Abstract
Psychophysiological hallmarks of posttraumatic stress disorder (PTSD) include exaggerated fear responses, impaired inhibition and extinction of conditioned fear, and decreased discrimination between safety and fear cues. This increased fear load associated with PTSD can be a barrier to effective therapy thus indicating the need for new treatments to reduce fear expression in people with PTSD. One potential biological target for reducing fear expression in PTSD is the hypothalamic-pituitary-adrenal (HPA) axis, which is dysregulated in PTSD. Recent translational rodent studies and cross-sectional clinical studies have shown that dexamethasone administration and the resulting suppression of cortisol in individuals with PTSD leads to a decrease in the fear responses characteristic of PTSD. These data, taken together, suggest that dexamethasone may serve as a novel pharmacologic intervention for heightened fear responses in PTSD. We conducted a double-blind, placebo-controlled trial to test our hypothesis that dexamethasone administration and the concomitant suppression of HPA axis hyperactivity would attenuate fear expression and enhance fear extinction in individuals with PTSD. Study participants (n=62) were recruited from Grady Memorial Hospital in Atlanta, GA. Participants were randomized to receive dexamethasone or placebo prior to fear conditioning and extinction, in a counterbalanced design (treatments separated by a week). Both PTSD− (n=37) and PTSD+ (n=25) participants showed significant startle increases in the presence of the danger signal during placebo and dexamethasone treatments (all p<0.05). However, only PTSD− control participants showed decreases in fear-potentiated startle across extinction blocks during both conditions (p’s≤ 0.001), with PTSD+ participants showing deficits in fear extinction and safety discrimination in the placebo condition. Notably, extinction and discrimination deficits in PTSD+ subjects were markedly reversed with dexamethasone (p<0.001). These data suggest that dexamethasone may serve as a pharmacological agent with which to facilitate fear extinction and discrimination in individuals with PTSD.
Keywords: PTSD, dexamethasone, fear extinction, safety discrimination, fear-potentiated startle
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that occurs in some individuals after exposure to a traumatic life event and increases individual vulnerability to adverse health outcomes across military and civilian populations (Dedert et al., 2010). Improving the present understanding of the neurobiological underpinnings of PTSD is crucial to the discovery of more effective treatment options for individuals suffering from this chronic and debilitating disorder. PTSD is a heterogeneous disorder consisting of avoidance, re-experiencing, and hyperarousal symptoms along with negative symptoms of cognition and mood. The hallmark psychophysiological characteristic of PTSD is dysregulation of the normal fear response. More specifically, individuals with PTSD consists have an exaggerated fear response (termed fear load, see (Norrholm et al., 2015), impaired inhibition of conditioned fear (Jovanovic and Ressler, 2010), and deficient fear extinction (Galatzer-Levy et al., 2016; Norrholm et al., 2011; Peri et al., 2000). Critically, the increased fear load associated with PTSD (Norrholm et al., 2015) may interfere with treatment and the achievement of remission in individuals who are unresponsive or incompletely responsive to conventional treatments for PTSD. As a consequence, there is an unmet need for interventions that address this gap in treatment efficacy.
One potential biological target for reducing fear in PTSD is the hypothalamic-pituitary-adrenal (HPA) axis and the actions of glucocorticoids, both of which are dysregulated in those with PTSD (Yehuda, 2009). While studies characterizing basal cortisol levels in PTSD have been equivocal (Meewisse et al., 2007), enhanced glucocorticoid negative feedback inhibition of the HPA axis in response to a dexamethasone suppression test has been repeatedly described in PTSD (Yehuda et al., 2004a). This enhanced suppression of endogenous cortisol in response to dexamethasone administration in individuals with PTSD occurs in tandem with lower levels of the GR co-chaperone FKBP5 (Yehuda et al., 2009a), and increased concentrations of corticotropin-releasing hormone (CRH) (Baker et al., 2005; de Kloet et al., 2008b) and glucocorticoid receptors (GRs, (Matic et al., 2013)) that can facilitate augmented glucocorticoid sensitivity (Yehuda et al., 2004a). Importantly, dexamethasone administration and the resulting suppression of cortisol in individuals with PTSD also results in a reduction of heightened fear responses that is characteristic of the PTSD (Jovanovic et al., 2010; Jovanovic et al., 2011). Because peripheral levels of cortisol have not been consistently associated with PTSD, the current status of the literature has been focused on glucocorticoid receptor sensitivity, rather than hormone levels (Yehuda et al., 2009a; Yehuda et al., 2004a). Thus, the current study used dexamethasone suppression of cortisol, which specifically targets GR hypersensitivity as a mechanism of deregulation fear responses in PTSD, rather than absolute values of baseline or suppressed cortisol levels. We hypothesize that dexamethasone suppression prior to extinction may serve to clamp the HPA hypersensitivity and increased glucocorticoid sensitivity previously reported to increase PTSD risk.
