Abstract
INTRODUCTION
Long-term results comparing percutaneous transluminal angioplasty with or without stenting (PTA/S) and open surgical bypass for chronic limb-threatening ischemia (CLTI) in patients who have had no prior intervention are lacking.
METHODS
All patients undergoing a first-time lower extremity revascularization for CLTI by vascular surgeons at our institution from 2005 to 2014 were retrospectively reviewed. Outcomes included perioperative complications, wound healing, restenosis, primary patency, re-intervention, major amputation, RAS events (i.e., re-intervention, major amputation, or stenosis), and mortality. Outcomes were evaluated using chi-squared, Kaplan-Meier, and Cox regression analyses.
RESULTS
Of the 2,869 total lower extremity revascularizations performed between 2005 and 2014, 1,336 fit our criteria of a first-time lower extremity intervention for CLTI (668 bypass procedures and 668 PTA/S procedures). Bypass patients were younger (71 vs. 72 years, P=.02) and more often male (62% vs. 56%; P<.02). Total mean hospital length of stay (LOS) was significantly longer following a first-time bypass (10 vs. 8 days, P<.001), as were mean preoperative LOS (4 vs. 3 days, P<.01) and post-operative LOS (7 vs. 5 days, P<. 001). There was no difference in perioperative mortality (3% vs. 3%, P=.63). Surgical site infection occurred in 10% of bypass patients. Freedom from re-intervention was significantly higher in patients undergoing a first-time bypass procedure (62% vs. 52% at 3 years, P=.04), as was freedom from restenosis (61% vs. 45% at 3 years, P<.001). Complete wound healing at six-month follow-up was significantly better following an initial bypass (43% vs. 36%; P<.01). A Cox regression model of all patients showed that re-intervention was predicted by a first-time PTA/S (Hazard Ratio (HR) 1.6; 95% Confidence Interval [CI] 1.3–2.1) and both preoperative femoropopliteal TASC C and TASC D lesions (2.0[1.3–3.1] and 1.8 [1.3–2.7], respectively). Major amputation among all patients was predicted by an initial presentation of gangrene (2.5 [1.3–5.0]), dialysis dependence (1.9 [1.3–2.9]), diabetes (2.0 [1.1 –3.8]), and preoperative femoropopliteal TASC D lesions (2.1 [1.1 –4.0]), and was not predicted by procedure type.
CONCLUSIONS
In this retrospective analysis, bypass for the primary treatment of CLTI showed improved six-month wound healing, higher freedom from restenosis, improved patency rates, significantly fewer re-interventions, and higher survival than PTA/S within three years, but was associated with increased total hospital LOS and wound infection. Perioperative mortality and amputation rates were similar between procedure types.
INTRODUCTION
There are currently two treatments available for patients with chronic limb-threatening ischemia (CLTI): open surgical bypass and percutaneous transluminal angioplasty with or without stenting (PTA/S). Although promoted for its long-term anatomical patency and clinical durability, bypass has also been shown to escalate morbidity and increase resource use.1–4 Support for balloon angioplasty, on the other hand, highlights the benefits of lower procedural morbidity and mortality, faster procedural times, and a reduced hospital stay.5 Proponents of PTA/S additionally claim that failed angioplasty does not threaten successive surgery and preserves collaterals; however, recent data have not only proven otherwise, but have also illustrated higher rates of restenosis.6–9
Previous studies have attempted to compare bypass and PTA/S for various degrees of lower extremity limb ischemia with varying methodological problems.10–20 Published in 2005, the randomized BASIL trial attempted to offer answers to similar concerns, concluding no difference between bypass-first and angioplasty-first strategies up to two years, after which overall survival and amputation free survival were better following bypass.21 The BASIL trial has been criticized, however, for its strict eligibility requirements and for its low number of patients with infrapopliteal disease. Further, patients within their cohort included those with a prior intervention and subsequent clinical failure, limiting the study’s ability to make recommendations for patients undergoing a first-time revascularization.
Conflicting evidence continues to amplify controversy regarding which treatment, if any, is both associated with a better clinical outcome and is a more effective use of healthcare resources in patients potentially suitable for both treatments with legs threatened by CLTI. With the increasing proficiency of endovascular techniques, the incidence of PTA/S as a first line therapy for CLTI has similarly increased. With varying success rates reported throughout the literature, however, it remains to be determined as to whether endovascular techniques have better long-term limb salvage rates as compared to open surgical bypass. Due to the inconclusive information regarding first-time lower extremity interventions for CLTI, we sought to describe our institution’s long-term experience with both endovascular and bypass repair, in hopes to find a statistically preferred treatment to minimize re-intervention, stenosis, amputation, and mortality following any first-time lower extremity revascularization for CLTI.
METHODS
We performed a retrospective review of all patients with CLTI undergoing a first-time lower extremity intervention by the Division of Vascular and Endovascular Surgery at Beth Israel Deaconess Medical Center (BIDMC). We individually reviewed the medical records of all open surgical bypass and all PTA/S interventions from January 2005 to October 2014. Patients who received previous interventions on the ipsilateral limb (whether at BIDMC or at an outside institution) or interventions involving the iliac arteries and above were excluded. Patients undergoing a concomitant procedure, including endarterectomy, profundaplasty, thrombectomy, atherectomy, or patch, were included and adjusted for in our analysis. Primary outcomes included 30-day mortality, wound healing, restenosis (>3.5× step-up by duplex), primary patency, re-intervention, major amputation, RAS events (a composite variable denoted by re-intervention, major amputation, or stenosis), and mortality. Demographics, discharge medications, comorbidities, and perioperative complications were also recorded. The decision of intervention type was surgeon dependent and varied over time with the acquisition of endovascular skills: In general, primary angioplasty with selective stenting was done so at the clinical judgment of the attending physician at the time of the procedure. Routine statin use was introduced over time. PTA/S patients were generally treated with clopidogrel for one month post-operatively and aspirin indefinitely. Technical success following PTA/S was defined as <30% residual stenosis and no flow limiting dissection. Technical success following a bypass procedure was defined as a patent graft at completion of the procedure, no significant defect in the vein on angioscopy, and continuous wave doppler interrogation.
