Abstract
Objectives
High-dose, hypofractionated radiotherapy (HFRT) is sometimes used to treat malignancy in the head-and-neck (HN), both in the curative and palliative setting. Its safety and efficacy have been reported in small studies and are still controversial.
Materials and Methods
We retrospectively evaluated the outcomes and toxicities of HFRT, including ultra-high-dose fractionation schemes (≥8 Gray per fraction), for HN malignancies.
Results
A total of 62 sites of measurable gross disease in 48 patients were analyzed. The median follow-up was 54.3 months among five survivors and 6.0 months in the remaining patients. Median RT dose was 30 Gray in 5 fractions; 20/62 lesions (32%) received dose-per-fraction of ≥8 Gray. Overall response rate at first follow-up was 79%. One-year local-progression free rate was 50%. On multivariate analysis for locoregional control, dose-per-fraction ≥6 Gray was associated with control (p=0.04) and previous radiation was associated with inferior control (p= 0.04). Patients who achieved complete response to RT had longer survival than those who did not (p=0.01). Increased toxicity rates were not observed among patients treated with dose-per-fraction ≥8 Gray; only re-irradiation increased toxicity rates.
Conclusion
Despite the poor prognostic features noted in this cohort of patients with HN malignancies, HFRT was associated with high response rates, good local control, and acceptable toxicity. Sites that were treated with 6 Gray per fraction or higher and had not been previously irradiated had the best disease control. A prospective trial is warranted to further refine the use and indications of HFRT in this setting.
INTRODUCTION
Hypofractionated radiation therapy (HFRT) involves the use of high doses per fraction to achieve improved tumor control. A more desirable therapeutic ratio has been achieved for HFRT in recent years through the use of image-guided RT (IGRT), which allows improved certainty regarding daily treatment setup and dose delivered to organs at risk. IGRT has in turn facilitated the use of stereotactic body radiation therapy (SBRT), which allows delivery of highly conformal, high doses of radiotherapy to a clinical target. Preclinical data show that high-dose single-fraction RT operates via a unique mechanism involving injury of tumor endothelial cells that is distinct from conventionally fractionated RT and independent of tumor histologic subtype.[1] Clinical data show that HFRT improves local tumor control beyond that possible using conventional fractionation, for various scenarios including early-stage lung cancer,[2, 3] radioresistant histologies such as melanoma and renal cell cancers,[4–7] and oligometastatic disease.[8–12]
In recent years, a growing body of literature has reported on the safety and feasibility of HFRT for tumors of the head and neck (HN).[13–20] Most of these series include patients with recurrent, unresectable HN cancers who had been previously irradiated. These studies have found promising overall response rates up to 80% and 1-year local control rates in the range of 50%. Despite promising tumor control, severe toxicities have occurred in patients receiving HFRT for re-irradiation, including carotid blowout and hemorrhage.[14, 19] Carotid blowout in particular is a severe complication of high-dose radiation therapy in which there is physical rupture of the carotid artery and hemorrhage. This hemorrhage is potentially fatal if not addressed emergently. Several groups have reported on outcomes of HFRT used to treat a heterogeneous group of HN malignancies, including primary, recurrent, and metastatic tumors.[20, 21] These studies have also shown high response rates and 2-year local control rates of 30% to 40%, with limited toxicity in patients who had not been previously irradiated.
In our institution, HFRT is routinely offered to the most challenging HN disease presentations: those with “radioresistant” histologies (such as melanoma and renal cell carcinoma), cases of disease recurrence in a previously irradiated field, or bulky lesions for which rapid palliation is desired. After the year 2004, IGRT became available in our center. In recent years, SBRT has been routinely used for treatment of lesions with proximity to critical structures. As we have gained experience with HFRT in HN cancers, our dose prescribed per fraction has increased over time. In this study, we report on the outcomes and toxicity of HFRT for various malignancies with measurable gross disease in the HN. We also analyze the outcomes of patients who received an ultra-high dose (≥8 Gray per fraction) hypofractionated regimen to determine if these more intensive regimens were associated with improved outcomes.
MATERIAL AND METHODS
We reviewed all cases of hypofractionated HN RT, which we defined as a dose of 5Gy or more per fraction, treated at our center from January 1997 to July 2014. We excluded patients who did not complete the prescribed course of radiotherapy and those who were treated to bone-only sites including the clivus. We therefore identified a total of 123 patients treated to 163 lesions in the HN. Within this group, we limited our analysis to patients with measurable gross disease, thereby excluding postoperative treatments following gross total resections, and at least one follow-up visit 30 days or more after completing HFRT.
Patients were offered HFRT to the HN tumor if they had one or both of the following features, as determined by the attending radiation oncologist: 1) radioresistant histology that would benefit from higher doses per fraction, 2) prior RT at the same site and not a candidate for salvage surgical resection or conventionally fractionated external beam RT, or 3) no prior RT at the site, but cannot tolerate surgical resection or conventionally fractionated RT to curative doses. Patients were treated with either “definitive” or “palliative” intent. Patients treated “definitively” did not have evidence of metastatic disease and were technically considered “curable” despite being ineligible for surgical or other modalities of treatment. Patients treated with “palliative” intent had other metastatic or locoregional disease and were considered “incurable” even if this course of RT were to lead to a complete response of the treated lesion. Pretreatment evaluation consisted of a complete history and physical examination, comprehensive metabolic panel, complete blood count, computed tomography (CT) of the HN and chest, magnetic resonance imaging (MRI) as indicated, and whole body positron-emission tomography (PET) as indicated. Our institutional review board approved a waiver of written informed consent for this retrospective study.
