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and expressed in arbitrary units.
Real-time PCR
Total RNA extraction from endothelium-denuded pulmonary arteries and reverse transcription were performed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), respectively. Quantitative real-time PCR analysis was performed based on the SYBR Green assay (SYBR Green Master Mix; Roche Applied Science, Indianapolis, IN, USA) using gene-specific primers for rat CaSR and GAPDH with a Bio-Rad CFX384 Real-Time System C1000 Thermal Cycler system (Bio-Rad Laboratories, Hercules, CA, USA).
Assessment of right ventricular (RV) hypertrophy and myocardium fibrosis
RV hypertrophy was expressed as the ratio of the weight of the RV wall to that of the free left ventricular (LV) wall and ventricular septum (LV + S). The RV wall was separated from the LV wall and ventricular septum. The wet weights of the RV, free LV wall and ventricular septum were determined. Formalin-fixed and paraffin-embedded sections (3 mm) were prepared from the right ventricles of the rats and mice. Masson staining4 was performed to examine RV myocardial fibrosis.
Statistical analysis
Composite data are shown as the mean ± s.e. The statistical significance between two groups was determined by Student's t-test. The statistical significance among groups was determined by the Scheffe test after one-way analysis of variance. Differences were considered significant at P<0.05 or P<0.01.
Results
The extracellular Ca2+-induced increase in [Ca2+]cyt is significantly enhanced in primary PASMCs from MCT-PH rats and HPH mice
We first examined and compared the effects of extracellular Ca2+ restoration on changes in the [Ca2+ ]cyt in PASMCs from control subjects, MCT-PH rats and HPH mice.
In control PASMCs superfused with a Ca2+ -free solution (plus 1 mmoll–1 EGTA) for 10 min, extracellular Ca2+ (2.2 mmoll–1) restoration in the bath solution had little effect on the [Ca2+]cyt (Figures 1a, b and 2a, b). In similarly treated PASMCs from MCT-PH rats or HPH mice, however, the restoration of extracellular Ca2+ resulted in a significant increase in [Ca2+]cyt in 95% of the cells tested (Figures 1a, b and 2a, b). The Ca2+ transient elicited by Ca2+ restoration was significantly larger in MCT-PH and HPH cells than in control cells.
Figure 1.
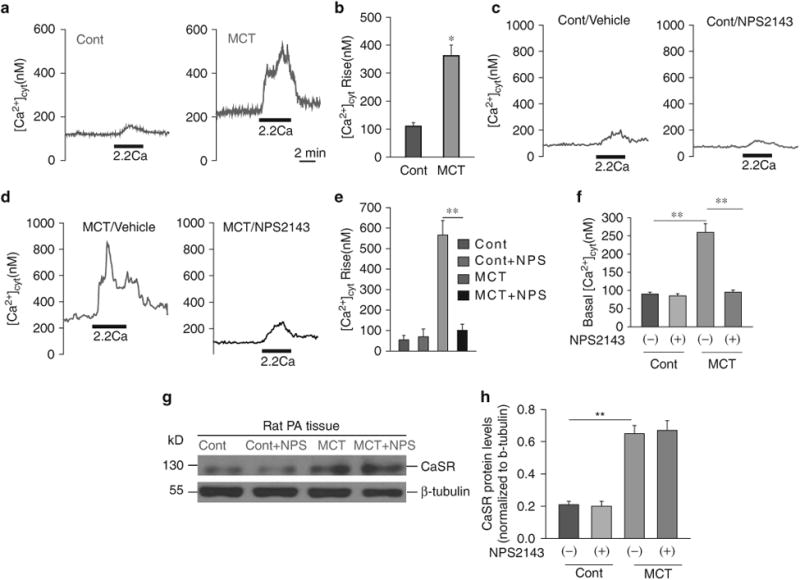
The extracellular Ca2+-induced increase in cytosolic Ca2+ concentration ([Ca2 +]cyt) is significantly enhanced in primary pulmonary arterial smooth muscle cells (PASMCs) from monocrotaline (MCT)-pulmonary hypertension (PH) rats. Representative traces (a) and summarized data (b) showing an extracellular Ca2+ -induced increase in [Ca2+ ]cyt in PASMCs from control (Cont) and MCT rats (*P<0.05 vs. Cont). Representative traces (c, d) and summarized data (e) showing the amplitude of extracellular Ca2 + -induced [Ca2 +]cyt increases (n =41) and the basal [Ca2 + ]cyt level (f) (n =42) in PASMCs isolated from normal rats and MCT-injected rats that were treated with vehicle (-NPS) or 10 μM NPS2143 (+ NPS), a synthetic calcilytic. **P<0.01 vs. Cont (—/+NPS). Western blot analysis of the CaSR (g) in membrane proteins isolated from the pulmonary arteries (PAs) of control rats (Cont, n = 6) and rats with MCT-induced pulmonary hypertension (MCT, n=5). Summarized data (mean±s.e.) showing Ca2+-sensing receptor (CaSR) protein levels (h), normalized to the level of β-tubulin. **P<0.01 vs. Cont; P40.05 for MCT+NPS2143 vs. MCT. A full color version of this figure is available at the Hypertension Research journal online.
