Abstract
Objective
Hot flashes are experienced by most midlife women. Emerging data indicate that they may be associated with endothelial dysfunction. No studies have tested whether hot flashes are associated with endothelial function using physiologic measures of hot flashes. We tested whether physiologically-assessed hot flashes were associated with poorer endothelial function. We also considered whether age modified associations.
Methods
272 nonsmoking women reporting either daily hot flashes or no hot flashes, aged 40-60, and free of clinical cardiovascular disease underwent ambulatory physiologic hot flash and diary hot flash monitoring; a blood draw; and ultrasound measurement of brachial artery flow mediated dilation to assess endothelial function. Associations between hot flashes and flow mediated dilation were tested in linear regression models controlling for lumen diameter, demographics, cardiovascular disease risk factors, and estradiol.
Results
In multivariable models incorporating cardiovascular disease risk factors, significant interactions by age (p<.05) indicated that among the younger tertile of women in the sample (ages 40-53), the presence of hot flashes [beta(standard error)=-2.07 (.79), p=.01], and more frequent physiologic hot flashes were associated with lower flow mediated dilation [for each hot flash: beta(standard error)=-.10(.05), p=.03, multivariable]. Associations were not accounted for by estradiol. Associations were not observed among the older women (ages 54-60) or for prospective-reported hot flash frequency, severity, or bother. Among the younger women, hot flashes explained more variance in flow mediated dilation than standard cardiovascular disease risk factors or estradiol.
Conclusions
Among younger midlife women, frequent hot flashes were associated with poorer endothelial function and may provide information about women's vascular status beyond cardiovascular disease risk factors and estradiol.
Keywords: endothelial function, hot flashes, vasomotor symptoms, menopause
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women.1 As women typically manifest with clinical CVD postmenopausally,2 there has been a longstanding interest in the role of the menopause transition and its correlates in the development of CVD in women. The focus of this work has largely been on the hormonal changes of menopause, including declining levels of the ovarian estrogen estradiol (E2). However, recent data has considered other menopause-related factors in the CVD risk among midlife women.
Hot flashes are the hallmark symptom of the menopause transition. They are reported by 70% of women,3 and for a third of women, they are frequent or severe.3, 4 Newer data indicate that hot flashes often start earlier than previously thought, during the late reproductive years5 and persist for a decade or more.6, 7 While hot flashes are well-established to impact a woman's quality of life, emerging work also links hot flashes to indicators of CVD risk.
The vascular endothelium, the single cell layer lining the vessel, is critical to multiple aspects of vascular health and function, and endothelial injury and dysfunction is an initiating event in atherosclerosis.8-10 The endothelium has long been shown to be sensitive to reproductive factors in women, including reproductive hormones such as E2,11 as well as to many standard CVD risk factors.12, 13 Newer data have begun to link the menopausal symptom of hot flashes to markers of poorer endothelial function.9, 10, 14
However, the existing literature on hot flashes and endothelial function has key limitations. Most existing work has employed brief, retrospective assessments subject to many biases,15, 16 including memory biases and other psychological factors impacting symptom reporting, which themselves are associated with poorer endothelial function.17 More advanced physiologic measures of hot flashes18 and prospective assessment self-report measures of hot flashes completed at the time of the hot flash (rather than recalled at the end of the day)19 do exist and should be employed to address these questions. Further, the endothelium is well-known to be sensitive to E2, yet the E2 assays employed in existing work have limited sensitivity at the low levels observed among menopausal women.20 Finally, there has been little consideration of factors that may modify hot flash-endothelial associations. The timing of hot flashes can vary dramatically across women, with some indication that early-occurring hot flashes may be those most relevant to CVD risk.14, 21 Similar to relations between a range of female reproductive factors (e.g., hormone therapy, hot flashes) and markers of CVD risk in women,21, 22 any modifying role of chronologic or ovarian aging in these associations requires careful consideration.
We tested the associations between rigorously and prospectively-assessed hot flashes and endothelial function as assessed by brachial artery flow mediated dilation (FMD), a well-validated and widely-used index of endothelial function (lower FMD indicating poorer function).23-26 We considered the role of CVD risk factors and endogenous E2 concentrations measured via state-of-the-art methods20 in these associations. Finally, we considered a priori how age or menopause stage may modify hot flash-FMD associations.