Radiation treatment design
All patients underwent either CT or PET-CT simulation. CT images were obtained using 2–3 mm slice thickness. Patients were immobilized in either a three-point or five-point thermoplastic face mask. Intravenous contrast was used for the simulation scan when indicated. Following CT-simulation, target volume was defined using available diagnostic CT, MRI, and/or PET images alongside the planning scan. For patients undergoing PET-CT simulation, the target volume was defined using the hypermetabolic tumor volume on the fused PET-CT scan. Treatments were targeted to local disease without elective nodal treatment. Any clinically or radiographically measurable gross disease was defined as the gross tumor volume (GTV). CTV was typically a 3–10 mm three-dimensional expansion on the GTV. The planning target volume (PTV) expansion on the CTV was dependent on the alignment technique, but was typically 2–3 mm for cases receiving IGRT with kV planar imaging or cone beam CT.
Dose was generally fractionated and delivered daily or every other day. Total dose and fractionation was selected by the attending radiation oncologist, based on field size, tumor location, prior radiation dose, and patient functional status. Treatment planning for 3D-conformal and IMRT treatments was performed using our in-house treatment-planning system. Dose was prescribed to the isodose line best covering the PTV, while also protecting normal tissues. The permitted normal tissue doses were defined by the attending radiation oncologist and were a function of fractionation scheme and any prior RT to the HN.
Response assessment
We determined objective response to HFRT using both clinical assessment and follow-up imaging. Patients were followed every 1–3 months on an outpatient basis. Follow-up evaluation consisted of an interval history and clinical examination focusing on the HN, often with fiberoptic endoscopy. Imaging included CT, MRI, and/or PET/CT routinely performed on a 3 to 6 month schedule. The follow-up interval was calculated from the last date of RT. Response was characterized using the RECIST criteria [22]: complete response (CR) if all tumor disappeared on follow-up evaluation, partial response (PR) if tumor exhibited at least >30% decrease in sum of diameters, stable disease (SD) if there was no change in tumor volume, and progression of disease (PD) if there was any increase in tumor size >20% and >5mm relative to pre-RT tumor volume. In certain cases progression was determined by the treating physician using primarily clinical examination.
Statistical analysis
We calculated in-field progression rate, locoregional progression-free rate (LRPF), overall survival (OS), and toxicity. All events were indexed to the final date of RT treatment. In-field progression was defined as failure within the treatment field, and locoregional progression was defined as progression either in the treatment field or in a regional lymph node group or neighboring site. OS was calculated from the date of completion of the initial course of HFRT, to the date of death. Patients without events were censored at last follow-up. Complications were scored per the Common Terminology Criteria for Adverse Events, version 4 (CTCAEv.4).[23] The Kaplan-Meier method was used for the generation of survival curves, and differences in survival curves were compared statistically with the log-rank test. Univariate and multivariate analyses of potential prognostic factors were performed using the Cox proportional hazards model. The chi-square test was used to compare rates between subgroups of patients. All provided p values are two-sided with an α-level of 0.05 considered significant. All statistical analysis was accomplished with the SPSS software package, version 22 (IBM, Armonk, NY).
RESULTS
Patient characteristics
Baseline characteristics for the initial cohort of 163 lesions in 123 patients, as well as for the analyzed group of 48 patients with 62 lesions, are available in Table 1. Predominant histologies of the 62 HN lesions were cutaneous melanoma (19%), mucosal melanoma (18%), squamous cell carcinoma (10%), and sarcomas (11%). Primary tumor site was nasal cavity in 18%, skin in 18%, and non-skin, metastatic cancer originating outside of the HN in 23%. The HN had been previously irradiated in 24% of cases.
TABLE 1.
Pretreatment clinical characteristics.
| Characteristic | Entire cohort (n=163), N (%) | Intact tumors (n=62), N (%) |
|---|---|---|
| Age, years (median, range) | 67.6 (18–95) | 70.2 (34–95) |
| Primary tumor location | ||
| Skin | 68 (42) | 11 (18) |
| Nasal cavity | 30 (18) | 11 (18) |
| Metastatic, non-skin cancer | 20 (12) | 14 (23) |
| Oral cavity | 9 (6) | 2 (3) |
| Paranasal sinus | 7 (4) | 4 (6) |
| Neck | 7 (4) | 3 (5) |
| Thyroid | 6 (4) | 6 (10) |
| Parotid | 4 (2) | 4 (6) |
| Nasopharynx | 3 (2) | 2 (3) |
| Orbit | 2 (1) | 0 (0) |
| Masticator space | 2 (1) | 2 (3) |
| Oropharynx | 1 (0.6) | 0 (0) |
| Hypopharynx | 1 (0.6) | 0 (0) |
| Larynx | 1 (0.6) | 1 (2) |
| Salivary Gland | 1 (0.6) | 1 (2) |
| Unknown primary | 1 (0.6) | 1 (2) |
|
| ||
| Histologic diagnosis | ||
| Cutaneous melanoma | 73 (45) | 12 (19) |
| Mucosal melanoma | 38 (23) | 11 (18) |
| Squamous cell carcinoma | 11 (6) | 6 (10) |
| Sarcomas | 10 (6) | 7 (11) |
| Carcinoma, NOS | 7 (4) | 6 (10) |
| Thyroid | 6 (4) | 6 (10) |
| Renal cell carcinoma | 6 (4) | 3 (5) |
| Adenoid cystic | 3 (2) | 3 (5) |
| Esthesioneuroblastoma | 3 (2) | 3 (5) |
| Neuroendocrine | 2 (1) | 2 (3) |
| Salivary gland carcinoma | 2 (1) | 2 (3) |
| Non-small cell lung cancer | 2 (1) | 1 (2) |
| Prior head and neck RT | 18 (11) | 18 (24) |
Abbreviation: RT, radiotherapy; NOS, not otherwise specified.