Figure 2.
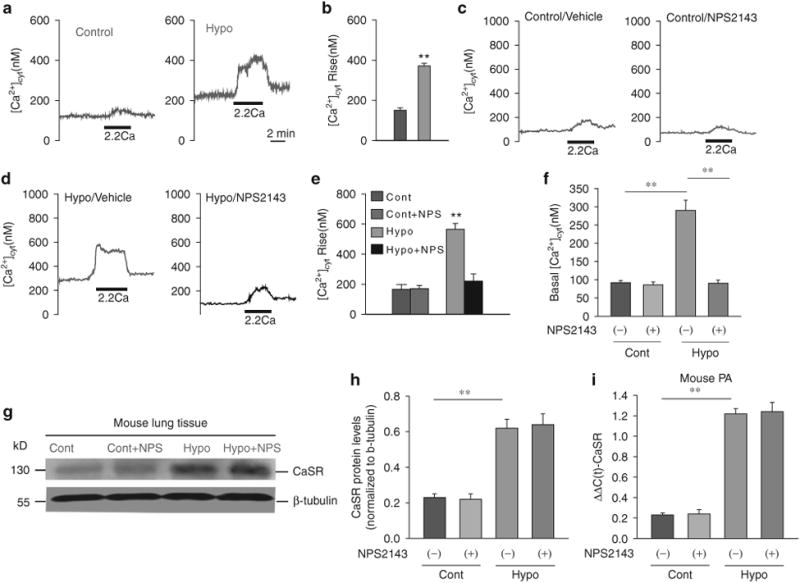
The extracellular Ca2+-induced increase in cytosolic Ca2+ concentration ([Ca2+ ]cyt) is significantly enhanced in primary pulmonary arterial smooth muscle cells (PASMCs) from hypoxia-induced pulmonary hypertension (HPH) mice. Representative traces (a) and summarized data (b) showing an extracellular Ca2+ -induced increase in [Ca2+]cyt in control (Cont) and HPH mouse PASMCs. Representative traces (c, d) and summarized data (e) showing the amplitude of extracellular Ca2+ induced [Ca2+]cyt increases (n = 43) and the basal [Ca2+ ]cyt (n = 42) level (f) in PASMCs isolated from normal rats and HPH mice that were treated with vehicle (-NPS2143) or 10 μM NPS2143 (+ NPS), a synthetic calcilytic. **P<0.01 vs. Cont (—/+NPS). Western blot analysis of the CaSR (g) in membrane proteins isolated from whole lung tissue. Summarized data (mean±s.e.) showing the CaSR protein levels (h), normalized to the level of β-tubulin. **P<0.01 vs. Cont; P40.05 for Hypo + NPS2143 vs. Hypo. Real-time-PCR analyses of the Ca2+-sensing receptor (CaSR) (i) in pulmonary arteries (PAs) isolated from normal mice (Cont, n=6) and mice with HPH (hypo, n=6). **P<0.01 vs. Cont; P40.05, Hypo+ NPS2143 vs. Hypo. A full color version of this figure is available at the Hypertension Research journal online.