Typical patient follow-up interval and modality was every three to four months for two years and every six months thereafter with arterial duplex ultrasound imaging and ankle-brachial indices (ABI) with forefoot pulse volume recordings (PVR) and/or toe pressures. Prior to 2008, only PVR and ABI information was recorded; after 2008, in order to obtain more quantitative analyses of flow to the forefoot, most surgeons routinely recorded toe pressures. Criteria for restenosis >75% was a >3.5 fold increase in peak systolic velocity by duplex or angiographic measurement. Intervention was performed for symptomatic graft restenosis and threatened asymptomatic grafts (peak systolic velocity ratio >3.5 – 4 or low graft velocities <30cm/second). Generally, patients did not undergo re-interventions for an asymptomatic restenosis post PTA/S alone; however, we were more likely to re-intervene with PTA/S for an asymptomatic in-stent restenosis with peak systolic velocity ratio of >3.5–4. We were less likely to re-intervene percutaneously for a symptomatic restenosis if the disease was extensive and restenosis was rapid, as we feel this has a low likelihood of deriving a durable benefit. Symptom recurrence and disease progression were determined by the attending surgeon at follow-up.
We included patients whose disease severity was distinctly identifiable as CLTI and who underwent either an angioplasty with or without stenting or an open surgical bypass. Indications for intervention included tissue loss (i.e., gangrene or ulcer) or rest pain. Limbs presenting with more than one indication were assigned as having only the most severe symptom: Gangrene was considered most severe, ulcer moderately severe, and rest pain least severe. Femoropopliteal lesion anatomy and severity was defined according to the modified Trans Atlantic Inter-society Consensus (TASC II) classification, while tibial lesion information was defined by TASC I, as no updated TASC class for tibial lesions was included in the modified TASC II.22, 23
All analyses were performed on a per-limb basis. Pearson chi-squared and Fisher exact test were used for comparisons of categorical variables. Continuous variables were compared using Student t-test or Mann-Whitney U test, as appropriate. Treatment outcomes during the course of follow-up were analyzed using Kaplan-Meier analysis, and time-to-failure curves were compared with the log-rank test. Bivariate and multivariable Cox regression models were used to assess predictor variables for time-dependent outcomes. Statistical significance was defined as P<.05. All statistical tests were done using STATA 13 (StataCorp, College Station, Tex). The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent due to the retrospective design.
RESULTS
Patient Characteristics
Of the 2,869 total lower extremity revascularizations performed between January 2005 to October 2014, 1,533 procedures were excluded from the analysis: 663 interventions performed on limbs with non-CLI symptoms, 437 re-interventions, and 433 limbs that had undergone a previous intervention. Ultimately, 1,336 met our inclusion criteria (i.e., a first-time lower extremity intervention for CLTI): 668 undergoing a first-time bypass procedure and 668 undergoing a first-time PTA/S procedure. The proportional distribution of procedure type over our decade-long study progressed from bypass in over three-quarters of the yearly CLTI revascularizations to a more even distribution, with angioplasty slowly becoming the more common practice (Figure I). Bypass-first patients, as compared to the PTA/S-first patients, were younger (71 years vs. 72 years; P=.02), more often male (62% vs. 56%; P=.02), and more often white (82% vs. 74%; P<.001) (Table I). Bypass-first patients more commonly smoked (both current and prior history) (26% vs. 16% [P<.001] and 68% vs. 53% [P<.001], respectively) and suffered from COPD (14% vs. 10%, P=.01), while PTA/S-first patients more commonly suffered from dialysis dependence (17% vs. 23%, P=.01) and hypertension (82% vs. 89%, P<.001). Although patients presenting with gangrene were statistically similar between groups (30% vs. 27%; P=.36), ulcerations were significantly less common in the bypass-first cohort (48% vs. 57%, P=.001) and rest pain was more common (23% vs. 16%, P=.001). Discharge medications differed between procedure types as well: Bypass-first patients were less often prescribed aspirin (82% vs. 86%; P=.046), clopidogrel (30% vs. 84%; P<.001), any antiplatelet (86% vs. 97%; P<.001), and dual antiplatelets (25% vs. 68%; P<.001), and were more commonly prescribed statins (81% vs. 75%; P<.01). Finally, bypass-first interventions were performed significantly more often in patients with both pre-operative femoropopliteal TASC D lesions (31% vs. 13%; P<.001), as well as pre-operative tibial TASC D lesions (37% vs. 27%; P<.001).
Figure I. Beth Israel Deaconess Medical Center procedural distribution for first-time lower extremity revascularizations for chronic limb-threatening ischemia (CLTI) from 2005–2014.
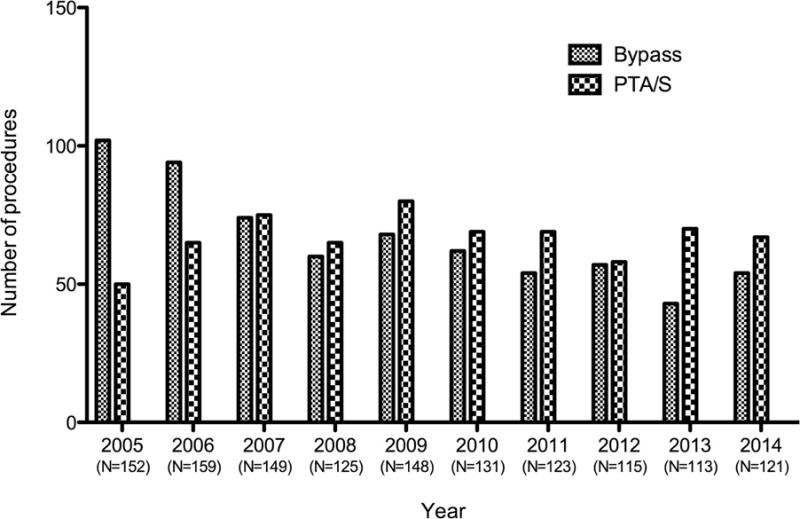
PTA/S, percutaneous transluminal angioplasty with or without stenting
Table I.