Treatment characteristics
Details of treatment, including sites and doses prescribed, are summarized in Table 2. Median age at HFRT was 70.2 years (range, 34–95). Patients were treated to a variety of HN sites, with the neck (36%) being the most common. Treatment intent was definitive in 52%, and palliative in 48%. Median prescribed dose was 30 Gy (range, 18–48) in 5 fractions (range, 1–6). When dose was converted into equivalent dose in 2-Gy fractions (EQD2)[23] using an α/β ratio of 3 for radioresistant histologies (melanoma and renal cell carcinoma) and an α/β ratio of 10 for other histologies, median EQD2 was 50 Gy (range, 24–108).
TABLE 2.
Details of head and neck sites treated with HFRT.
| Characteristic | Entire cohort (n=163), N (%) | Intact tumors (n=62), N (%) |
|---|---|---|
| Treated Sites | ||
| Neck | 55 (34) | 22 (36) |
| Sinonasal | 34 (21) | 9 (15) |
| Skin | 29 (18) | 10 (16) |
| Parotid | 16 (10) | 6 (10) |
| Orbit | 7 (4) | 6 (10) |
| Mandible | 5 (3) | 3 (5) |
| Ear | 4 (2) | 2 (3) |
| Oral cavity | 4 (2) | 0 (0) |
| Nasopharynx | 3 (2) | 2 (3) |
| Oropharynx | 3 (2) | 0 (0) |
| Hypopharynx | 1 (0.6) | 1 (2) |
| Maxilla | 1 (0.6) | 0 (0) |
| Thyroid | 1 (0.6) | 1 (2) |
| PTV Treated, cc, median (range) | 112 (8 – 606cc) | |
| Peri-radiotherapy chemotherapy | ||
| None | 32 (52) | |
| Yes, Before RT only | 9 (15) | |
| Yes, Before, During, After RT | 8 (13) | |
| Yes, After RT only | 3 (5) | |
| Yes, Before and After RT only | 9 (15) | |
| Months received, median (range) | 6.1 (1.8 – 11.4) | |
| RT Doses Received | ||
| 30Gy in 5 fx (6Gy per fx) | 27 (44) | |
| 36Gy in 6 fx (6Gy per fx) | 11 (18) | |
| 30Gy in 3 fx (10Gy per fx) | 10 (16) | |
| 24Gy in 3 fx (8Gy per fx) | 4 (6) | |
| 25Gy in 3 fx (5Gy per fx) | 3 (5) | |
| 27Gy in 3 fx (9Gy per fx) | 2 (3) | |
| 36Gy in 3 fx (12Gy per fx) | 1 (2) | |
| 48Gy in 4 fx (12Gy per fx) | 1 (2) | |
| 24Gy in 1 fx (24Gy per fx) | 1 (2) | |
| 20Gy in 2 fx (10Gy per fx) | 1 (2) | |
| 18Gy in 3 fx (6Gy per fx) | 1 (2) | |
Abbreviations: HFRT, hypofractionated radiotherapy. RT, radiation therapy. Gy, Gray. Fx, fractions. PTV, planning target volumes.
Dose per fraction of 8 Gy or more was used in 20 (33%) cases. Three patients were treated with 5 Gy per fraction to a total dose of 25 Gy. The majority of patients (92%) were treated with intensity-modulated radiotherapy (IMRT) or 3D conformal RT. IGRT with on-board 2D or 3D techniques was used in 56% of patients, all treated in 2007 or later. All patients with melanoma or renal cell carcinoma (n=26) received HFRT with EQD2 ≥50 Gy. Patients with melanoma and renal cell carcinoma histologies were significantly more likely to receive an EQD2 ≥50 Gy than patients with other histologies (p ≤ 0.001).
Response to Treatment and Recurrences
Among the 48 patients with 62 treated lesions, median follow-up was 54.3 months among five surviving patients and 6.0 months in remaining patients. Response to RT is outlined in Table 3. At first follow-up visit after RT, 79% of target lesions demonstrated response, 16% had stable disease, and 3% had progression of disease. The two patients with progressive disease had anaplastic thyroid cancer metastatic to the orbit, and cutaneous squamous cell carcinoma metastatic to the parotid gland.
TABLE 3.