Inhibition of the CaSR with NPS2143 in vivo inhibits the extracellular Ca2+-induced [Ca2+]cyt increase in primary PASMCs isolated from MCT-PH rats and HPH mice
Four weeks after the initial experiments, we examined and compared the effects of extracellular Ca2+ restoration on changes in the [Ca2+]cyt in primary PASMCs isolated from control subjects, NPS2143-treated control (from days 14 to 24) subjects, MCT-PH rats, HPH mice, NPS2143-treated MCT-PH rats (from days 14 to 24) and NPS2143-treated HPH mice (from days 14 to 24). No difference was found in the basal or increased [Ca2+]cyt between control and NPS2143-treated subjects (Figures 1c–f and 2c–f). However, in vivo treatment with NPS2143 significantly decreased the basal or increased [Ca2+]cyt in primary PASMCs isolated from MCT-PH rats and HPH mice (Figures 1d–f and 2d–f)
The CaSR expression in the pulmonary artery is greater in MCT-PH rats and HPH mice than in controls, but treatment with NPS2143 does not change the CaSR expression in MCT-PH rats or HPH mice
To investigate the potential mechanism of the extracellular Ca2+-mediated increase in [Ca2+]cyt, we compared the protein and mRNA expression levels of CaSR in pulmonary arteries from control and PH animals. As shown in Figures 1g and h, the CaSR protein expression in MCT-PH rat pulmonary arteries was significantly higher than that in control subjects. The CaSR protein expression in lung tissue from HPH mice was significantly higher than that in control subjects (Figures 2g and h). The CaSR mRNA expression in pulmonary arteries from HPH mice was significantly higher than that in control subjects (Figure 2i). However, treatment with NPS2143 did not change the expression of the CaSR in MCT-PH rats and HPH mice (Figures 1g, h and 2g–i).
Hemodynamic changes after NPS2143 treatment in MCT-PH rats and HPH mice
I.p. NPS2143 injection (4.5mg kg–1 per day on days 14–24) had little effect on the RVSP and ±dp/dtmax in control rats (Cont) but significantly inhibited the increase in RVSP and ±dp/dtmax in MCT-PH rats (Figures 3a–d). Furthermore, i.p. NPS2143 injection (1 mg kg–1 per day on days 14–24) had little effect on the RVSP and ±dp/dtmax in normoxic control mice (Cont) but significantly inhibited the increase in RVSP and ±dp/dtmax in hypoxic mice (Hypo) (Figures 4a–d). There were no significant changes in SBP between control rats with or without NPS2143 treatment or between MCT-PH rats with or without NPS2143 treatment (Figure 3e). Moreover, there were no significant changes in SBP between normoxic mice with or without NPS2143 treatment or between hypoxic mice with or without NPS2143 treatment (Figure 4e).
Figure 3.
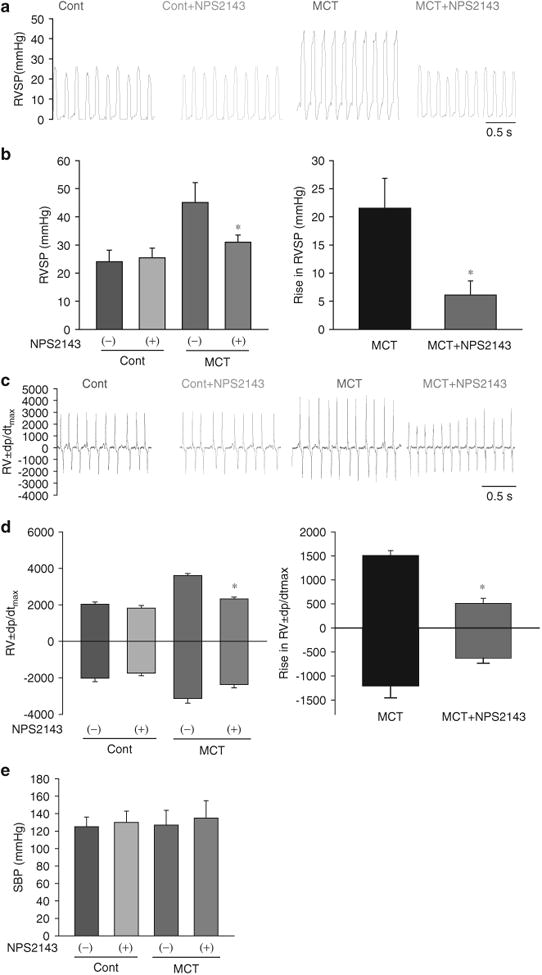
Hemodynamic changes after treatment with NPS2143 in monocrotaline (MCT)-pulmonary hypertension (PH) rats. Representative traces (a) and summarized data (b) showing that NPS2143 attenuated the increase in right ventricular systolic pressure (RVSP). Representative traces (c) and summarized data (d) showing that NPS2143 attenuated the increase in ±dp/dtmax. *P<0.05 for MCT +NPS2143 vs. MCT. There were no significant changes in systemic blood pressure (SBP) in control rats with or without NPS2143 treatment and MCT-induced pulmonary hypertension rats with or without NPS2143 treatment (e). A full color version of this figure is available at the Hypertension Research journal online.