While these data suggest that dexamethasone may serve as a pharmacological intervention for heightened fear responses in PTSD, no studies to date have tested dexamethasone’s ability to modulate fear responses or facilitate fear extinction in those with PTSD in a within-subjects design. Thus, in the current study, we undertook a double-blind, placebo-controlled trial to test the hypothesis that dexamethasone administration and the concomitant suppression in cortisol would result in decreased fear expression and enhanced fear extinction in individuals with PTSD.
Methods
Participants
Study participants were recruited from Grady Memorial Hospital in Atlanta, GA between June 2011 and December of 2013. Study participants were English-speaking men and women between the ages of 18 and 65 years who were enrolled in the Grady Trauma Project, a large project assessing trauma exposure and clinical symptoms in a low income urban population. Exclusion criteria included pregnancy, positive urine toxicology (tetrahydrocannabinol, benzodiazepines, cocaine, and opiates), hearing impairment as assessed by an audiometer (Grason-Stadler, Model FS1710), active psychosis and major medical illnesses as determined by a medical professional during a history and physical examination. Upon determining eligibility for the study and obtaining consent for the current study, participants were randomly assigned to drug condition and intervention order. All study procedures were reviewed and approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight Committee.
Psychological Assessment
As part of the Grady Trauma Project, all participants provided self-reported levels of PTSD symptoms using the modified PTSD Symptom Scale (PSS), a psychometrically valid, 17-item self-report measure assessing PTSD symptoms. For the present study, the PSS was used to determine a the categorical definition of PTSD status based on DSM-IV criteria (APA, 2000), if participants reported any level of severity of at least one re-experiencing symptom, three avoidance and/or numbing symptoms, and two hyperarousal symptoms (Falsetti et al., 1993). Because the PSS is a self-report measure and not a structured diagnostic interview, this should be considered as a probable PTSD diagnosis (Binder et al., 2008). The Traumatic Events Inventory (TEI), was used to assess lifetime adult trauma history by detailing the frequency and type of trauma(s) experienced by study participants (Gillespie et al., 2009). Total level of trauma exposure was measured by a sum score reflecting the total number of different types of trauma (e.g., car accident, sexual assault, and natural disaster) to which a participant had been exposed over the course of their life. The Childhood Trauma Questionnaire (CTQ, (Bernstein et al., 1997) is a 25-item, self-report inventory assessing three domains of childhood abuse (sexual, physical, and emotional), and two domains of childhood neglect (physical and emotional). The CTQ was used to capture childhood trauma exposure that occurred at or before 18 years of age. The Beck Depression Inventory (BDI) - II is a 21-item self-report measure assessing depressive symptoms in the last two weeks (Beck et al., 1996). Demographic information included participant sex, age, self-identified race, education, and income.