Demographics and comorbidities among 1,336 first-time revascularizations for chronic limb-threatening ischemia (CLTI)
|
|
|||
|---|---|---|---|
|
Bypass (N=668) |
PTA/S (N=668) |
P-value | |
| Demographics, No. (%) | |||
| Age, mean (SD) | 70.8 (12.5) | 72.3 (12.7) | 0.02 |
| Male sex | 415 (62) | 374 (56) | 0.02 |
| Race | <.001 | ||
| White | 549 (82) | 493 (74) | |
| Non-white | 118 (18) | 171 (26) | |
| Comorbidities, No. (%) | |||
| Coronary artery disease | 348 (53) | 326 (50) | 0.35 |
| Hypertension | 543 (82) | 583 (89) | <.001 |
| Diabetes | 490 (73) | 506 (76) | 0.32 |
| Dialysis dependence | 115 (17) | 148 (23) | 0.01 |
| Hyperlipidemia | 392 (59) | 410 (63) | 0.16 |
| History of myocardial infarction | 183 (28) | 161 (25) | 0.22 |
| History of CABG | 182 (27) | 149 (23) | 0.06 |
| Congestive heart failure | 198 (30) | 210 (32) | 0.38 |
| COPD | 95 (14) | 64 (10) | 0.01 |
| BMI | 27.5 (6.4) | 27.8 (6.3) | 0.47 |
| Current smoker | 172 (26) | 103 (16) | <.001 |
| Smoking history | 451 (68) | 345 (53) | <.001 |
| Indication, No. (%) | |||
| Rest pain | 152 (23) | 105 (16) | 0.001 |
| Ulcer | 319 (48) | 381 (57) | 0.001 |
| Gangrene | 197 (30) | 182 (27) | 0.36 |
| Discharge medications, No. (%) | |||
| Aspirin | 532 (82) | 552 (86) | 0.046 |
| Clopidogrel | 192 (30) | 517 (84) | <.001 |
| Statin | 537 (81) | 494 (75) | <.01 |
| Antiplatelet (any) | 563 (86) | 631 (97) | <.001 |
| Dual antiplatelet | 161 (25) | 438 (68) | <.001 |
| Femoropopliteal TASC Class, No. (%) | |||
| None | 66 (12) | 133 (21) | <.001 |
| TASC A | 89 (16) | 129 (21) | 0.05 |
| TASC B | 137 (25) | 217 (35) | <.001 |
| TASC C | 89 (16) | 63 (10) | <.01 |
| TASC D | 167 (31) | 84 (13) | <.001 |
| Tibial TASC Class, No. (%) | |||
| None | 104 (20) | 43 (6.9) | <.001 |
| TASC A | 50 (9.4) | 98 (16) | <.01 |
| TASC B | 90 (17) | 162 (26) | <.001 |
| TASC C | 93 (18) | 156 (25) | <.01 |
| TASC D | 195 (37) | 169 (27) | <.001 |
PTA/S, percutaneous transluminal angioplasty with or without stenting; CABG, coronary artery bypass grafting; BMI, body mass index; COPD, chronic obstructive pulmonary disease; TASC, Trans-Atlantic Inter-Society Consensus
In bypass-first patients, the distal targets were primarily infrapopliteal (44% in the tibial and peroneal arteries and 25% in the inframaleolar dorsalis pedis, posterior tibial, plantar, or tarsal arteries) and single-segment great saphenous vein conduits were used in over three-quarters of procedures (77%; Table II). Non-reversed great saphenous vein was most the most common conduit (41%), while any single-segment vein conduit was used in 82% of the first-time bypass procedures. Twelve percent of bypass-first procedures used composite vein, and 6% used prosthetic material. Of the 668 first-time PTA/S procedures, the most common distal lesions treated were in the tibial and peroneal arteries (55%). Further, approximately 44% of all angioplasty interventions were multi-level. Of all PTA/S-first procedures, the superficial femoral artery was intervened on the most (63%), 27% of which included a stent (Figure II). Approximately 38% of all PTA/S-first procedures included stenting. The median follow-up for bypass was 18 months (range <1–114) and 14 months for PTA/S (range <1–118).
Table II.
Procedural details among 668 first-time bypass procedures for CLTI
|
|
||
|---|---|---|
| Variable (N=668) | No. | % |
| Inflow artery | ||
| Femoral | 494 | 74 |
| Popliteal | 173 | 26 |
| Tibial | 1 | .2 |
| Outflow artery | ||
| Above-knee popliteal | 120 | 18 |
| Below-knee popliteal | 93 | 14 |
| Tibial | 237 | 36 |
| Peroneal | 53 | 7.9 |
| Dorsalis pedis | 137 | 21 |
| Plantar or tarsal | 28 | 4 |
| Conduit | ||
| In situ saphenous vein | 153 | 23 |
| Reversed saphenous vein | 88 | 13 |
| Non-reversed saphenous ve | in 268 | 41 |
| Arm vein | 35 | 5.3 |
| Composite vein | 77 | 12 |
| Synthetic | 38 | 5.8 |
Figure II. Proportion of lower extremity vessels intervened on in 668 first-time PTA/S procedures for chronic limb-threatening ischemia (CLTI).
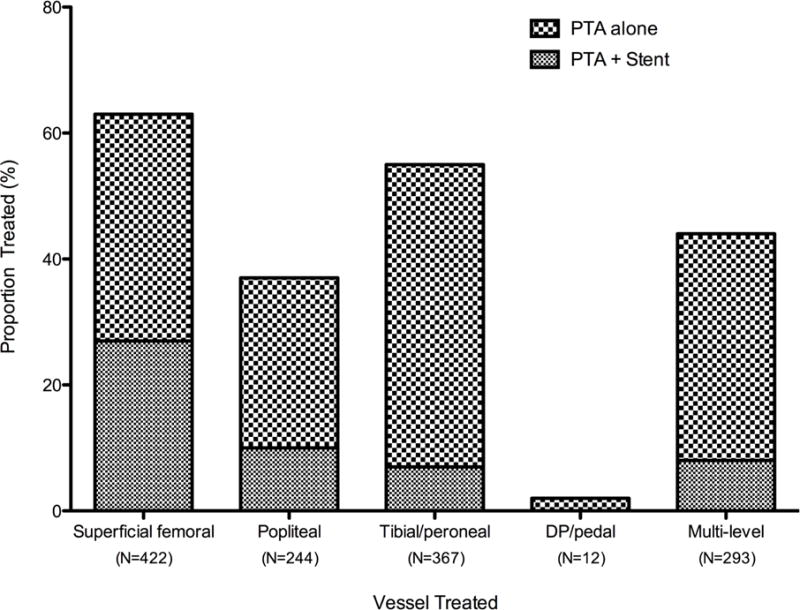
PTA/S, percutaneous transluminal angioplasty with or without stenting
Perioperative Outcomes
Perioperative mortality did not differ between procedure types, occurring in 22 bypassfirst (3.3%) and 19 PTA/S-first patients (2.8%) (P=.63). There was a higher overall complication rate following a bypass, which was primarily due to surgical site infections (10%; Table III). Bypass-first patients had a significantly longer total mean hospital length of stay (LOS) (10 vs. 7 days, P<.001), mean pre-operative LOS (4 vs. 3 days, P<.01), and mean post-operative LOS (7 vs. 5 days, P<.001). Additionally, within 30 days of the index procedure, bypass-first patients underwent partial foot or toe amputations less often than PTA/S-first patients (9% vs. 14%, P<.01). Although lower among bypass-first patients, 30-day transmetatarsal amputations (4% vs. 5%, P=.60) and wound debridements (8% vs. 9%, P=.40) did not differ between procedure types.