Figure 4.
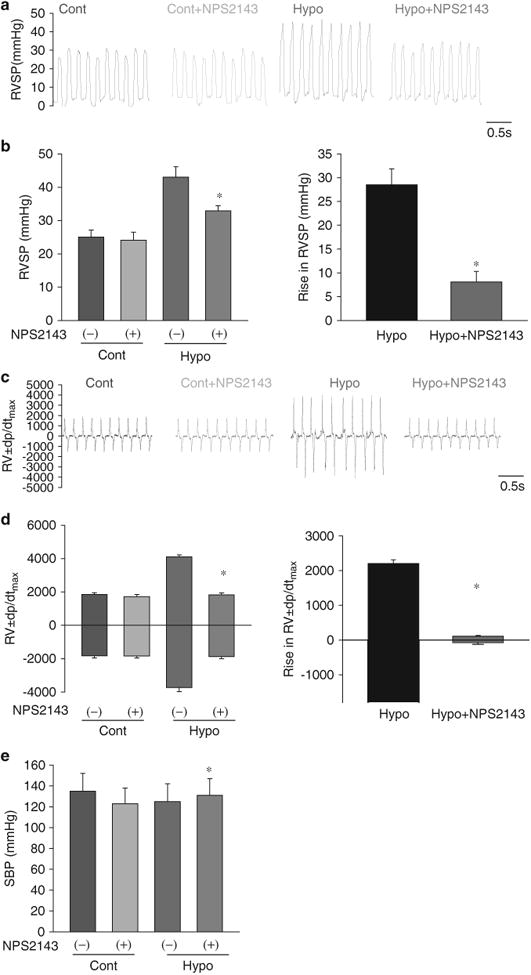
Hemodynamic changes after treatment with NPS2143 in hypoxia-induced pulmonary hypertension (HPH) mice. Representative traces (a) and summarized data (b) showing that NPS2143 attenuated the increase in right ventricular systolic pressure (RVSP). Representative traces (c) and summarized data (d) showing that NPS2143 attenuated the increase in ±dp/dtmax. *P<0.05 for Hypo +NPS2143 vs. Hypo. There were no significant differences in systemic blood pressure (SBP) between control mice with or without NPS treatment or between HPH mice with or without NPS treatment (e). A full color version of this figure is available at the Hypertension Research journal online.
Hemodynamic changes after an acute application of NPS2143 in rats (14 days after MCT injection) and mice (14 days after exposure to chronic hypoxia)
The i.p. injection of NPS2143 (4.5 mg kg–1) attenuated the increase in RVSP at 80 min after NPS2143 administration in MCT-PH rats (Figure 5a). In addition, the i.p. injection of NPS2143 (1 mg kg–1) attenuated the increase in RVSP 80 min after NPS2143 administration in HPH mice (Figure 5c). There were no significant changes in heart rate between control rats with or without NPS2143 treatment or between the PH animal models (rats or mice) with or without NPS2143 treatment (Figures 5b and d).