Initial responses to head and neck HFRT (n=62).
| Characteristic | N (%) |
|---|---|
| Response | |
| Complete | 6 (10) |
| Partial | 43 (69) |
| Stable disease | 10 (16) |
| Progressive disease | 2 (3) |
| Unknown | 1 (2) |
| Modality to determine response | |
| CT | 10 (16) |
| PET/CT | 10 (16) |
| MRI | 16 (26) |
| CT and MRI | 2 (3) |
| Clinical examination only | 24 (39) |
Abbreviations: CT, computed tomography. PET, postiron emission tomography. MRI, magnetic resonance imaging.
Among the 59 cases with either disease response or stability following HFRT, 10 cases remained progression-free, in both locoregional and distant sites. The most common site of relapse was distant-only in 34% of cases. Isolated locoregional failure in the HN without distant relapse occurred in 23 cases (39%), including 12 (19%) with isolated in-field recurrence without any regional or distant failure. In patients who recurred, median times to both isolated in-field progression and isolated LRP was 6.2 months (range, 0 – 33.9).
Overall Survival
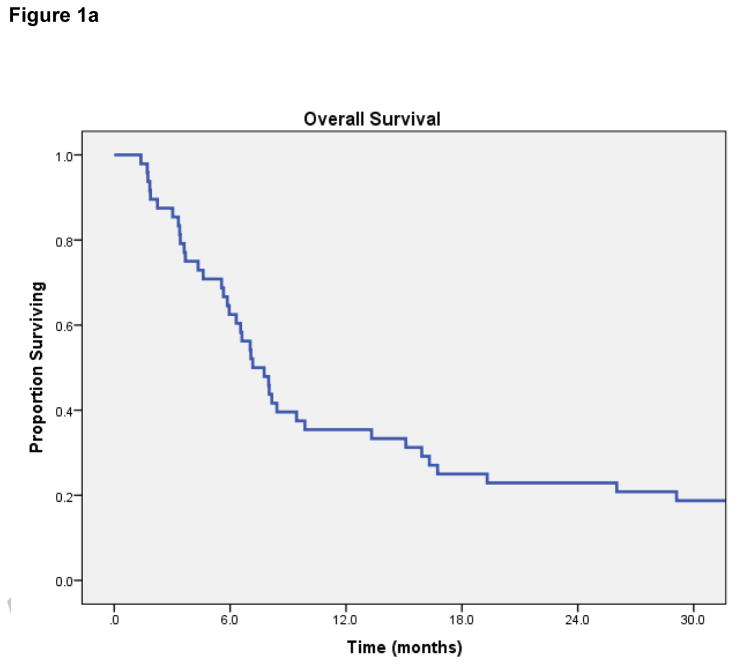
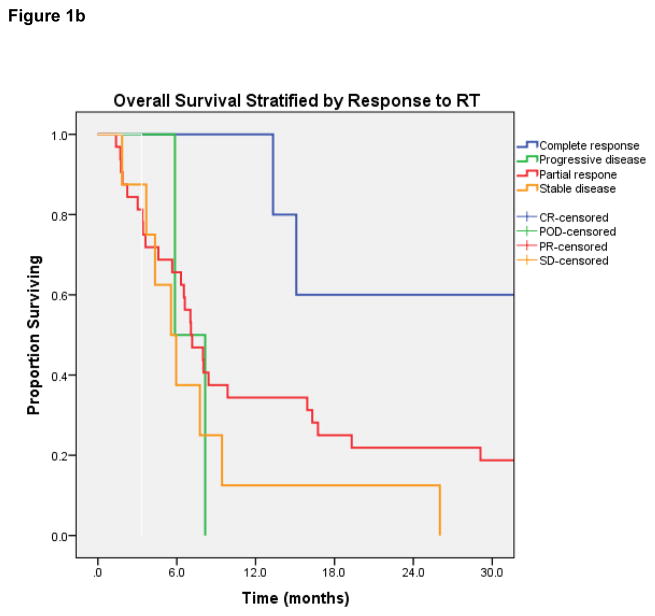
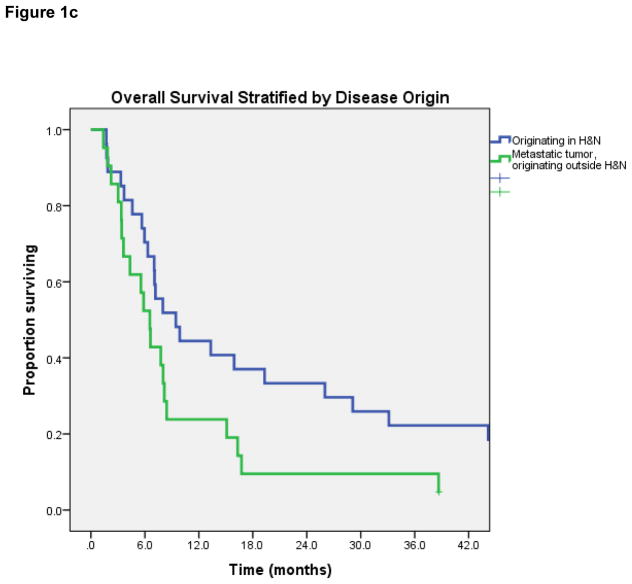
Median OS for all 48 patients was 7.2 months (95% Confidence Interval [CI] 5.6 – 8.8 months) [Figure 1a]. On log-rank testing, patients with complete response to treatment had far superior OS to those with less than complete response: 1-year OS was 100%, vs 34% in partial-response group (p=0.03) [Figure 1b]. Patients with metastatic tumors that had traveled to the head-and-neck also had inferior OS (median 6.5 vs 9.4 months, p= 0.05) [Figure 1c]. No difference in OS was observed based on dose per fraction ≥6 Gy or ≥8 Gy, age, chemotherapy use, EQD2, re-irradiation, or histology.