Methods
We recruited 304 late perimenopausal (2-12 months amenorrhea) and postmenopausal (≥12 months amenorrhea)27 nonsmoking women aged 40-60 from the community via advertisements, mailings, and online message boards. By design, half of the women reported daily hot flashes or night sweats (“flashers”), and half reported no hot flashes or night sweats in the past three months (“non-flashers”). Exclusion criteria included hysterectomy and/or bilateral oophorectomy; history of heart disease, stroke, arrhythmia, gynecological cancer, pheochromocytoma, pancreatic tumor, kidney failure, seizures, Parkinson's disease, Raynaud's Phenomenon; current pregnancy; or having used the following medications in the past 3 months: oral/transdermal estrogen or progesterone (hormone therapy, hormonal contraceptives, hormone cream/patch/ring, hormone-eluting intra uterine device), selective estrogen receptor modulators, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, gabapentin, insulin, beta blockers, calcium channel blockers, alpha-2 adrenergic agonists, or other antiarrhythmic agents. Also excluded were women who had undergone endometrial ablation (preventing menopause staging), endarterectomy, or lymph node removal or who were undergoing chemotherapy, hemodialysis, or peritoneal dialysis. Of the 304 women, 32 women were excluded due to missing FMD data. An additional four women were excluded from expanded models due to missing data for homeostatic model assessment (HOMA, N=2) and low density lipoprotein cholesterol (LDL-C, N=2). Excluded women did not differ on any study variables than included women. Thus, 272 women were included in primary models, and 268 women in expanded models.
Participants underwent physical measurements, ambulatory hot flash monitoring, a blood draw, and a carotid artery ultrasound. All study procedures were reviewed and approved by the University of Pittsburgh Institutional Review Board, and all participants provided written, informed consent. Height and weight were measured via a fixed stadiometer and a calibrated balance beam scale and body mass index calculated (kg/m2). Seated blood pressure (BP) was measured via a Dinamap device after 10-min rest. Medical and reproductive history was assessed by standard instruments. Menopause status was obtained from reported menstrual bleeding patterns.27 Parity was classified by the total reported number of live births. Use of medications [e.g., antihypertensives, lipid-lowering medications, diabetes, inhaled beta agonists] were reported and considered as covariates. Depressive symptoms were assessed by the Center for Epidemiologic Studies Depression scale,28 anxiety via the State-Trait Anxiety Inventory,29 and sleep quality via the Pittsburgh Sleep Quality Index.30 Leisure-time physical activity was assessed via the International Physical Activity Questionnaire.31
All women completed ambulatory hot flash monitoring with an electronic hot flash diary (three days), wrist actigraph (three days), and a physiologic hot flash monitor (24 hours). The physiologic hot flash monitor, the VU-AMS (VU University Amsterdam, the Netherlands),32, 33 is a portable ambulatory monitor that quantifies hot flashes via sternal skin conductance, a validated measure of hot flashes.18, 34 An electronic hot flash diary (Palm Z22) was completed at the time of a waking subjectively-experienced hot flash, where women reported the occurrence, severity, and bother of each waking hot flash.19 Women also pressed event mark buttons on the VU-AMS monitor and actigraph when experiencing a hot flash, providing date and time-stamped hot flash reports. Participants wore the VU-AMS monitor for 24 hours, after which time they removed it and stored it in a provided case. For the remaining two days, participants reported their hot flashes via diary (self-reported occurrence, severity, bother) and actigraph (self-reported occurrence). After monitoring, hot flash data were downloaded, reviewed, and scored via UFI software (DPSv3.7; Morro Bay, CA) according to standard, validated methods18, 34, 35 that have established reliability in the present laboratory (ĸ =.86).36 The frequency of physiologic or self-reported hot flashes was calculated as the number of hot flashes divided by monitoring time; women not showing or reporting hot flashes, respectively, were assigned a zero value. Average severity and bother over the monitoring period was calculated over diary entries. In addition to reporting on their hot flashes as they occurred, on the first day of the protocol women completed a questionnaire similar to that used in epidemiologic investigations3, 16 whereby they were asked to report on the frequency (number of days with hot flashes and number of hot flashes per day), severity (1=not at all to 4=severe), and bother (1=not at all to 4=a lot) associated with hot flashes as recalled over the prior two weeks.