Figure 5.
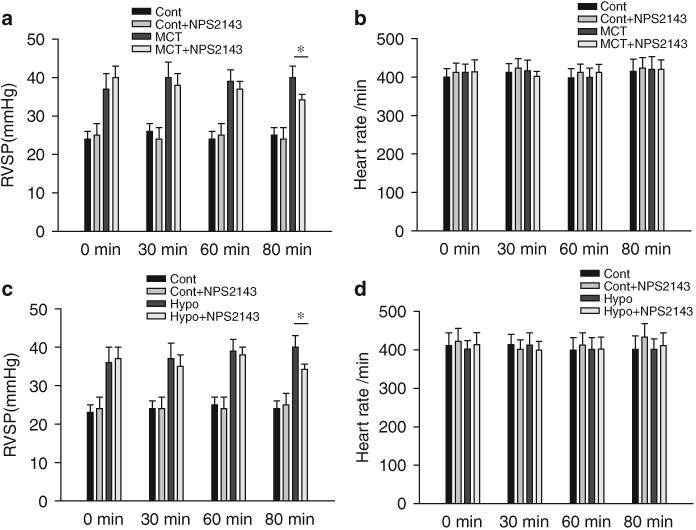
Hemodynamic changes after acute NPS2143 administration in rats (14 days after monocrotaline (MCT) injection) and mice (14 days after exposure to chronic hypoxia). Summarized data (a, c) showing that right ventricular systolic pressure (RVSP) decreased 80min after NPS2143 administration in MCT-pulmonary hypertension (PH) rats (14 days after MCT injection) and mice exposed to chronic hypoxia (14 days after exposed to hypoxia) (*P<0.05 for MCT+ NPS2143 vs. MCT; *P<0.05 for Hypo + NPS2143 vs. Hypo). There were no significant differences in heart rate between control rats with or without NPS treatment or between pulmonary hypertension model animals (rats or mice) with or without NPS treatment (b, d).
RV hypertrophy and myocardial fibrosis is inhibited by NPS2143 in MCT PH rats and HPH mice
To test the in vivo therapeutic effects of the CaSR antagonist on the remodeling of the right ventricle, we examined and compared the Fulton index (RV/LV + S ratio) and degree of RV myocardial fibrosis in control and PH animals with and without NPS2143 treatment. The averaged Fulton index (RV/LV+S ratio, mean±s.e.) showed that RV hypertrophy is significantly inhibited in MCT-PH rats and HPH mice treated with NPS2143 (Figures 6a, b and 7a, b). NPS2143 attenuated myocardial fibrosis in the right ventricle in MCT PH rats and HPH mice (Figures 6c, d and 7c, d).
Figure 6.
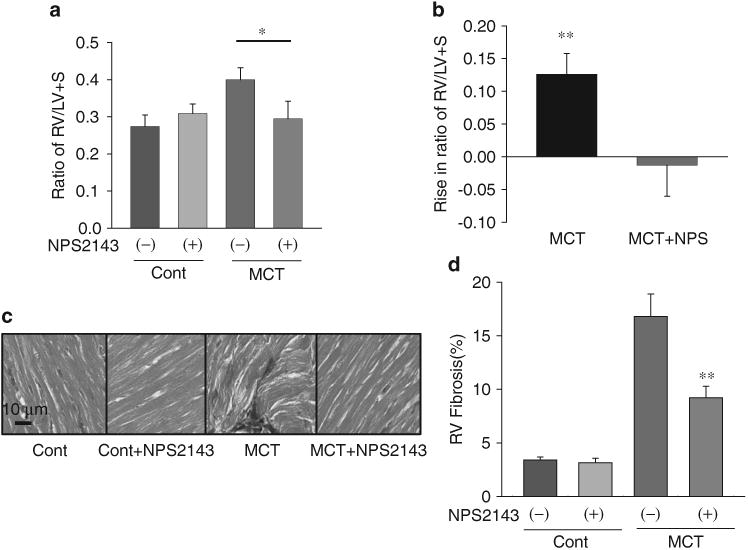
Right ventricular (RV) hypertrophy and myocardial fibrosis is inhibited by NPS2143 in monocrotaline (MCT)-pulmonary hypertension (PH) rats. Averaged Fulton index (RV/LV+S ratio, mean±s.e.) (a) and Rise in ratio of RV/LV+S (b) showing that RV hypertrophy is significantly inhibited in MCT-PH rats treated with NPS2143. *P<0.05 vs. MCT alone. Representative Masson staining images (c) and summarized data (d) showing that NPS2143 attenuated myocardial fibrosis in the right ventricles of MCT-PH rats. **P<0.01 vs. MCT alone. LV, left ventricular. A full color version of this figure is available at the Hypertension Research journal online.