Table III.
Perioperative (30-day or in-hospital) complications following any first-time revascularization for CLTI
|
|
|||
|---|---|---|---|
|
Perioperative Complications No. (%) |
Bypass (N=668) |
PTA/S (N=668) |
P-value |
| Mortality | 22 (3.3) | 19 (2.8) | .63 |
| Myocardial infarction | 9 (1.4) | 11 (1.7) | .94 |
| Surgical site infection | 70 (10) | N/A | N/A |
| Hematoma | 53 (7.9) | 28 (4.2) | <.01 |
Long-term Outcomes
Complete wound healing at six-month follow-up was significantly better following an initial bypass as compared to an initial PTA/S (43% vs. 36%; P<.01). On survival analysis, bypass patients had a higher freedom from restenosis when compared to PTA/S (61% vs. 45% at 3 years; P<.001) (Figure III). After adjusting for baseline characteristics (i.e., age, gender, race, procedure year, hypertension, indication for intervention, concomitant procedures, diabetes, dialysis dependence, COPD, CHF, history of smoking, femoropopliteal TASC class, tibial TASC class, and discharge medications), multivariable predictors of restenosis for all patients included a first-time PTA/S intervention (Hazard Ratio (HR) 1.7; 95% Confidence Interval [CI] 1.4–2.2), a history of smoking (1.3 [1.1–1.7]), diabetes (1.4 [1.1 –1.9]), and preoperative femoropopliteal TASC C or TASC D lesions (1.9 [1.3–2.9] and 1.5 [1.1–2.2], respectively). Additionally, freedom from re-intervention was significantly higher among bypass-first patients (62% vs. 52% at 3 years; P=.04) (Figure IV), where independent predictors of re-intervention included a first-time PTA/S (1.6 [1.3–2.1 ]), as well as preoperative femoropopliteal TASC C or TASC D lesions (2.0 [1.3–3.1] and 1.8 [1.3–2.7], respectively). When this same model is stratified by procedure type, only preoperative femoropopliteal TASC C and TASC D lesions prove significant for re-intervention in both bypass-first (2.6 [1.3–5.4] and 1.8 [1.2–3.5], respectively) and PTA/S-first patients (1.8 [1.1–3.2] and 2.0 [1.1–3.4], respectively). Finally, primary patency was shown to be significantly higher among bypass-first patients (72% vs. 63% at 3 years; P=.02), where independent predictors among all patients included a first-time PTA/S (1.5 [1.1–2.1]) and preoperative femoropopliteal TASC C or TASC D lesions (1.8 [1.1–3.1] and 1.9 [1.1–3.1], respectively).
Figure III. Freedom from re-stenosis following any first-time revascularization for CLTI.
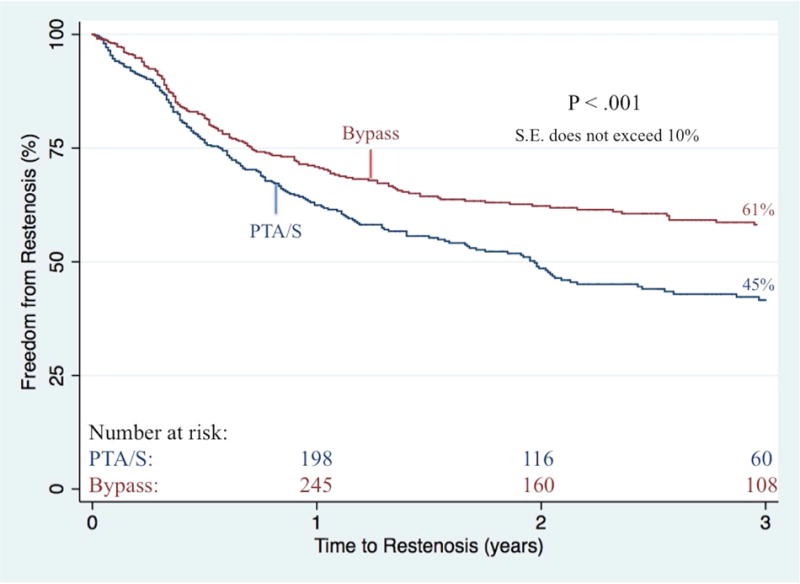
PTA/S, percutaneous transluminal angioplasty with or without stenting
S.E. standard error
Figure IV. Freedom from re-intervention following any first-time revascularization for CLTI.