Experimental Design
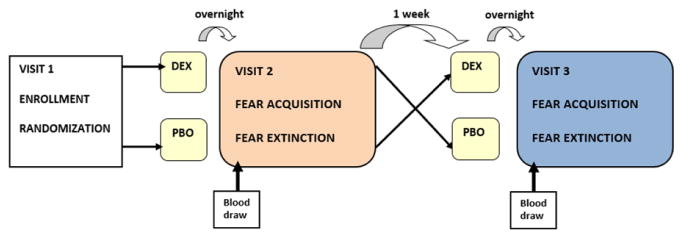
A double-blind, crossover design study was conducted with two groups (Group: PTSD+, PTSD−) and two within-subjects treatment conditions (Drug: placebo, DEX; see Figure 1). To suppress endogenous cortisol levels in the DEX condition, a single 0.5mg dose of dexamethasone was taken by the participant the night prior to the study visit as previously described to specifically target GR hypersensitivity as a mechanism of deregulation of fear responses in PTSD, rather than absolute values of baseline or suppressed cortisol levels (Jovanovic et al., 2010; Koopmans et al., 1992; Yehuda et al., 2004b). The treatment conditions were separated by one week and counterbalanced to control for order effects. Each condition consisted of fear-potentiated startle test (the testing visit included fear acquisition and fear extinction assessment as outlined below). Participants were randomized by study pharmacist to order of treatment condition (placebo vs. dexamethasone) and received a pill from the hospital pharmacy and told to take it at 11 p.m. the night prior to the next visit. The study coordinator also called the participant to remind them to take the pill the day prior to their scheduled visit. The hospital pharmacist maintained a randomization list, and was not involved in any of the other study procedures. The next day, a blood sample was collected at 8 a.m. and the fear-potentiated startle session was conducted. Upon completion of the startle visit, the participant was scheduled for the next visit one week later and given the second pill. Both participants and assessors were blind to treatment condition throughout the study.
Figure 1.
Diagram of the design for the current randomized, placebo-controlled, double-blind study to assess the effects of dexamethasone (DEX) versus placebo (PBO) on fear responses in individuals with (PTSD+) and without PTSD (PTSD−).
Fear-Potentiated Startle Paradigm
FPS was measured by the relative increase in the acoustic startle reflex in the presence of conditioned stimuli that were paired with aversive unconditioned stimuli (Jovanovic et al., 2012). Differential fear conditioning included two distinct cues: the reinforced conditioned stimulus (CS+, also referred to as the danger signal) and a non-reinforced conditioned stimulus (CS−, also referred to as the safety signal). Startle testing: As previously described (Jovanovic et al., 2012), the eyeblink component of the acoustic startle response was measured by electromyography (EMG) recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel. The startle probe was a 108-dB (A) SPL, 40ms burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones. The startle response data was acquired using Biopac MP150 for Windows (Biopac Systems, Inc.) as described in our previous studies. All data was sampled at 1000 Hz and amplified with a gain of 5000 using the EMG module of the Biopac system. The acquired data were rectified and filtered between 28 Hz and 500 Hz using MindWare software (MindWare Technologies, Ltd.) and exported for statistical analyses. A maximum amplitude of the eyeblink muscle contraction 20–200 ms after presentation of the startle probe was used as a measure of the acoustic startle response. Fear acquisition: The FPS consisted of an initial habituation phase wherein CS’s were presented without any reinforcement (Norrholm et al., 2015). The acquisition phase consisted of three blocks with four trials of each type of CS (reinforced conditioned stimulus, CS+; non-reinforced conditioned stimulus, CS−; noise probe alone, NA) for 12 trials per block and a total of 36 trials. Both CS’s were colored shapes (i.e. blue square, purple triangle) presented on a computer monitor for six seconds each. The CS’s differed between the two study visits and were counterbalanced across subjects. The unconditioned stimulus (US; aversive stimulus) was a 250-msec air blast of 140-psi intensity to the larynx that has been shown in our previous studies to produce a robust fear-potentiated startle response (Norrholm et al., 2015). The air blast was delivered from a compressed air tank via polyethylene tubing and controlled by a solenoid switch. The inter-trial intervals throughout the acquisition phase were randomized to be between nine and 22 seconds in duration. Fear extinction: As previously described (Norrholm et al., 2015), the fear extinction session occurred ten minutes after acquisition. Neither the CS+ nor the CS− was paired with the US. The extinction session consisted of four blocks of four trials of each type (NA, CS+ (unreinforced), and CS−) in each block. The first two trials of fear acquisition and extinction were categorized as early and the last two trials of each session were categorized as late fear acquisition and extinction as previously described (Norrholm et al., 2015).
Cortisol and Dexamethasone Sampling and Assays
On the morning of each FPS visit a blood sample was collected and stored at −80 degrees C until time of assay. Cortisol suppression due to dexamethasone was assessed by subtracting post-dexamethasone cortisol concentrations from cortisol concentrations the morning after placebo treatment. Plasma cortisol and dexamethasone were assayed at the Yerkes National Primate Research Center Biomarkers Core using mass spectrometry (Franke et al., 2011). Dexamethasone was assayed in order to verify compliance.