Figure 1.
Figure 1a. Overall Survival.
Figure 1b. Overall survival stratified by response to radiation therapy.
Figure 1c. Overall survival stratified by primary site of tumor (metastatic vs H&N).
Five patients out of 48 were alive at last follow-up. In 43 patients who died, cause was head-and-neck disease in 13 patients and due to other cause including systemic disease in 30 cases.
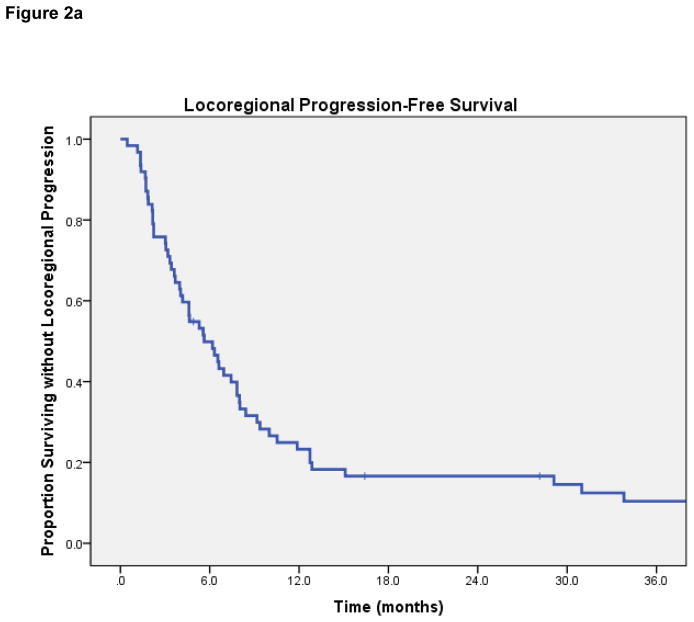
Locoregional progression-free rate
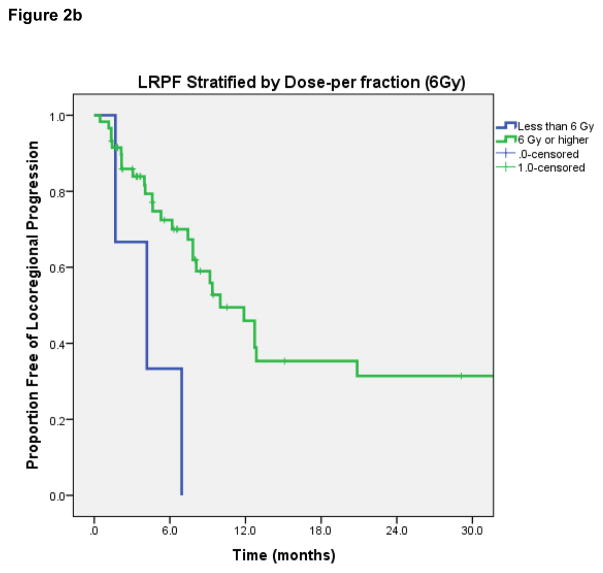
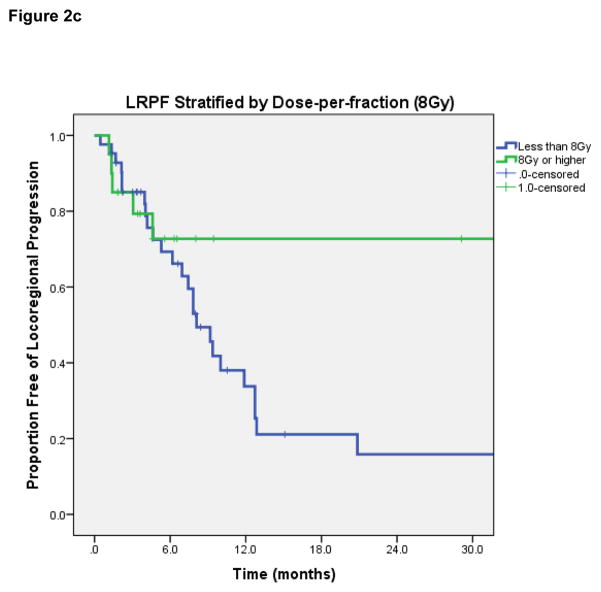
LRPF rates were 70% (95% CI 57–83%) at 6-months, 43% (95% CI 27–59%) at 12-months, and 29% (95% CI 13–45%) at 2-years. On log-rank test, lesions with complete response after HFRT had superior LRPF than patients with initial PR or less response (1-year rate 67% if CR vs 42% if PR, p=0.009). Dose-per-fraction ≥6 Gy was associated with improved LRPF (1-year rate 46% vs 0%, p=0.01); dose-per-fraction ≥8 Gy was also significantly associated with improved LRPF (1-year rate 73% vs 34%, p=0.038) [Figures 2b and 2c]. Patients receiving re-irradiation had worse LRPF (median LRPF 12.7 months vs 6.2 months, p=0.017), which may be attributed to the lower dose per fraction and smaller radiation field treated in patients receiving re-irradiation. No significant differences in LRPF were observed based on tumor histology, receipt of chemotherapy, PTV volume, metastatic vs. primary HN tumor, or EQD2.