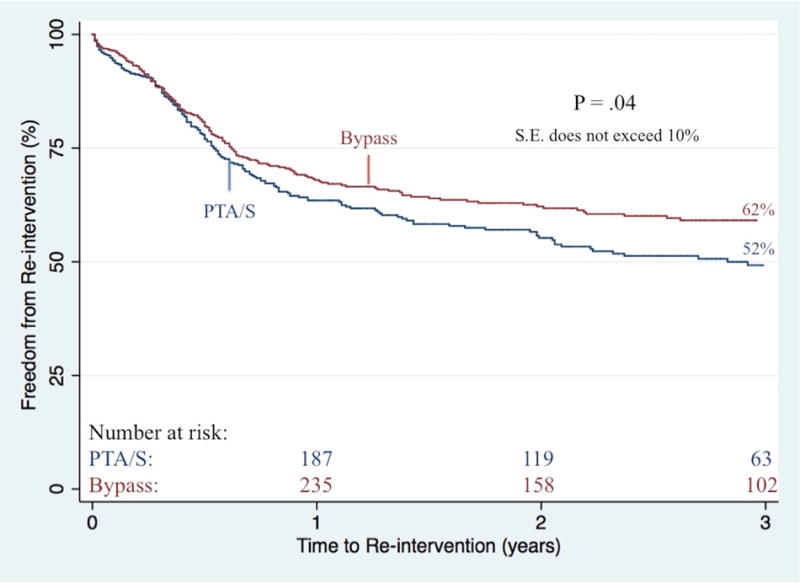
PTA/S, percutaneous transluminal angioplasty with or without stenting
S.E. standard error
Bypass-first patients less commonly underwent any partial foot or toe amputations (23% vs. 30%, P<.01). When further stratifying by indication for intervention, partial foot or toe amputations did not differ between bypass-first and PTA/S-first rest pain patients (9.2% vs. 9.5%; P=.93) nor ulcer patients (19% vs. 25%; P=.08); however, there was a significantly lower proportion of bypass-first patients with gangrene undergoing any form of minor amputation (38% vs. 53%; P<.01). Freedom from major amputation did not differ between bypass and PTA/S groups neither during six-months (93% vs. 92%, respectively; P=.88), nor throughout follow-up (81% vs. 85% at 3 years; P=.40; Figure V). Among bypass-first patients, major amputation was predicted by gangrene as the indication for intervention (4.1 [1.3–12.6]), COPD (3.3 [1.5–7.3]), synthetic conduits (2.6 [1.2–5.8]), dialysis dependence (2.1 [1.1–4.0]), preoperative femoropopliteal TASC D lesions (6.2 [1.7–22.8]), and preoperative tibial TASC C and TASC D lesions (5.8 [2.1–16.2] and 4.0 [1.5–10.8], respectively). Conversely, among PTA/S-first patients, major amputation was predicted by patients presenting with gangrene (3.0 [1.1–8.5]), dialysis dependence (1.9 [1.1–3.2]), and CHF (1.9 [1.1–3.2]), while discharge aspirin administration was protective (0.4 [0.2–0.8]). Ultimately, the risk of experiencing a RAS event was significantly greater in patients undergoing a first-time PTA/S (1.7 [1.3–2.2]) and in patients with either pre-operative femoropopliteal TASC C or TASC D lesions (2.0 [1.4–2.9] and 1.5 [1.1–2.2], respectively). Freedom from a RAS event within 3 years was 47% in bypass-first patients and 34% in PTA/S-first patients (P<.001) (Figure VI). Finally, survival was higher in the bypass-first patients (61% vs. 52% at 3 years, P<.01) (Figure VII), where all patient mortality was predicted by age (1.1 [1.0–1.2]), PTA/S-first interventions (1.4 [1.1–1.8]), initial presentation of gangrene (1.6 [1.2–2.2]), dialysis dependence (1.9 [1.5–2.3], a history of COPD (1.4 [1.1–1.9]) and CHF (1.7 [1.4–2.1]). Discharge antiplatelet administration was protective against mortality (0.6 [0.4–0.9]).
Figure V. Freedom from major amputation following any first-time revascularization for CLTI.
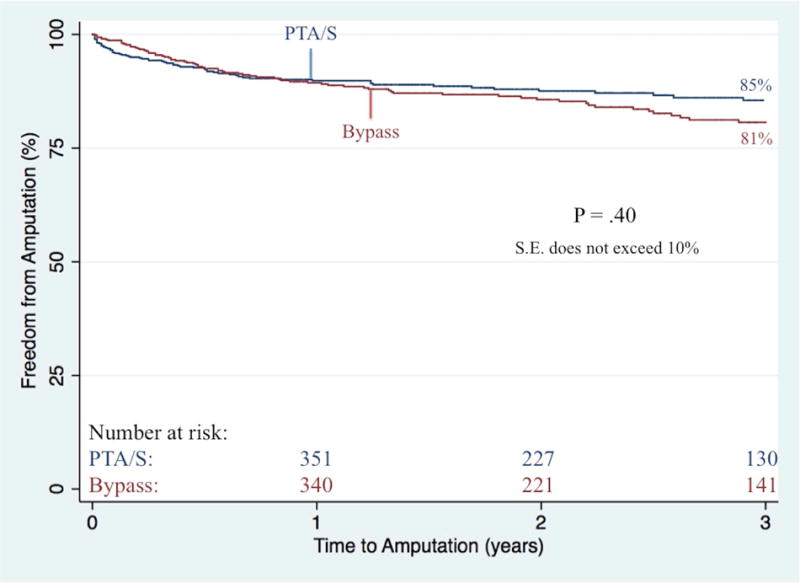
PTA/S, percutaneous transluminal angioplasty with or without stenting
S.E. standard error
Figure VI. Freedom from re-intervention, major amputation, or stenosis (RAS events) following any first-time revascularization for CLTI.
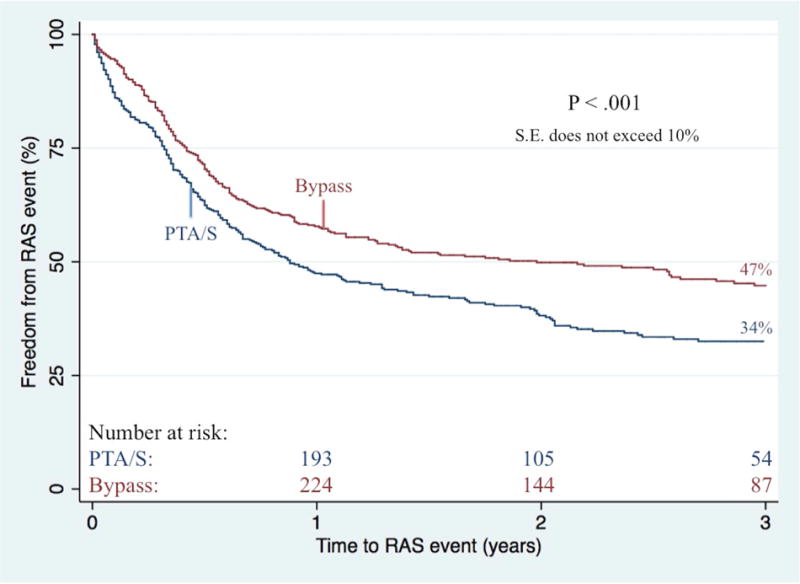
PTA/S, percutaneous transluminal angioplasty with or without stenting
S.E. standard error
Figure VII. Overall survival following any first-time revascularization for CLTI.