After an overnight fast, FMD was measured after 10 minutes of supine rest by high resolution B-mode ultrasound imaging of the right brachial artery, 2-10 cm proximal to the antecubital crease, by trained sonographers using a standardized protocol. Images were obtained at rest (baseline) and after 5 minutes of forearm blood flow occlusion (postdeflation) with a pneumatic tourniquet set to 50 mm Hg above the participant's systolic blood pressure. For baseline diameters, digitized images were captured on the R wave for 20 seconds. Immediately after deflation, images were captured on the R wave for three minutes. The arterial diameter was measured as the distance between the anterior and posterior arterial wall media-adventitia interfaces using edge-detection software. All images for this study were read by a single trained reader using the Brachial Analysis System (MIA, University of Iowa) software.37 The reading software allows continuous tracking of the brachial artery diameter across these images so that the peak change in diameter can be accurately determined. FMD was calculated as the maximum percentage of change in arterial diameter, relative to baseline. This methodology at this laboratory has shown reproducibility (ICC's=0.70-0.72).17
Phlebotomy was performed after a 12-hr overnight fast. Estradiol was assessed via liquid chromatography-tandem mass spectrometry, the gold standard method to measure estradiol at the low levels of the postmenopause (lower limit of quantitation=2.5pg/mL; lower limit of detection=1.0 pg/ml).38 Glucose, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured enzymatically (Vital Diagnostics, Lincoln, RI). Total cholesterol was determined enzymatically and LDL-C calculated using the Friedewald formula.39 Insulin was measured via radioimmunoassay. HOMA, reflecting insulin resistance, was calculated.40 C-reactive protein was measured using a high sensitivity reagent set (Beckman Coulter, Brea, CA) and interleukin-6 with an R&D Systems (Minneapolis, MN) via high sensitivity ELISA.
For data analyses, estradiol, HOMA, triglycerides, C-reactive protein, and interleukin-6 values were natural log transformed and leisure time physical activity square root transformed for analysis. Hot flash rates were calculated as the number of hot flashes/monitoring time. Differences between participants by included/excluded status were tested using linear regression, Wilcoxon rank sum, and chi-square tests. Associations between hot flash status or hot flash frequency and brachial artery measures (FMD, lumen diameter) were tested in linear regression models. Covariates were entered as factors associated with FMD in the present study at p<.20: baseline lumen diameter, age, race/ethnicity, leisure time physical activity, state anxiety, parity, beta agonist medications (Model 1), and next addition of CVD and demographic risk factors of body mass index, systolic BP, triglycerides, LDL-C, HOMA, education, and BP-lowering, lipid-lowering, and diabetes medications (Model 2). Estradiol was added to Model 2 in a separate step. All variables were treated as continuous variables with the exception of race/ethnicity (white/nonwhite), education (< or ≥ college), and medication use (yes/no). To avoid issues of collinearity, BP and lipid variables with the strongest associations with the outcome were selected for inclusion in Model 2. Interactions were tested by cross product terms in multivariable models, with age and time since the last reported menstrual period considered as continuous variables. Significant interactions were probed in stratified models, with age tertiled according to the sample distribution for illustration. R2 values were derived from linear regression models. Secondary models included additional covariates added to Model 2 (sleep quality, depressive symptoms, interleukin-6, C-reactive protein). Residual analysis and diagnostic plots were conducted to verify model assumptions. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). Models were 2-sided at α=0.05.
Results
Participants were on average 54 years old, white, college educated, and postmenopausal (Table 1). Although being on average overweight, the average CVD risk factor profile was relatively favorable. Participants showed an average of 6 physiologic hot flashes/24 hours on physiologic monitoring and reported an average of 2 hot flashes (waking hours). Physiologically-monitored and self-reported hot flash frequency during wake were moderately to highly correlated (ρ=.69, p<.0001), consistent with other work.41
Table 1. Sample characteristics (N=272).