Figure 2.
Figure 2a. Locoregional Progression-Free Survival.
Figure 2b. Locoregional progression-free rate, stratified by dose-per-fraction of 6Gy or higher.
Figure 2c. Locoregional progression-free rate, stratified by dose-per-fraction of 8Gy or higher.
A subgroup analysis was performed on the 46 patients who had both 1) not received previous radiation, and 2) were treated with dose-per-fraction of 600cGy or higher. LRPF rates were 78% (95% CI 63–93%) at 6-months, 56% (95% CI 34–77%) at 12 months, and 45% (95% CI 22 – 67%) at 2-years. Dose-per-fraction of 8Gy or higher was marginally associated with improved LRPF, although not statistically significant (1-year rate 83% vs 45%, p=0.127). Other factors tested were not associated with differences in LRPF.
In-field control
Median in-field control was 11.9 months, with a 6-month estimate of 81% (95% CI 68- 93%). Dose-per-fraction ≥6 Gy was associated with improved in-field control (12-month rate 54% vs 0%, p<0.001). No significant difference was seen among patients receiving 8Gy or higher (p=0.18). In-field control was not significantly worse in patients receiving re-irradiation (12-month rate 60% vs 30%, p=0.10). No significant differences in in-field control were observed based on EQD2, chemotherapy, PTV volume, tumor histology, and metastatic vs. primary HN tumor.
A subgroup analysis was again performed on the 46 patients without re-irradiation, and with dose-per-fraction of 600cGy or higher. Median in-field control was 33.8 months, with a 6-month estimate of 86% (95% CI 72 – 99%). No significant difference in in-field control was seen among patients based on dose 8Gy or higher (p=0.12), EQD2, chemotherapy, PTV volume, tumor histology, and metastatic vs primary HN tumor.
Cox Regression Analyses
Factors with p-value <0.10 on log-rank analysis for in-field control (Dose ≥6 Gy, re-irradiation) and LRPF (Dose ≥6 Gy, dose ≥8 Gy, re-irradiation) were included in univariate and multivariate Cox regression analysis. For in-field control, only dose-per-fraction ≥6 Gy (HR, 0.1; p = 0.004; 95% CI, 0.02 – 0.42) remained significant. For LRPF, re-irradiation (HR, 2.2; p = 0.036; 95% CI, 1.1 – 4.5) and dose-per-fraction ≥6 Gy retained significance (HR, 0.27; p = 0.04; 95% CI, 0.08 – 0.95).
Toxicity
The highest acute toxicity that occurred was grade 1 in 37 cases (60%), grade 2 in 6%, and grade 3 in one case. No acute grade 4 or 5 toxicities occurred. Late grade 2 toxicities were recorded in 13% of cases, and 3% percent experienced late grade 3 complications, both involving retinopathies and vision loss. No carotid blowout or hemorrhage developed following HFRT. On Chi-square test, sites that were re-irradiated were significantly more likely to experience grade ≥2 late toxicity (p <0.001) but not grade ≥2 acute toxicity. Dose ≥8 Gy per fraction was not associated with an increase in grade ≥2 acute or late toxicities. No increased grade ≥2 late toxicity was seen in patients treated before routine use of on-board imaging in 2004.
DISCUSSION
Locoregional control of malignancies in the head-and-neck is of paramount importance for quality of life and survival. Uncontrolled HN disease progression causes significant morbidity, justifying a more aggressive approach for recurrent disease or palliation of metastatic disease. In the setting of HN disease that cannot be surgically resected, the use of IGRT and SBRT has paved the way for radiotherapy dose-escalation, thereby improving the therapeutic ratio and locoregional disease control. Unfortunately, and especially for recurrent HN disease, RT comes with a risk of severe toxicities.[24–30] Hypofractionated IGRT with doses of 4–10 Gy per fraction has been used extensively in non-HN tumor sites and is considered a safe and effective treatment, particularly for radioresistant histologies and previously irradiated disease.[31, 32] Preclinical and clinical data show that higher doses per fraction overcome the traditional radioresistance of certain histologies.[4, 6, 7] HFRT represents an attractive treatment option for unresectable disease, because of an increase in local control over conventionally fractionated RT.[4] Another advantage of HFRT in the palliative setting is the shorter overall treatment time, which minimizes disruption of important systemic therapies. A small body of literature has described the use of HFRT for heterogeneous tumors in the HN, but more widespread application in the HN has yet to occur.
In our study, we find that patients who achieve CR to treatment have not only improved locoregional disease control, but also improved overall survival. Our finding underscores the great importance of local control for HN cancers. We also find that the dose-per-fraction of 6Gy or higher with a median of 5 fractions is associated with improved outcomes compared to lower dose/fractionation schemes. In our study, patients who were re-irradiated had uniformly worse outcomes. These patients may benefit from a more aggressive multidisciplinary approach involving maximal resection, re-RT, and systemic therapy. For those who cannot undergo resection, a growing body of evidence shows that dose-escalated HFRT using SBRT to 40Gy or higher is associated with improved local control [15, 16, 21].