Statistical Analyses
Sociodemographic data were analyzed using t-tests and chi-square test. Data were analyzed using mixed-model analysis of variance (ANOVA) to assess differences in startle magnitude during late acquisition between NA and CS+ as a within-subjects factor during acquisition with PTSD diagnosis as a between-groups variable. Discrimination between danger and safety cues during late acquisition was tested in an ANOVA comparing FPS to CS+ and CS− and PTSD diagnosis. A separate ANOVA was used to compare FPS during early CS+ and late CS+ during extinction, again with PTSD diagnosis as between-groups variable. The ANOVAs were repeated for each condition (placebo and dexamethasone). Group differences were analyzed with and without co-varying for trauma exposure and depression symptoms to see whether these variables accounted for the observed differences in startle measures. Correlations were used to assess whether cortisol levels were associated with dexamethasone-induced differences in fear acquisition and extinction. Based on our previous work using the same fear extinction paradigm, a sample size of n=24 per group is necessary to detect a decrease in fear during extinction with 80% power and 0.05 alpha. The data were analyzed using SPSS (v.24) and alpha level was set at p<0.05 for statistical significance.
Results
Sociodemographics
Sixty-eight individuals met inclusion criteria, were enrolled and completed the current study. Five participants (2 PTSD− and 3 PTSD+) were not compliant in taking the study medication the night prior to the study visit. Of the remaining 63 participants, 24 participants received a diagnosis of PTSD (PTSD+) and 39 participants did not (PTSD−; Table 1). There were no significant differences between groups with respect to age, sex, race, education, or income (all p>0.05; see Table 1). While both groups of participants reported exposure to trauma during their lifetime, individuals with PTSD had significantly higher rates of childhood and adult trauma, as well as greater PTSD and depressive symptoms (all p<0.001; Table 1).
Table 1.
Mean ± SEM and frequency of participant sociodemographic characteristics broken down by PTSD status.
| PTSD− (n=39) | PTSD+ (n=24) | p-value | |
|---|---|---|---|
| Trauma and Symptoms | |||
| CTQ Total | 35.79 ± 2.09 | 52.87 ± 3.02 | <0.001* |
| TEI Total Experienced | 2.74 ± 0.28 | 5.25 ± 0.49 | <0.001* |
| PSS Total | 7.87 ± 1.17 | 26.67 ± 2.07 | <0.001* |
| BDI Total | 12.69 ± 1.55 | 24.33 ± 2.53 | <0.001* |
| Percent (%) | Percent (%) | ||
| Demographics | |||
| Age | 42.41 ± 2.05 | 43.08 ± 2.24 | 0.83 |
| Race | 0.69 | ||
| African American | 94.9 | 95.8 | |
| Other | 5.1 | 4.2 | |
| Sex | 0.62 | ||
| Male | 30.8 | 25.0 | |
| Female | 69.2 | 75.0 | |
| Education | 0.74 | ||
| < High School (HS) | 10.8 | 17.4 | |
| HS or GED | 45.9 | 39.1 | |
| > HS | 43.3 | 43.5 | |
| Monthly Income | 0.30 | ||
| <$500 | 54.1 | 73.9 | |
| $500–$999 | 13.5 | 8.7 | |
| >$1000 | 32.4 | 17.4 |
Asterisks denote significant differences between PTSD− and PTSD+ participants (p<0.001).
Cortisol and Dexamethasone Concentrations
Assays for dexamethasone revealed that dexamethasone levels were undetectable during the placebo condition and averaged 1.13±0.15 ng/mL during the dexamethasone condition across all participants, with no differences between PTSD groups. Assays for cortisol revealed that mean cortisol levels were 2.17±0.42 μg/dL during the placebo condition and 0.70±0.32 μg/dL during the dexamethasone condition across all participants. There was no significant difference in baseline cortisol levels between PTSD− and PTSD+ participants (p=0.82). Administration of dexamethasone significantly suppressed cortisol levels (F=295.11, p<0.001), but did so in a manner independent of PTSD diagnosis (p=0.53). The percent cortisol suppression due to dexamethasone administration did not differ between those without (59.0%±5.3) and with PTSD (60.1%±6.9). Controlling for depression symptoms or degree of trauma exposure did not change the results.