| Total Sample | Age ≤ 53 | Age 53.1-56 | Age >56 | |
|---|---|---|---|---|
| Age, years, M (SD)* | 54.1 (3.9) | 50.2 (2.8) | 55.1 (0.8) | 58.4 (1.0) |
| Race/ethnicity, N (%) | ||||
| White | 198 (72.8) | 73 (67.0) | 63 (74.1) | 62 (79.5) |
| African american | 59 (21.7) | 30 (27.5) | 18 (21.2) | 11 (14.1) |
| Other (Asian, Hispanic, biracial) | 15 (5.5) | 6 (5.5) | 4 (4.7) | 5 (6.4) |
| Education, N (%) | ||||
| High school, some college, vocational | 114 (41.9) | 47 (43.1) | 38 (44.7) | 29 (37.2) |
| College graduate | 79 (29.0) | 33 (30.3) | 23 (27.1) | 23 (29.5) |
| Postgraduate | 79 (29.0) | 29 (26.6) | 24 (28.2) | 26 (33.3) |
| Parity, median (iqr) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (0.0, 2.0) | 2.0 (1.0, 3.0) |
| State anxiety, M (SD)a | 32.2 (9.9) | 33.5 (10.3) | 31.8 (9.6) | 30.8 (9.4) |
| Depressive symptoms, Median (IQR)a | 5.5 (2.0, 11.0) | 6.0 (3.0, 11.0) | 6.0 (2.0, 12.0) | 4.5 (1.0, 10.0) |
| Leisure time physical activity, Median (IQR)a | 396.0 (0, 1386.0) | 330.0 (0.0, 1470.0) | 495.0 (66.0, 1097.0) | 346.5 (0.0, 1050.0) |
| Body mass index, M (SD) | 29.0 (6.8) | 29.1 (6.7) | 28.9 (8.0) | 29.0 (5.6) |
| Systolic blood pressure, mmhg, M (SD)* | 119.5 (14.4) | 117.0 (13.2) | 118.4 (12.0) | 124.1 (17.1) |
| Diastolic blood pressure, mmhg, M (SD) | 69.9 (9.0) | 70.8 (9.6) | 69.2 (8.2) | 69.5 (9.2) |
| Menopause stage N (%)* | ||||
| Perimenopausal | 44 (16.2) | 36 (33.0) | 7 (8.2) | 1 (1.3) |
| Postmenopausal | 228 (83.8) | 73 (67.0) | 78 (91.8) | 77 (98.7) |
| High density lipoprotein cholesterol, mg/dl, M (SD) | 62.7 (14.7) | 60.7 (15.1) | 64.4 (15.5) | 63.8 (13.1) |
| Low density lipoprotein cholesterol, mg/dl, M (SD) | 131.0 (33.8) | 126.0 (30.7) | 134.7 (31.7) | 134.1 (39.2) |
| Triglycerides, mg/dl, Median (IQR) | 95.5 (71.5, 130.0) | 94.0 (74.0, 128.0) | 96.0 (74.0, 126.0) | 100.5 (70.0, 143.0) |
| Homeostatic model assessment, Median (IQR) | 2.7 (1.7, 3.2) | 2.1 (1.6, 2.9) | 2.1 (1.6, 3.4) | 2.4 (1.8, 3.1) |
| Estradiol, pg/ml, Median (IQR)* | 5.0 (2.0, 10.1) | 5.4 (2.9, 16.0) | 4.8 (2.0, 8.0) | 4.0 (2.0, 8.0) |
| Medications, N (%) | ||||
| Lipid-lowering* | 36 (13.2) | 7 (6.4) | 12 (14.1) | 17 (21.8) |
| Blood pressure-lowering | 45 (16.5) | 16 (14.7) | 13 (15.3) | 16 (20.5) |
| Diabetes medication | 10 (3.7) | 3 (2.8) | 2 (2.4) | 5 (6.4) |
| Beta-agonists | 14 (5.2) | 7 (6.4) | 3 (3.5) | 4 (5.1) |
| Hot flash status, N (%)* | ||||
| Yes | 136 (50) | 41 (37.6) | 40 (47.1) | 55 (70.5) |
| No | 136 (50) | 68 (62.4) | 45 (52.9) | 23 (29.5) |
| Physiologically detected hot flashes, 24 hour, M (SD)* | 9 (9) | 9 (9) | 10 (9) | 7 (9) |
| Self-reported hot flashes, waking, M (SD)* | 2 (3) | 3 (3) | 2 (3) | 1 (2) |
| Flow mediated dilation, %, M (SD) | 7.4 (4.3) | 7.6 (3.9) | 7.4 (4.3) | 7.1 (4.0) |
| Baseline lumen diameter, mm, M (SD) | 3.5 (0.5) | 3.5 (0.5) | 3.4 (0.5) | 3.5 (0.5) |
State anxiety assessed by Spielberger State-Trait Anxiety Inventory, sample range: 20-63; depressive symptoms assessed by Center for Epidemiologic Studies Scale, sample range: 0-51; leisure time physical activity assessed by International Physical Activity Questionnaire, sample range: 0-6399
Differs by age status p<.05
FMD did not differ between “flashers” [M(SE)=7.37 (4.18)] and “nonflashers” [M(SD)=7.60 (3.91), p=.32, Model 2 covariates]. Further, neither the frequency of physiologic hot flashes [B(SE)=-.01 (.03), p=.70] nor self-reported hot flashes [b(SE)=-.13(.08), p=.09, Model 2 covariates] were significantly related to FMD.