Our study also adds to the HN HFRT literature by including 20 patients treated with a dose per fraction of 8 Gy or above. On log-rank testing and univariate analysis, this dose range is associated with a trend to improved local control, although the analysis is limited by small patient numbers and low life-expectancy of this highly unfavorable patient population. With the rapid advances in SBRT planning and delivery, we can now safely treat gross tumor burden in the HN with doses that provide optimal local control. Our sample size is small and longer follow-up may reveal late toxicities; our results are nevertheless promising. We are in the process of generating a prospective trial of hypofractionated RT using SBRT for tumors in the HN, with the aims of evaluating toxicity, efficacy, and potentially broadening the indications for these treatments.
Several series have found severe toxicities associated with SBRT for reirradiation of HN tumors. Cengiz et al[14] treated 46 patients with recurrent previously irradiated HN cancers to a median dose of 30 Gy in 5 fractions and found that 17% of patients had carotid blowout and 15% died of carotid bleeding. These patients had recurrent disease involving the carotid artery, which received full prescription dose. A Korean group treated 36 patients with previously irradiated HNC to doses of 18–40 Gy in 3–5 fractions; 3 developed necrosis associated with treatment.[13] A group from Kyoto[19] treated 21 recurrent HN cancers with CyberKnife SBRT to a median dose of 30 Gy in 3–8 fractions. At 24 months, OS was 50%, but two patients died of pharyngeal hemorrhage at 5 and 28 months. The Henry Ford Hospital group[21] treated 37 HN lesions with fractionated SBRT of 36–48 Gy in 5–8 fractions, and 18 lesions with single-fraction SBRT. They achieved 1-year tumor control rates of 61% to 83% and reported three grade 3 and four grade 4 toxicities, including fistulae that resulted from disease progression or regression. Unger et al[33] treated 65 patients with previously irradiated HN lesions to a median SBRT dose of 30 Gy in 5 fractions. Response was achieved in 81%. Severe toxicity including arterial bleeding, neuropathy, trismus, dysphagia, and necrosis developed in 11% of patients.
Other groups have achieved high response rates with acceptable toxicities. The University of Michigan published one of the earliest reports of HN SBRT[17] in which 13 patients were treated with SBRT to doses of 12–18 Gy in a single fraction or 30 Gy in 5–6 fractions. At 6-months, nine patients showed response and no patients had acute or subacute radiation toxicity. The Pittsburgh experience[15] with CyberKnife for recurrent HN squamous cell carcinoma included 22 patients treated to a median dose of 24 Gy in 1–8 fractions. They report only two grade 2–3 acute toxicities and no late toxicities at a median follow-up of 19 months. The same group reported results of SBRT reirradiation to the HN in 85 patients[16]: at 2 years, local control was 31% and OS was 16%. They found only four grade 3 acute toxicities, and no grade 3 or greater late toxicities. Comet et al[18] treated 43 previously irradiated HN lesions to 36 Gy in 6 fractions. At a median follow-up of almost 26 months, 79% of patients achieved response and four patients had grade 3 toxicity. The authors note that they did not treat tumors invading more than one-third of the carotid artery.
Controversies do exist regarding the appropriateness of the linear-quadratic model in the setting of high-dose radiotherapy and radiosurgery.[35, 36] Studies of single-fraction radiosurgery show that cell killing at dose levels above 10 Gy occurs partially as a consequence of vascular endothelial damage, in addition to the classical model of single- and double-strand DNA breaks [1, 6]. The LQ model therefore underestimates the biological effects of a single-fraction dose that is “biologically equivalent” to a conventionally fractionated course, with equivalence calculated using the EQD2 model.[35] Several groups have proposed LQ models that better fit the observed in vitro cell kill from higher doses per fraction of radiosurgery[36], but these models are not yet widely accepted for clinical use. We currently do not have another reliably validated model to help us compare conventional fractionation and high-dose hypofractionation. We therefore used the LQ model and EQD-2Gy in order to facilitate comparison between the hypofractionated doses used in our study and a “biologically equivalent” conventionally fractionated course.
Several potential sources of bias exist in this retrospective study. First, the decision to use HFRT versus conventional RT was chosen by the individual treating physician and not according to any formal criteria. We did find that patients with radioresistant histologies were more likely to receive a higher EQD2, a finding that is consistent with clinical practice. Another limitation of our study is the short survival and short median follow-up of patients who died, which may underestimate the number of late toxicities we encounter. However, with a median overall survival of 7.2 months, our length of follow-up is appropriate for this patient population with poor overall prognosis.
Our study shows that hypofractionated RT can be delivered safely and provides relatively high local control for challenging cancers in the HN and that a higher dose-per-fraction is associated with improved in-field control. More stringent selection criteria for HFRT, combined with more tailoring of dose and fractionation schedules to the individual lesion and patient, can likely reduce the development of late severe toxicities. A prospective dose-escalation trial is warranted to find the optimal dose scheme in these patients.
TABLE 4.
Sites of first recurrence in 59 treated lesions who initially had response or stable disease.
| Location | N (%) |
|---|---|
| Local (in-field) only | 12 (19) |
| Regional only | 9 (15) |
| Distant only | 21 (34) |
| Local and regional | 4 (6) |
| Local and distant | 1 (2) |
| Regional and distant | 3 (5) |
| Local, regional, and distant | 1 (2) |
| No recurrence | 11 (18) |
Highlights.