Fear Acquisition
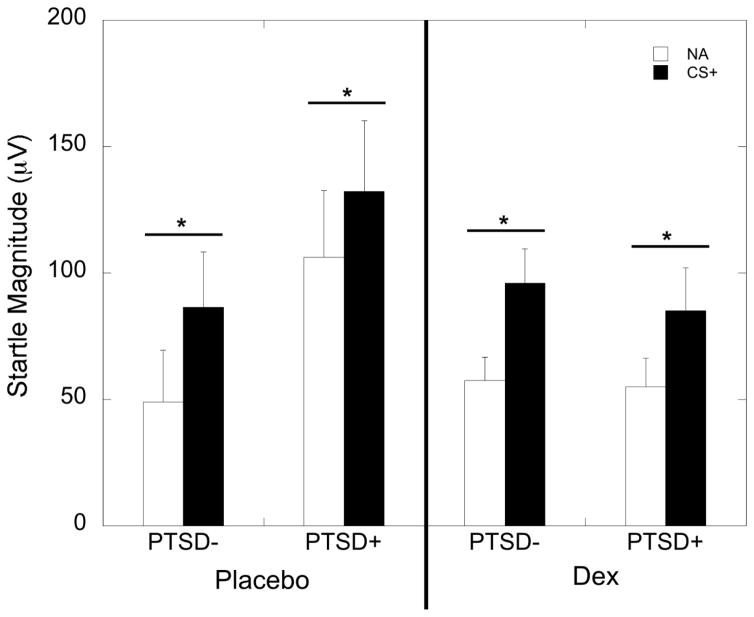
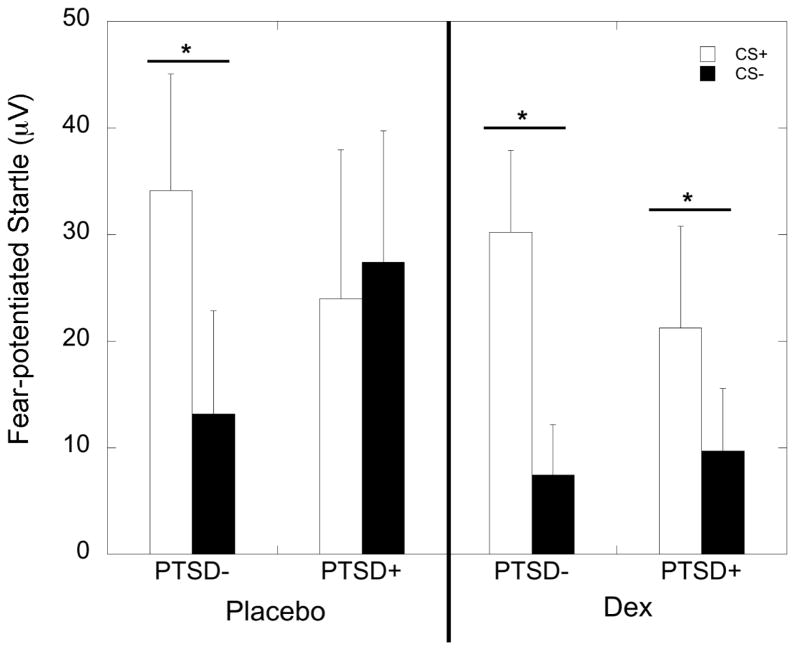
ANOVA with startle magnitude and PTSD diagnosis during the placebo condition showed a significant increase in startle to the CS+ relative to the NA trials (F=12.40, p=0.001) demonstrating that fear to the CS+ was acquired, with no interaction or main effect of PTSD diagnosis (Figure 2). During the dexamethasone condition, both groups again showed a significant increase in startle to the CS+ (F=24.74, p<0.001), with no effect of PTSD diagnosis (Figure 2). Comparison of FPS to CS+ and CS− during placebo revealed an interaction between trial type and PTSD diagnosis (F=5.32, p=0.025), with the PTSD− group showing a significantly lower FPS response to the CS− than to the CS+ (F=10.48, p=0.003), while the PTSD+ group did not (F=0.16, p=0.69). After dexamethasone, all participants showed lower FPS to the CS− than to the CS+ (10.62, p=0.002), with no interaction effect with PTSD (Figure 3). We examined correlations between the percent cortisol suppression and post-dexamethasone FPS to CS+ and CS, separately in each PTSD group, and found a negative correlation between FPS to danger and cortisol suppression in the PTSD− group (r=−0.52, p=0.005), but not in the PTSD+ group (r=0.15, p=0.55). FPS to the CS− was not correlated with cortisol suppression. The order of dexamethasone or placebo administration did not influence fear acquisition and did not interact with PTSD status (all p>0.05).