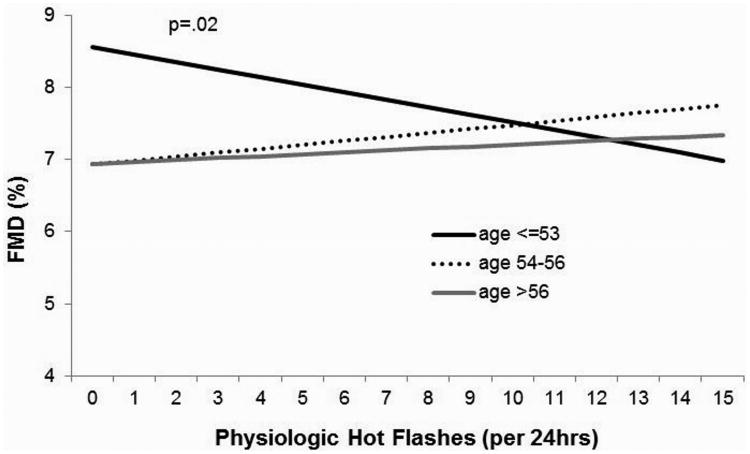
However, age significantly modified the association between hot flash status and FMD (p value interaction: p=.02, Model 1 covariates; p=.03, Model 2 covariates), such that among the younger tertile of women (age 40-53), “flashers” had significantly lower FMD than “nonflashers” (Table 2). Age also moderated the association between physiologically-monitored hot flash frequency and FMD (p value interaction: p=.03, Model 1 covariates; p=.035, Model 2 covariates), such that a greater frequency of physiologic hot flashes were associated with poorer FMD among the younger women (Table 2, Figure 1). CVD risk factors and other Model 2 covariates did not account for these associations. In contrast, we did not observe significant age interactions for self-reported hot flash frequency in relation to FMD. Notably, among the younger tertile of women, hot flash status accounted for more variance in FMD than all other covariates with the exception of baseline lumen diameter (Table 3).
Table 2. Relation between hot flashes and FMD by age tertile.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Age ≤53 | Age 54-56 | Age ≥57 | Age ≤53 | Age 54-56 | Age ≥57 | |
|
| ||||||
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Hot flash status (yes/no) | -1.90 (.72)b | 1.06 (.89) | -.38 (.94) | -2.15 (.79)b | .67 (.96) | -.44 (1.04) |
| Frequency of physiologic hot flashes | -.08 (.04)a | .07 (.05) | .02 (.05) | -.10 (.05)a | .05 (.06) | .03 (.05) |
Model 1: Age, baseline lumen diameter, race/ethnicity, state anxiety, parity, physical activity, beta agonist medication
Model 2: Model 1 plus education, SBP, BMI, HOMA, LDL, triglycerides, lipid lowering medication, blood pressure lowering medication, diabetes medication
All variables were treated as continuous variables with the exception of race/ethnicity (white or nonwhite), education (< or ≥ college), and medication use (yes/no for each medication)
BMI, body mass index; FMD, flow mediated dilation; HOMA, homeostatic model assessment; LDL, low-density lipoprotein; SBP, systolic blood pressure
p<.05,
p<.01
Figure 1. FMD and physiologic hot flashes by age.
Covariates: age, race, baseline diameter, physical activity, parity, state anxiety, body mass index, systolic BP, education, HOMA, LDL, triglycerides, medications (beta agonist, lipid-lowering, blood pressure-lowering, diabetes medications)
All variables were treated as continuous variables with the exception of race/ethnicity (white or nonwhite), education (< or ≥ college), and medication use (yes/no for each medication)
CVD, cardiovascular disease; FMD, flow mediated dilation; HOMA, homeostatic model assessment; LDL, low-density lipoprotein; BP, blood pressure
Table 3. Percent of variance (R2) in FMD explained by each variable in multivariable models among women aged ≤ 53.