Figure 7.
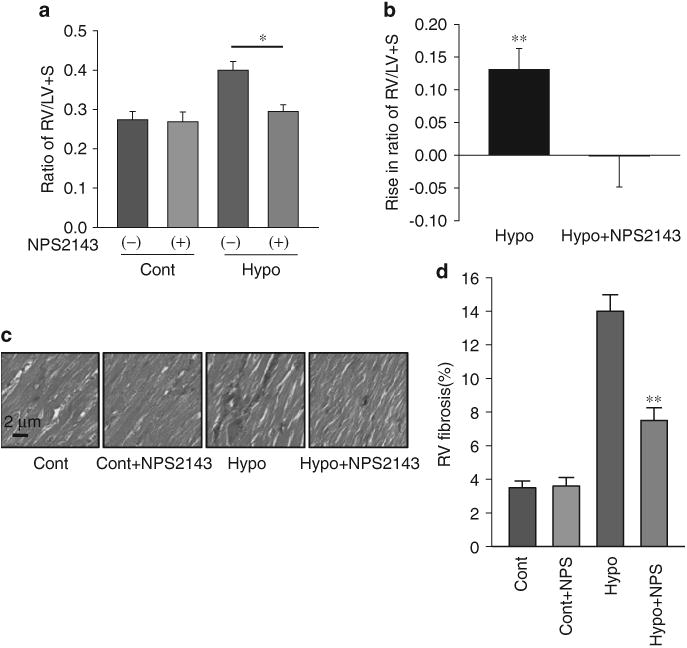
Right ventricular (RV) hypertrophy and myocardial fibrosis is inhibited by NPS2143 in hypoxia-induced pulmonary hypertension (HPH) mice. Averaged Fulton index (RV/LV+ S ratio, mean±s.e.) (a) and Rise in ratio of RV/LV+ S (b) showing that RV hypertrophy is significantly inhibited in HPH mice treated with NPS2143. *P<0.05 vs. hypoxia alone. Representative Masson staining images (c) and summarized data (d) showing that NPS2143 attenuated myocardial fibrosis in right ventricles from HPH mice. **P<0.01 vs. hypoxia alone. LV, left ventricular. A full color version of this figure is available at the Hypertension Research journal online.
Discussion
To further understand the role of the CaSR in the mechanism of PH, we investigated the rescue effects of NPS2143 in MCT-PH rats and HPH mice. In summary, we found the following: (1) the extracellular Ca2+-induced increase in [Ca2+]cyt is significantly enhanced in primary PASMCs from MCT-PH rats and HPH mice. NPS2143 rescues the extracellular Ca2+-induced increase in the [Ca2+]cyt in PASMCs from MCT-PH rats and HPH mice; (2) NPS2143 rescues the increase in RVSP and dp/dtmax in rats and mice exposed to hypoxia and (3) NPS2143 rescues the hypertrophy and right ventricle myocardial fibrosis in MCT-PH rats and HPH mice. Collectively, the observations in this study indicate that blockade of the upregulated CaSR with NPS2143 rescues the development of pulmonary arterial hypertension in MCT-PH rats and HPH mice. The data in this study and our previous study show that the upregulation and activation of the CaSR has an important role in the course of PH, and the CaSR antagonist NPS2143 is a potential treatment for PH.