Figure 2.
Mean ± SEM of fear-potentiated startle (FPS) responses to noise alone (NA) and danger signal (CS+). Both PTSD− and PTSD+ participants showed increased startle response to the danger signal compared to the noise alone during both the placebo and dexamethasone (DEX) treatment conditions.
Figure 3.
Mean ± SEM of fear-potentiated startle (FPS) responses to danger (CS+) and safety (CS−) signals during the fear acquisition paradigm. PTSD− participants showed significant discrimination between the safety and danger signals during both the placebo and dexamethasone (DEX) treatment conditions. In contrast, PTSD+ subjects only showed discrimination during dexamethasone treatment.
Fear Extinction
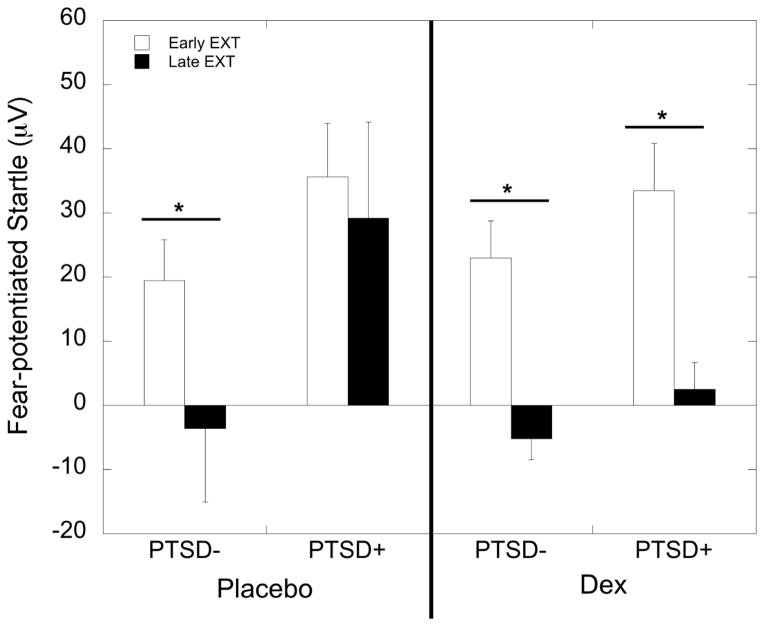
ANOVA with FPS to CS+ during early vs. late extinction during the placebo condition showed a main effect of PTSD (F=5.08, p=0.028), but no effect of extinction. The effect of PTSD remained significant after co-varying for trauma exposure and depression symptoms (p=0.021). When the two groups were analyzed separately, the PTSD− group showed a significant decrease in FPS from early to late extinction (F=16.05, p<0.001), whereas the PTSD+ group did not (F=0.06, p=0.81; Figure 4). In the dexamethasone condition, there was a significant effect of extinction (F=41.79, p<0.001), but no main or interaction effect with PTSD diagnosis. Separate analyses in each group showed that both groups decreased in FPS from early to late extinction (F=23.29, p<0.001 in PTSD− and F=20.64, p<0.001 in PTSD+; Figure 4). Cortisol suppression due to dexamethasone was not associated with the decreased FPS during the late extinction blocks in those with (r=−0.09, p=0.73) or without PTSD (r=0.14, p=0.46). The order of dexamethasone or placebo administration did not influence fear extinction and did not interact with PTSD status (all p>0.05).