| Variable | R2 |
|---|---|
| Age | 1.2 |
| Baseline lumen diameter | 6.4 |
| Race | 1.9 |
| Education | .30 |
| Body mass index | .20 |
| Systolic blood pressure | .01 |
| Low-density lipoprotein cholesterol | .30 |
| Triglycerides | 1.6 |
| Homeostatic model assessment | .10 |
| State anxiety | 4.2 |
| Parity | .10 |
| Physical activity | 2.6 |
| Medications | |
| Lipid-lowering | 2.4 |
| Blood pressure-lowering | .20 |
| Diabetes | .20 |
| Beta agonist | 4.7 |
| Hot flash status (yes/no) | 5.6 |
FMD, flow mediated dilation
All variables were treated as continuous variables with the exception of race/ethnicity (white or nonwhite), education (< or ≥ college), and medication use (yes/no for each medication)
We next considered the role of estradiol. Estradiol was not significantly related to FMD [b(SE)=.01(.28), p=.96, multivariable]. Estradiol did not reduce the magnitude or significance of hot flash-age interactions with FMD nor did it reduce the association between hot flashes and FMD among the youngest tertile of women in multivariable models (data not shown).
We conducted several additional analyses. First, we considered the variance accounted for by physiologic hot flash frequency instead of hot flash status among the younger women in multivariable models; physiologic hot flash frequency accounted for 4.1% of the variance, greater than that of all standard CVD risk factors (see Supplemental Table 1). Second, we considered interactions between hot flashes and menopause stage, race/ethnicity (white/nonwhite), or time since last menstrual period in relation to FMD. There were no significant interactions for race/ethnicity or the time since the last menstrual period. However, there was some evidence of an interaction between physiologic hot flash frequency and menopause stage (interaction p value: p=.048; Model 1 covariates; p=.06; Model 2 covariates), such that associations between physiologic hot flashes and FMD were strongest among the perimenopausal women [b(SE)=-.16(.06), p=.02], controlling for age and other Model 1 covariates. Findings in perimenopausal women persisted with adjustment for Model 2 covariates [b(SE)=-.18(.08), p=.03]. Third, we considered physiologically-detected hot flashes during sleep versus wake among the younger women. The most pronounced associations between hot flashes and FMD were observed for waking [b(SE)=-.12(.05), p=.01] rather than sleep hot flashes [b(SE)=-.09(.14), p=.51; Model 1 covariates]. Fourth, neither diary-reported severity nor bother associated with hot flashes was associated with FMD nor did they show interactions by age in relation to FMD. We also examined questionnaire-reported hot flashes (frequency, severity, bother, past two weeks) in relation to FMD. None of these indices were significantly related to FMD for the sample as a whole. However, there was some indication of an interaction between hot flash bother and age in relation to FMD (p=.058; Model 2 covariates); in age stratified analyses, greater hot flash-related bother was associated with lower FMD among the younger tertile [age ≤53 b(SE)=-.90 (.41), p=.03; Model 2 covariates] but not the older tertiles of women [age 54-56 b(SE)=-.06, p=.90; age>56 b(SE)=-.56(.64), p=.38; Model 2 covariates]. Finally, we considered additional covariates of inflammatory markers, depressive symptoms, and sleep quality, but these markers did not explain any of the observed associations (data not shown).
Discussion
This study was the first to test relations between physiologically-assessed hot flashes and endothelial function as assessed by brachial artery FMD. We found that both the reported presence of hot flashes as well as more frequent physiologically-assessed hot flashes were associated with lower FMD among younger midlife women. Associations between hot flashes and FMD were not observed among the older women in the sample. Observed associations were not explained by a range of potentially confounding or explanatory factors, including standard CVD risk factors or endogenous estradiol. Thus, for younger midlife women, hot flashes appear to be accompanied by poorer endothelial function.
The endothelium is the single cell layer lining the vessel which is critical to multiple aspects of vascular health.8 Reproductive hormonal factors such as estradiol have long been shown to be associated with endothelial function.11 The present study shows that menopausal hot flashes are associated with poorer endothelial function independent of estradiol among younger women only. With its rigorous quantification of hot flashes, CVD risk factors, and estradiol, this study is the most definitive on the topic to date. Notably, impairment in endothelial function is an initiating event in the atherosclerotic process,42 and thereby frequent hot flashes may mark emerging vascular dysfunction among early midlife women.