The specific aim of this study was to determine whether chronic treatment with NPS2143 could rescue the PH phenotype. We found that NPS2143 attenuated extracellular Ca2+-induced increases in [Ca2+]cyt in primary PASMCs freshly isolated from MCT-PH rats and HPH mice but had no effect on PASMCs from normal rats or mice. To examine the effects of NPS2143 on the expression of the CaSR in pulmonary arteries from MCT-PH rats and HPH mice, western blot analysis was performed in pulmonary arteries from MCT-PH rats. Real-time PCR was used to detect the mRNA expression of CaSR in pulmonary arteries from HPH mice. We found that treatment with NPS2143 did not change the expression of the CaSR in MCT-PH rats and HPH mice. However, NPS2143 specifically inhibited the function of the CaSR in disease-associated constriction. In addition, the pharmacological rescue effects of NPS2143 on PH were examined in MCT-PH rats and HPH mice. Our data show that NPS2143 reversed or normalized the hemodynamics inflicted by PH in the two animal models (increases in the RVSP and ±dP/dt-max), RV hypertrophy and RV myocardial fibrosis. These findings will help to clarify the interplay between CaSR function and the PH phenotype, illustrating that the CaSR may be a novel target for future clinical treatments.
Furthermore, we examined whether NPS2143 affects SBP in rescue experiments. Our data show that treatment with NPS2143 did not affect SBP. Instead, the data suggest that this approach is selective for the abnormal pulmonary circulation and provides evidence for the absence of vasodilatory effects because NPS2143 presumably caused a decrease in pulmonary arterial vascular resistance. Moreover, it is unknown how changes in the PTH level in MCT-PH rats and HPH mice occur during NPS2143 treatment. In the parathyroid gland, the CaSR controls calcium homeostasis by regulating the release of PTH. The cross talk between the CaSR and PTH during PH should be examined to clarify this mechanism.
As PH was shown to require 14 days to develop after MCT injection (rats) or exposure to chronic hypoxia (mice)3 in our previous study, acute treatment experiments were performed in rats 14 days after MCT injection and 14 days after exposure to chronic hypoxia in mice in this study. I.p. NPS2143 injection attenuated the increase in RVSP 80 min after NPS2143 administration in MCT-PH rats and HPH mice. The data further support the potential effects of NPS2143 in the treatment of PH.
Data regarding the pharmacodynamics and pharmacokinetics of NPS2143 would explain why blocking the CaSR produced an effect that took a minimum of 80 min to occur.21 A previous study22 demonstrated changes in the plasma [Ca2+] in rats i.v. infused with NPS2143 (0.1μmol kg–1 per minutes) for 2 h. Approximately 30 min later, the plasma [Ca2+] began to decrease until 60 min after injection. However, the plasma [Ca2+] began to increase until 3 h after injection. In our study, NPS2143 was administered to the rats and mice by i.p. infusion, which takes more time than i.v. infusion to produce an effect. Thus, it is reasonable to predict that the maximum plasma [Ca2+] should occur >60 min after NPS2143 injection. Therefore, the in vivo pharmacodynamics and pharmacokinetics contribute more directly to the timing of the NPS2143 blocking effect than do other mechanisms.
The mechanistic link between CaSR dysfunction and the development of pulmonary vascular disease remains unclear. Our previous study showed a pathogenic role for the CaSR in PH, and it will be interesting to investigate whether the CaSR might be a potent therapeutic target in patients with PH. More interestingly, our group recently reported that dihydropyridine derivatives increase the [Ca2+ ]cyt by potentiating the activity of the CaSR in PASMCs independently of their blocking (or activating) effect on Ca2+ channels.23 Therefore, it is possible that the use of dihydropyridine Ca2+ channel blockers (for example, nifedipine) to treat idiopathic pulmonary arterial hypertension patients with upregulated CaSRs in PASMCs may exacerbate PH. However, it is unclear how the function and expression of the CaSR are regulated in PASMCs. Do some signaling pathways contribute to the regulation of the CaSR in PH? Further investigation should be conducted to clarify this mechanism. In our study, the potential demonstration of a reversal of the cardiovascular changes inflicted during the development of PH by an antagonist of the CaSR is clearly important.