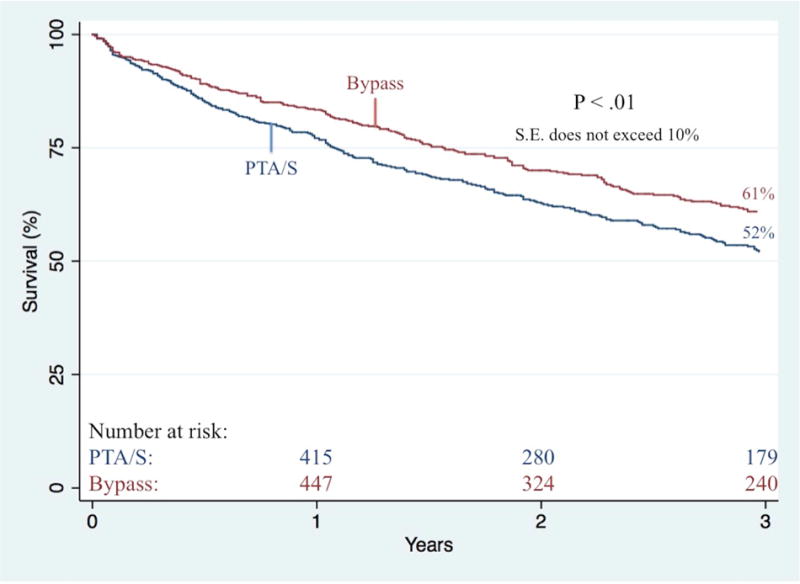
PTA/S, percutaneous transluminal angioplasty with or without stenting
S.E. standard error
Throughout follow-up, among all 1,336 procedures, 375 underwent a re-intervention within 3 years (43%): 175 bypass-first and 200 PTA/S-first (38% vs. 48%, respectively; P=.04) (Table IV). An endovascular re-intervention was the most common procedure in both groups, yet occurred more often in PTA/S-first patients (22% vs. 39%, P<.001). Conversely, bypass re-interventions did not differ between bypass-first and PTA/S-first patients (21% vs. 16%; P=.18). Overall, approximately half of the PTA/S patients underwent a re-intervention due to recurring and/or non-healing ulcers (48%, versus 34% in bypass patients; P<.01), while significantly more patients with a first-time bypass procedure underwent a re-intervention on an asymptomatic duplex-detected stenosis (22% vs. 9%, P<.001).
Table IV.
Re-intervention details at three years following any first-time revascularization for CLTI
|
|
|||
|---|---|---|---|
|
Variables No. (%)a |
Bypass (N=668) |
PTA/S (N=668) |
P-Value |
| Re-intervention | |||
| Any | 175 (38) | 200 (48) | .04 |
| Bypass | 85 (21) | 65 (16) | .18 |
| PTA/S | 94 (22) | 140 (39) | <.001 |
| Indication for Re-intervention | |||
| Asymptomatic/duplex | 40 (22) | 18 (9.0) | <.001 |
| Rest Pain | 43 (24) | 36 (18) | .14 |
| Ulcer | 62 (34) | 99 (48) | <.01 |
| Gangrene | 34 (19) | 52 (25) | .12 |
Percentages are three-year Kaplan-Meier estimates
PTA/S, percutaneous transluminal angioplasty with or without stenting
Sensitivity Analyses
In order to control for potential study and cohort limitations, we performed several sensitivity analyses aimed toward mitigating any potential confounding effects. As previously mentioned and illustrated in Figure I, procedure type varied over time as the acquisition of endovascular skills improved; in order to account for these potential technical improvements, we constructed a separate analysis with the same primary outcomes limited to the years in which PTA/S was the more common procedure type (2007–2014). Restriction of the analysis to these years did not yield any substantial differences in wound healing, restenosis, primary patency, re-intervention, major amputation, RAS events, or survival between bypass-first and PTA/S-first interventions for CLTI. Further, as ulcerations were significantly less common in the bypass-first cohort, we performed additional (and separate) sensitivity analyses stratifying our multivariable models by ulcer-only patients and tissue loss-only patients, which also did not appreciably alter any primary outcomes. Finally, as PTA/S-first patients were more commonly dependent on dialysis, we performed a final sensitivity analysis where patients on dialysis (N=263) were removed.
Removal of these patients did alter the unadjusted and adjusted rates of death, both shown to no longer depict a significant difference between bypass-first and PTA/S-first patients (P=.25 and P=.20, respectively). Additionally, following the removal of these patients, the unadjusted six-month wound healing rates were shown to no longer be statistically different between groups (P=.09); when adjusting for baseline characteristics, however, the six-month wound healing rates remain significantly improved following bypass-first interventions (P<.01).
DISCUSSION
Our data show that, in patients undergoing a first-time lower extremity intervention for CLTI, bypass-first patients had significantly improved wound healing, greater freedom from restenosis and re-intervention, higher primary patency rates, and lower mortality than those who underwent a first-time PTA/S. Importantly, in our experience, re-interventions in PTA/S-first patients were more commonly performed on ongoing wound problems, while re-interventions on bypass patients occurred more commonly for asymptomatic duplex-detected lesions. Although a bypassfirst approach was associated with a significantly greater total, pre-operative, and post-operative length of hospital stay as well as more perioperative complications (primarily due to surgical-site infections), perioperative mortality did not differ between procedure types and long-term mortality was significantly less frequent following an index bypass. Further, as expected, limb salvage did not differ between procedure types. Importantly, preoperative femoropopliteal TASC D lesions proved predictive for major amputation among all patients, while femoropopliteal TASC D and tibial TASC C and TASC D lesions proved predictive of major amputation in bypass-first patients. Finally, among bypass-first patients, vein conduits proved most effective in decreasing major amputation rates. Overall, these data illustrate that, when compared to a firsttime PTA/S procedure, a bypass-first procedure provides a more durable and long-term repair with a significantly higher freedom from restenosis, a significantly higher freedom from re-intervention, better wound healing, and greater survival.
Previously, the randomized BASIL trial concluded that bypass surgery-first and balloon angioplasty-first strategies provided equal outcomes for amputation-free survival up to two years, after which overall survival and amputation free survival were better following bypass.21 Furthermore, this study found that re-intervention increased in patients receiving bypass as a secondary treatment (which occurred mostly in patients receiving PTA/S as a primary treatment). The BASIL trial has been criticized, however, for its strict eligibility requirements, potentially impacting its real-world generalizability, and for its low number of patients with infrapopliteal disease. Further, the BASIL trial cohort included patients with a clinical failure after prior interventions and did not consist of exclusively first-time interventions; however, both our results and the BASIL trial findings raise the possibility that patients can benefit from a bypassfirst strategy, as opposed to an angioplasty-first strategy. Overall, our data suggest that the BASIL trial results may be more generalizable than previously assumed.