Hot flash-FMD associations showed pronounced effect modification by age, and to a lesser extent menopause stage. Effects primarily observed among the younger and perimenopausal women, and not among the older women in the sample. Notably, hot flashes were long thought to begin in the few years prior to the final menstrual period, yet newer data indicate that a substantial portion of women have hot flashes very early in midlife.5 These hot flashes may mark poorer vascular health, as in the present study, the FMD of the youngest women with hot flashes was similar to the FMD of women in the oldest tertile. Large prospective cohort studies (e.g., Study of Women's Health Across the Nation) similarly indicate that it is the women who have hot flashes early in the transition who have the highest subclinical atherosclerosis even when controlling for CVD risk factors.21 In the Women's Ischemia Syndrome Evaluation study, women who recalled earlier onset of their hot flashes had lower FMD.14 The reason for these patterns of findings is not immediately apparent, yet it is possible that effects for hot flashes are observed in younger women, while aging effects on endothelial function predominate in older women. Notably, there is some evidence that the predictive value of FMD for CVD is stronger in younger populations.23 Thus, different CVD markers may show different temporal relations to hot flashes, progressing from endothelial dysfunction to atherosclerosis to clinical CVD events. Further supporting this interpretation is findings indicating that hot flashes may be linked to clinical CVD events primarily in older women.43 Notably, effect modification by age is a frequent observation for relations between reproductive hormonal factors (e.g., hormone therapy) and CVD risk in women.22, 44, 45
The mechanisms that may link hot flashes to poorer endothelial function require elucidation. Hot flashes are conceptualized as thermoregulatory events occurring in the context of the altered thermoregulation observed during the menopause transition, possibly secondary to endogenous estradiol withdrawal. A large literature shows that exogenous estrogen administration improves endothelial function,11 particularly for younger midlife women.45 Endogenous estradiol, measured via state-of-the-art liquid chromatography-tandem mass spectrometry methods, was not related to FMD here, suggesting a potential differing vascular physiology of endogenous estradiol versus exogenous estrogen administration. Further, traditional or novel CVD risk factors linked to hot flashes46-48 did not explain observed associations. Notably, hot flashes may be associated with greater reactivity of the microvasculature, a response often mediated by the sympathetic nervous system.49 Emerging work also links the hypothalamic pituitary adrenal axis to hot flashes,50 which is also associated with poorer vascular health.51 These pathways should be considered in future work.
Several additional findings deserve mention. Somewhat stronger associations were observed for waking versus sleep hot flashes. The reason for this pattern of findings is not immediately apparent. However, this finding is broadly consistent with our prior work,9, 52, 53 potentially suggesting differing underlying physiologies or correlates of these two events. Prospectively reported frequency, severity, and bother associated with hot flashes were not associated with FMD. However, there was some indication that the general bother associated with hot flashes, as recalled over the prior two weeks, was associated with poorer endothelial function among the younger women in the sample, paralleling findings for physiologic hot flash frequency. Memory and reporting effects associated with recalled hot flashes are well established,15 particularly for self-reported bother,16 and should be kept in mind when interpreting these findings. However, it is possible that the overall perceived bother associated with hot flashes captures an aspect of the frequent physiologic hot flashes not captured by prospective self-report.
There are several limitations to this work. The majority of women in the sample were postmenopausal and early perimenopausal women were not included; future work should consider associations among these women. Brachial artery FMD is a validated measure indexing vascular responses largely mediated by the endothelium, but is not as direct a measure as more invasive measures.26 Moreover, participants were recruited to be reporting either no hot flashes in the past three months or daily hot flashes, and thereby associations among women reporting infrequent hot flashes could not be assessed. Larger studies with longitudinal outcomes should be conducted to further confirm findings. Finally, the sample included relatively few racial/ethnic minority women, particularly Hispanic and Asian women. Future work should examine these women.
This study had significant strengths. It provides the most rigorous test to date of relations between hot flashes and brachial artery FMD. FMD is a widely accepted and widely utilized noninvasive marker of endothelial function that correlates with later clinical events.23-26 Hot flashes were prospectively assessed by self-report and physiologic means, reducing the impact of memory and reporting. Highly symptomatic women were included and characterized. A range of possible mechanisms and confounders were rigorously assessed and considered here, including CVD risk factors, estradiol, as well as psychological factors associated with symptom reporting.54
Conclusions
In sum, the present investigation found that among nonsmoking younger midlife women (ages 40-53), hot flashes were associated with markers of poor endothelial function. Associations were not observed for the older women in the sample (ages 54-60). All associations were independent of CVD risk factors and of endogenous estradiol concentrations. These findings point to the potential value in considering the role of not only hormones, but also hot flashes, in the cardiovascular changes that occur early in the menopause transition,55 while also underscoring the potential role that the endothelium may play in the physiology of early hot flashes. With further replication and extension of this work, these findings may indicate that among early midlife women, frequent hot flashes may signal emerging vascular dysfunction.