Hypofractionated head and neck radiation therapy is controversial due to toxicity concerns.
62 sites of measurable head-and-neck tumors were treated with high-dose radiation therapy.
Dose per fraction of 6Gy or greater had improved locoregional control.
Re-irradiated patients had worse disease control after hypofractionated radiation.
No increased toxicity was seen in patients treated with higher doses per fraction.
Footnotes
Conflicts of Interest Statement
Dr. Yamada is a consultant for Varian Medical Systems and speaks for Continuing Medical Education Speakers’ Bureau.
All other authors report no potential conflicts of interest including no financial, personal, or other relationships with other people or organizations that could inappropriately influence this work. No grant support or funding was used for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124(5):1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 3.Onishi H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–31. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 4.Damast S, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. 2011;81(3):819–26. doi: 10.1016/j.ijrobp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Gerszten PC, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32(2):193–9. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 7.Truman JP, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5(9) doi: 10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae SH, et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012;106(2):138–43. doi: 10.1002/jso.23058. [DOI] [PubMed] [Google Scholar]
- 9.Milano MT, et al. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(5):1516–22. doi: 10.1016/j.ijrobp.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Milano MT, et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83(3):878–86. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol. 2006;16(2):77–84. doi: 10.1016/j.semradonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Salama JK, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res. 2008;14(16):5255–9. doi: 10.1158/1078-0432.CCR-08-0358. [DOI] [PubMed] [Google Scholar]
- 13.Roh KW, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1348–55. doi: 10.1016/j.ijrobp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Cengiz M, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011;81(1):104–9. doi: 10.1016/j.ijrobp.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Voynov G, et al. Frameless stereotactic radiosurgery for recurrent head and neck carcinoma. Technol Cancer Res Treat. 2006;5(5):529–35. doi: 10.1177/153303460600500510. [DOI] [PubMed] [Google Scholar]
- 16.Rwigema JC, et al. Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: updated report of the University of Pittsburgh experience. Am J Clin Oncol. 2010;33(3):286–93. doi: 10.1097/COC.0b013e3181aacba5. [DOI] [PubMed] [Google Scholar]
- 17.Ryu S, et al. Image-guided radiosurgery of head and neck cancers. Otolaryngol Head Neck Surg. 2004;130(6):690–7. doi: 10.1016/j.otohns.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Comet B, et al. Salvage Stereotactic Reirradiation With or Without Cetuximab for Locally Recurrent Head-and-Neck Cancer: A Feasibility Study. Int J Radiat Oncol Biol Phys. 2012;84(1):203–9. doi: 10.1016/j.ijrobp.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 19.Kodani N, et al. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res. 2011;52(1):24–31. doi: 10.1269/jrr.10086. [DOI] [PubMed] [Google Scholar]
- 20.Unger KR, et al. Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77(5):1411–9. doi: 10.1016/j.ijrobp.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui F, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2009;74(4):1047–53. doi: 10.1016/j.ijrobp.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. National Institutes of Health, Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. 2010 http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 23.Barton M. Tables of equivalent dose in 2 Gy fractions: a simple application of the linear quadratic formula. Int J Radiat Oncol Biol Phys. 1995;31(2):371–8. doi: 10.1016/0360-3016(94)E0126-5. [DOI] [PubMed] [Google Scholar]
- 24.De Crevoisier R, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16(11):3556–62. doi: 10.1200/JCO.1998.16.11.3556. [DOI] [PubMed] [Google Scholar]
- 25.De Crevoisier R, et al. Full dose reirradiation combined with chemotherapy after salvage surgery in head and neck carcinoma. Cancer. 2001;91(11):2071–6. doi: 10.1002/1097-0142(20010601)91:11<2071::aid-cncr1234>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Janot F, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26(34):5518–23. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 27.Wong LY, et al. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. 2003;25(11):953–9. doi: 10.1002/hed.10310. [DOI] [PubMed] [Google Scholar]
- 28.Wong SJ, Machtay M, Li Y. Locally recurrent, previously irradiated head and neck cancer: concurrent re-irradiation and chemotherapy, or chemotherapy alone? J Clin Oncol. 2006;24(17):2653–8. doi: 10.1200/JCO.2005.05.3850. [DOI] [PubMed] [Google Scholar]
- 29.Spencer SA, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30(3):281–8. doi: 10.1002/hed.20697. [DOI] [PubMed] [Google Scholar]
- 30.Lee N, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):731–40. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki H, et al. Reirradiation of head and neck cancer focusing on hypofractionated stereotactic body radiation therapy. Radiat Oncol. 2011;6:98. doi: 10.1186/1748-717X-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AM, Coleman C, Horasek S. Stereotactic radiosurgery for the treatment of primary sarcomas and sarcoma metastases of the spine. Neurosurgery. 2009;64(2 Suppl):A54–9. doi: 10.1227/01.NEU.0000339131.28485.4A. [DOI] [PubMed] [Google Scholar]
- 33.Unger KR. Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. International journal of radiation oncology, biology, physics. 2010;77(5):1411–9. doi: 10.1016/j.ijrobp.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 34.Ang KK, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys. 1994;30(4):795–8. doi: 10.1016/0360-3016(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 35.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–3. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Wang JZ, et al. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med. 2010;2(39):39ra48. doi: 10.1126/scitranslmed.3000864. [DOI] [PubMed] [Google Scholar]