The main purpose of our study was to assess the rescuing effect of NPS2143 on experimental PH models. Possible changes in hemodynamics, cardiac morphometric parameters and the extent of cardiac fibrosis were analyzed to determine whether NPS2143 had any protective effects on PH. We concluded that CaSR antagonists such as calcilytics and NPS2143, that is, negative allosteric modulators that indirectly stimulate PTH secretion via a decrease in CaSR activity, are potential drug candidates for the treatment of not only osteoporosis and other bone metabolism diseases24 but also PH. Moreover, our data provide strong evidence that the CaSR is necessary and sufficient for augmented Ca2+ signaling and excessive PASMC proliferation in PH animal models. Using two well-established animal models of PH (MCT-injected rats and chronic hypoxic mice), we found that i.p. injection of the CaSR antagonist NPS2143 significantly rescues increases in the RV/(LV+S) ratio and RV myocardial fibrosis. In conclusion, pharmacological blockade of the CaSR in the pulmonary vasculature by synthetic calcilytics may be a novel therapeutic approach for PH patients who do not respond to conventional drug therapy.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL066012 and HL098053), National Natural Science Foundation of China (NSFC81300040) and Application Foundation of Suzhou China (SYS201336).
References
- 1.Yuan Jason XJ, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 2.Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012;111:469–481. doi: 10.1161/CIRCRESAHA.112.266361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamura A, Yamamura H, Zeifman A, Yuan JXJ. Activity of Ca2+-activated Cl- channels contributes to regulating receptor- and store operated Ca2 + entry in human pulmonary artery smooth muscle cells. Pulm Circ. 2011;1:269–279. doi: 10.4103/2045-8932.83447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark F, Berry Adam J, Joseph Engler Y, Woo Timothy J, Pirolli Lawrence T, Vasant Bish, Jayasankar Kevin J, Morine Timothy J, Gardner Dennis E, Discher, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Firth AL, Remillard CV, Yuan JX. Trp channels in hypertension. Biochim Biophys Acta. 2007;1772:895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent camp-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1202–L1210. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, Thistlethwaite PA, Channick RN, Robbins IM, Loyd JE, Ghofrani HA, Grimminger F, Schermuly RT, Cahalan MD, Rubin LJ, Yuan JX. A functional single-nucleotide polymorphism in the trpc6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 10.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 11.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 12.Brown EM, Butters R, Katz C, Kifor O. Neomycin mimics the effects of high extracellular calcium concentrations on parathyroid function in dispersed bovine parathyroid cells. Endocrinology. 1991;128:3047–3054. doi: 10.1210/endo-128-6-3047. [DOI] [PubMed] [Google Scholar]
- 13.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular ca2_-sensing receptor. Proc Natl Acad Sci USA. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gowen M, Stroup GB, Dodds RA, James IE, Votta BJ, Smith BR, Bhatnagar PK, Lago AM, Callahan JF, DelMar EG, Miller MA, Nemeth EF, Fox J. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riccardi D, Finney BA, Wilkinson WJ, Kemp PJ. Novel regulatory aspects of the extracellular Ca2+-sensing receptor, car. Pflugers Arch. 2009;458:1007–1022. doi: 10.1007/s00424-009-0681-z. [DOI] [PubMed] [Google Scholar]
- 16.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 17.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 18.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 19.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol. 1993;264:L107–L115. doi: 10.1152/ajplung.1993.264.2.L107. [DOI] [PubMed] [Google Scholar]
- 20.Marshall CMA, Verhoeven AJ, Marshall BE. Pulmonary artery nadphoxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 21.Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 22.Nemeth EF. The search for calcium receptor antagonists(calcilytics) J Mol Endocrinol. 2002;29:15–21. doi: 10.1677/jme.0.0290015. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura A, Yamamura H, Guo Q, Zimnicka AM, Wang J, Ko EA, Smith KA, Pohl NM, Song S, Zeifman A, Makino A, Yuan JXJ. Dihydropyridine Ca2+ channel blockers increase cytosolic [Ca2 +] by activating Ca2 + -sensing receptors in pulmonary arterial smooth muscle cells. Circ Res. 2013;4:640–650. doi: 10.1161/CIRCRESAHA.113.300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth EF. Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]