Supplementary Material
Acknowledgments
Sources of Financial Support: This work was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647 and K24123565 to Thurston) and by the University of Pittsburgh Clinical and Translational Science Institute (NIH Grant UL1TR000005).
The National Institutes of Health funded this work and approved the initial study design, but was not involved in the conduct of the study; collection, management, analysis, or interpretation of the data; nor the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest: Thurston: None; Chang: None; Barinas-Mitchell: None; Jennings: None; Landsittel: None; von Känel: None; Matthews: None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916–47. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 3.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation (SWAN) Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32–43. doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for African American and Caucasian women. J Womens Health Gend Based Med. 2001;10(1):67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 6.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–9. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol. 1997;30(2):325–33. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechlioulis A, Kalantaridou SN, Naka KK, et al. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95(3):1199–206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 11.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665–86. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 12.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 13.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston RC, Johnson BD, Shufelt CL, et al. Menopausal symptoms and cardiovascular disease mortality in the Women's Ischemia Syndrome Evaluation (WISE) Menopause. 2016 Sep 26; doi: 10.1097/GME.0000000000000731. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu PB, Matthews KA, Thurston RC. How well do different measurement modalities estimate the number of vasomotor symptoms? Findings from the Study of Women's Health Across the Nation FLASHES Study. Menopause. 2014;21(2):124–30. doi: 10.1097/GME.0b013e318295a3b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause. 2008;15(5):841–7. doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65(3):402–9. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–15. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fisher W, Thurston RC. Measuring hot flash phenomenology using ambulatory prospective digital diaries. Menopause. 2016;23(11):1222–7. doi: 10.1097/GME.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 21.Thurston RC, El Khoudary SR, Tepper PG, et al. Trajectories of vasomotor symptoms and carotid intima media thickness in the Study of Women's Health Across the Nation. Stroke. 2016;47(1):12–7. doi: 10.1161/STROKEAHA.115.010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–31. doi: 10.1056/NEJMoa1505241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51(10):997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 26.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 29.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Craig CL, Marshall A, Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 32.de Geus EJ, Willemsen GH, Klaver CH, van Doornen LJ. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biol Psychol. 1995;41(3):205–27. doi: 10.1016/0301-0511(95)05137-6. [DOI] [PubMed] [Google Scholar]
- 33.Willemsen G, De Geus E, Klaver C, Van Doornen L, Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33(2):184–93. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 34.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–9. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 35.Thurston RC, Matthews KA, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46(2):285–92. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurston RC, Hernandez J, Del Rio JM, De la Torre F. Support vector machines to improve physiologic hot flash measures: application to the ambulatory setting. Psychophysiology. 2011;48(7):1015–21. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonka M, Liang W, Lauer R. Flow-mediated dilation in brachial arteries: computer analysis of ultrasound image sequences. CVD Prevention. 1998;1:147–55. [Google Scholar]
- 38.Santen RJ, Lee JS, Wang S, et al. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73(13):1318–21. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 40.Matthews D, Hosker J, Rudenski A, Naylor B, Teacher D, Turner R. Homeostasis model assessment: insulin resistance and B cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 41.Mann E, Hunter MS. Concordance between self-reported and sternal skin conductance measures of hot flushes in symptomatic perimenopausal and postmenopausal women: a systematic review. Menopause. 2011;18(6):709–22. doi: 10.1097/gme.0b013e318204a1fb. [DOI] [PubMed] [Google Scholar]
- 42.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 43.Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603–10. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342–53. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 45.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27(8):1782–7. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 46.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119(4):753–61. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2012;97(10):3487–94. doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gast GC, Grobbee DE, Pop VJ, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51(6):1492–8. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 49.Sassarini J, Fox H, Ferrell W, Sattar N, Lumsden MA. Hot flushes, vascular reactivity and the role of the alpha-adrenergic system. Climacteric. 2012;15(4):332–8. doi: 10.3109/13697137.2011.636847. [DOI] [PubMed] [Google Scholar]
- 50.Reed SD, Newton KM, Larson JC, et al. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol (Oxf) 2016;84(5):672–9. doi: 10.1111/cen.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68(5):657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 52.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352–8. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurston RC, Chang Y, Barinas-Mitchell E, et al. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47(12):2910–5. doi: 10.1161/STROKEAHA.116.014674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–75. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14. doi: 10.1097/gme.0b013e3182611787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.