A 2013 study conducted by Jones et al. evaluated the adverse effects of subsequent revascularizations following infrainguinal treatment failure in patients suffering from peripheral arterial disease.24 After comparing 2,350 patients undergoing a primary infrainguinal bypass with 1,154 patients undergoing secondary infrainguinal bypass (following a failed PTA/S or bypass), secondary bypass patients were shown to have inferior 1-year outcomes, including major adverse limb event (MALE)-free survival, and re-intervention- or amputation-free survival, regardless of the prior failed treatment type (PTA/S or bypass). Similar to our data, Jones et al. advocate appropriate patient selection rather than an “endovascular first” approach – an important finding, as recent studies have shown that distal targets can be altered from multiple percutaneous interventions.8, 9
Finally, a study conducted by Engelhardt et al. evaluated the initial treatment of 104 patients presenting with a first episode of CLTI to determine their amputation-free survival rate.25 In total, 65% received some form of revascularization: surgical arterial reconstruction in 55% and PTA/S in 45%. Twenty-two percent of limbs were initially treated non-operatively, and 4.3% died before conservative therapy could be initiated. In total, following the initial revascularization treatment, six limbs (22%) required further interventions for ongoing CLTI, including surgical reconstructions and secondary amputations. With a 3-year limb salvage rate of 73%, patient survival rate of 41%, and amputation-free survival rate of 31%, Engelhardt et al. concluded that two-thirds of all patients presenting with a first episode of CLTI should be considered for some form of direct revascularization. However, although the study suggests that many patients ill-qualified for bypass procedures may be instead offered endovascular options for revascularization, to date, no reliable formula to successfully identify such patient-selection factors exists.
Like the BASIL Trial, this study illustrates the importance of patient selection, especially in regards to cardiac risk and burden of disease. Although there were few significant differences in many comorbidities that constitute cardiac risk, dialysis dependence – an important cardiac risk factor that has been shown to increase MI and death after revascularization – was shown to be higher among the PTA/S-first patients, illustrating that there may be additional increases in cardiac risk within the PTA/S patients that is not presently captured within this analysis.26–28 Additionally, although uncategorized within this patient population, poor surgical candidates – shown to have higher rates of 30-day mortality, loss of patency, limb loss, and long-term mortality – are another important subset of patients that highlight one of the many challenges involved in treating CLTI.29 Although prior studies have seen an increase in 30-day and perioperative mortality among bypass patients, we saw similar rates between the two groups, truly emphasizing the burden of disease that PTA/S patients have.
Importantly, our study illustrates the increased risk for major amputation, RAS events, and re-intervention among limbs with femoropopliteal TASC C and TASC D lesions; however we believe that there are several additionally important factors that should play a role in selection of revascularization strategy. For example, as discussed in our previous work on the WIfI classification system, the extent of the foot disease and the severity of the wound play critical roles in understanding a patient’s risk of undergoing a future event.30 In addition, medical condition of patients and availability of adequate conduit are necessary factors when deciding on bypass versus PTA/S: In our practice, a relatively healthy patient with a good conduit, extensive tissue loss, and severe occlusive disease would typically dictate a bypass; however, our patient enrollment in the BEST Trial will hopefully better elucidate answers that this retrospective analysis cannot. Ultimately, based on our study and others such as the BASIL Trial, sites not enrolling in the BEST trial might strongly consider bypass in limbs with TASC C and D lesions. Notably, however, we now use drug-coated balloons and drug-eluting stents in the femoropopliteal vessels, which we expect to improve PTA/S outcomes, even among the more demanding TASC C and TASC D lesions.
There are important limitations to this study. First, it was a retrospective, single-center review where patients were allocated to treatment based on surgeon preference, which has changed over time. Second, as a retrospective study, there are many patient details – such as vein mapping information within the PTA/s-first patients – that are unreliably documented throughout the study period and may theoretically limit the conclusions that can be drawn. Prior to 2008, our institution routinely recorded PVRs and ABIs; however, PVRs are not quantitative measures and, as three-quarters of the patients within our study have diabetes, there is a predisposition for a number of limbs to have calcified tibial vessels and, therefore, have unreliable ABI information. After 2008, most surgeons within our institution began using toe pressures routinely; unfortunately, it is difficult to quantitatively compare patients in this regard when forefoot PVR, ABI, and toe pressure information is unobtainable in the high number of patients with toe lesions or missing toes. Ultimately, the various difficulties of recording valuable and dependable duplex surveillance on all patients throughout the study period caused a comparative shortcoming that is not uncommon in previous studies; hopefully future studies can provide more thorough information in this regard. Furthermore, our dataset does not contain cost-related data, and, therefore, does not help resolve the unmet need to better understand how the high vascular readmission and revascularization rates for PAD fit into the discussion of cost-effectiveness and cost saving. Finally, these data only include revascularization attempts and do not reflect outcomes for those patients treated with primary amputation or medical management as a contrast. Regardless, this study remains one of the largest reported analyses of initial treatment of CLTI comparing surgical bypass and PTA/S.
CONCLUSION
These data support the conclusions of the BASIL trial and suggest that, based on the reduced mortality, improved wound healing, and fewer future interventions, for appropriately selected patients, bypass may be preferred over the mid and long-term. Therefore, in relatively fit patients expected to live more than two years, the apparent improved durability and reduced re-intervention rate of open surgical bypass could outweigh the short-term considerations of increased morbidity, especially in those with an available and suitable single segment great saphenous vein conduit. Conversely, regardless of the high failure and re-intervention rate associated with PTA/S, patients who are expected to live for fewer than one-to-two years and have significant comorbidities may be better suited, when possible, for a PTA/S-first approach. As such, irrespective of the methodological differences between our study and the BASIL trial, the results of the latter may be more generalizable to the larger populations than initially expected. Ultimately, we hope that future studies focus on the comparison between first-time interventions for patients who are medically fit for bypass and have adequate single segment great saphenous vein, as we believe that further subgroup evaluation may better elucidate which procedure type best suits particular patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 42rd annual symposium of the Society for Clinical Vascular Surgery, Carlsbad, California, March 19, 2014 (Plenary)
References
- 1.Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg. 2005;42(3):449–55. doi: 10.1016/j.jvs.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Wolfle KD, Bruijnen H, Loeprecht H, Rumenapf G, Schweiger H, Grabitz K, et al. Graft patency and clinical outcome of femorodistal arterial reconstruction in diabetic and non-diabetic patients: results of a multicentre comparative analysis. Eur J Vasc Endovasc Surg. 2003;25(3):229–34. doi: 10.1053/ejvs.2002.1849. [DOI] [PubMed] [Google Scholar]
- 3.van der Zaag ES, Legemate DA, Prins MH, Reekers JA, Jacobs MJ. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. Eur J Vasc Endovasc Surg. 2004;28(2):132–7. doi: 10.1016/j.ejvs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Hobbs SD, Yapanis M, Burns PJ, Wilmink AB, Bradbury AW, Adam DJ. Peri-operative myocardial injury in patients undergoing surgery for critical limb ischaemia. Eur J Vasc Endovasc Surg. 2005;29(3):301–4. doi: 10.1016/j.ejvs.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Papavassiliou VG, Walker SR, Bolia A, Fishwick G, London N. Techniques for the endovascular management of complications following lower limb percutaneous transluminal angioplasty. Eur J Vasc Endovasc Surg. 2003;25(2):125–30. doi: 10.1053/ejvs.2002.1822. [DOI] [PubMed] [Google Scholar]
- 6.Lipsitz EC, Ohki T, Veith FJ, Rhee SJ, Kurvers H, Timaran C, et al. Fate of collateral vessels following subintimal angioplasty. J Endovasc Ther. 2004;11(3):269–73. doi: 10.1583/03-1149.1. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitz EC, Veith FJ, Ohki T. The value of subintimal angioplasty in the management of critical lower extremity ischemia: failure is not always associated with a rethreatened limb. J Cardiovasc Surg (Torino) 2004;45(3):231–7. [PubMed] [Google Scholar]
- 8.Joels CS, York JW, Kalbaugh CA, Cull DL, Langan EM, 3rd, Taylor SM. Surgical implications of early failed endovascular intervention of the superficial femoral artery. J Vasc Surg. 2008;47(3):562–5. doi: 10.1016/j.jvs.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Darling JD, McCallum JC, Curran T, Buck DB, Guzman R, Wyers M, et al. Consequences of Failed Tibial Endovascular Intervention. Journal of Vascular Surgery. 2014;59(6):102S–3S. [Google Scholar]
- 10.Salas CA, Adam DJ, Papavassiliou VG, London NJ. Percutaneous transluminal angioplasty for critical limb ischaemia in octogenarians and nonagenarians. Eur J Vasc Endovasc Surg. 2004;28(2):142–5. doi: 10.1016/j.ejvs.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SE, Wolf GL, Cross AP. Percutaneous transluminal angioplasty versus operation for peripheral arteriosclerosis. Report of a prospective randomized trial in a selected group of patients. J Vasc Surg. 1989;9(1):1–9. [PubMed] [Google Scholar]
- 12.Holm J, Arfvidsson B, Jivegard L, Lundgren F, Lundholm K, Schersten T, et al. Chronic lower limb ischaemia. A prospective randomised controlled study comparing the 1-year results of vascular surgery and percutaneous transluminal angioplasty (PTA) Eur J Vasc Surg. 1991;5(5):517–22. doi: 10.1016/s0950-821x(05)80338-x. [DOI] [PubMed] [Google Scholar]
- 13.Saketkhoo RR, Razavi MK, Padidar A, Kee ST, Sze DY, Dake MD. Percutaneous bypass: subintimal recanalization of peripheral occlusive disease with IVUS guided luminal reentry. Tech Vasc Interv Radiol. 2004;7(1):23–7. doi: 10.1053/j.tvir.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Desgranges P, Boufi M, Lapeyre M, Tarquini G, van Laere O, Losy F, et al. Subintimal angioplasty: feasible and durable. Eur J Vasc Endovasc Surg. 2004;28(2):138–41. doi: 10.1016/j.ejvs.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Clair DG, Dayal R, Faries PL, Bernheim J, Nowygrod R, Lantis JC, 2nd, et al. Tibial angioplasty as an alternative strategy in patients with limb-threatening ischemia. Ann Vasc Surg. 2005;19(1):63–8. doi: 10.1007/s10016-004-0136-0. [DOI] [PubMed] [Google Scholar]
- 16.Atar E, Siegel Y, Avrahami R, Bartal G, Bachar GN, Belenky A. Balloon angioplasty of popliteal and crural arteries in elderly with critical chronic limb ischemia. Eur J Radiol. 2005;53(2):287–92. doi: 10.1016/j.ejrad.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Surowiec SM, Davies MG, Eberly SW, Rhodes JM, Illig KA, Shortell CK, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41(2):269–78. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Trocciola SM, Chaer R, Dayal R, Lin SC, Kumar N, Rhee J, et al. Comparison of results in endovascular interventions for infrainguinal lesions: claudication versus critical limb ischemia. Am Surg. 2005;71(6):474–9. doi: 10.1177/000313480507100605. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 19.Tefera G, Hoch J, Turnipseed WD. Limb-salvage angioplasty in vascular surgery practice. J Vasc Surg. 2005;41(6):988–93. doi: 10.1016/j.jvs.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Treiman GS. Subintimal angioplasty for infrainguinal occlusive disease. Surg Clin North Am. 2004;84(5):1365–80. viii. doi: 10.1016/j.suc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–s296. [PubMed] [Google Scholar]
- 23.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Jones DW, Schanzer A, Zhao Y, MacKenzie TA, Nolan BW, Conte MS, et al. Growing impact of restenosis on the surgical treatment of peripheral arterial disease. J Am Heart Assoc. 2013;2(6):e000345. doi: 10.1161/JAHA.113.000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelhardt M, Boos J, Bruijnen H, Wohlgemuth W, Willy C, Tannheimer M, et al. Critical limb ischaemia: initial treatment and predictors of amputation-free survival. Eur J Vasc Endovasc Surg. 2012;43(1):55–61. doi: 10.1016/j.ejvs.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.O’Hare AM, Sidawy AN, Feinglass J, Merine KM, Daley J, Khuri S, et al. Influence of renal insufficiency on limb loss and mortality after initial lower extremity surgical revascularization. J Vasc Surg. 2004;39(4):709–16. doi: 10.1016/j.jvs.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Parvataneni KC, Piyaskulkaew C, Szpunar S, Sharma T, Patel V, Patel S, et al. Relation of Baseline Renal Dysfunction With Outcomes in Patients Undergoing Popliteal and Infrapopliteal Percutaneous Peripheral Arterial Interventions. Am J Cardiol. 2016;118(2):298–302. doi: 10.1016/j.amjcard.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Namini H, Seely L. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg. 2005;42(3):456–64. doi: 10.1016/j.jvs.2005.05.001. discussion 64–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo RC, Darling J, Bensley RP, Giles KA, Dahlberg SE, Hamdan AD, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg. 2013;57(6):1455–63. doi: 10.1016/j.jvs.2012.10.109. discussion 63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darling JD, McCallum JC, Soden PA, Meng Y, Wyers MC, Hamdan AD, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg. 2016;64(3):616–22. doi: 10.1016/j.jvs.2016.03.417. [DOI] [PMC free article] [PubMed] [Google Scholar]