Figure 4.
Mean ± SEM of fear-potentiated startle (FPS) responses during early and late blocks of the fear extinction paradigm. PTSD− participants showed significant decreases in FPS in response to the danger signal during both the placebo and dexamethasone (DEX) treatment conditions. In contrast, PTSD+ subjects only showed fear extinction during dexamethasone treatment.
Discussion
The current double-blind, placebo-controlled crossover trial indicated that administration of dexamethasone resulted in facilitation of fear discrimination and extinction specifically in individuals with PTSD. Dexamethasone administration resulted in suppression of cortisol concentrations, suggesting that HPA actions may be partially responsible for fear extinction deficits observed in PTSD (Milad et al., 2008). Translating these experimental findings to the clinical arena, our results suggest that dexamethasone administration may improve the efficacy of extinction-based therapies such as prolonged exposure for traumatized individuals with PTSD.
The finding of dexamethasone facilitation of extinction supports recent results from a translational rodent model of PTSD-like behavior in which dexamethasone administration enhanced fear extinction and extinction retention in a dose-dependent manner (Sawamura et al., 2016). Additional studies in rodents have shown the efficacy of dexamethasone in decreasing FPS and contextual fear during fear extinction paradigms (Yang et al., 2006). Furthermore, enhanced fear extinction upon dexamethasone treatment in rodents occurred in tandem with decreases in mRNA expression of FKBP5, a gene which codes for a co-chaperone of GR implicated in the pathophysiology of PTSD (Binder et al., 2008), within the amygdala along with alterations in FKBP5 epigenetic regulation (Sawamura et al., 2016). A recent cross-species examination of fear extinction implicates FKBP5 as integral to extinction mechanisms in rodents and humans (Galatzer-Levy et al., 2016). Thus, it is possible that the dexamethasone effects on FKBP5 regulation of GR are central to the extinction enhancement observed here. Finally, even though dexamethasone has high affinity for the glucocorticoid receptors, it may also influence FKBP5 interactions with limbic mineralocorticoid receptors that have been implicated in PTSD to mediate corticosteroid response (Matic et al., 2014). Future studies are necessary to determine the effects of dexamethasone on FKBP5 expression in individuals with PTSD.
While the current study found that dexamethasone facilitated fear extinction and safety discrimination between danger and safety cues in those with PTSD, it did not have a significant effect on fear acquisition (see Figure 2). Therefore, dexamethasone rescued extinction deficits associated with PTSD in a relatively specific manner. There were no differences on the influence of dexamethasone on fear conditioning between the PTSD and control groups. The inability to discriminate danger and safety cues in PTSD may, in part, arise from an over-generalization of stimuli and increased arousal (Jovanovic et al., 2012). We have previously shown in a naturalistic, between-group study that dexamethasone administration and parallel reductions in endogenous cortisol levels eliminate exaggerated FPS to danger cues in individuals with PTSD (Jovanovic et al., 2011). Additionally, administration of hydrocortisone, another exogenous GR agonist, prior to fear conditioning impairs eyeblink conditioning in individuals with PTSD (Vythilingam et al., 2006) and normalizes increased amygdala activation in individuals with PTSD as assessed by glucose metabolism PET (Yehuda et al., 2009b). In addition, GR activation facilitates fear memory consolidation (Roozendaal et al., 2009) and GR antagonism attenuates dysregulated fear responses in a rodent model of PTSD (Kohda et al., 2007). Importantly, glucocorticoid receptors are abundant in the amygdala, a region of the brain critical for fear conditioning whose hyperactivity has been consistently shown in those with PTSD (Shin et al., 2006), and thus can modulate the expression of fear as previously shown in rodents and non-human primates (Roozendaal et al., 2008). These studies are consistent with our finding that cortisol suppression was associated with less fear to the danger signal in controls. While our results show that a single, low dose of dexamethasone specifically facilitates fear extinction and safety discrimination in PTSD, future studies are necessary to characterize whether higher doses, different types (i.e. hydrocortisone) and longer duration treatments of corticosteroids also influence fear acquisition in PTSD.
Suppression of cortisol due to dexamethasone treatment occurred in parallel with facilitation of fear extinction in those with PTSD. However, the degree of cortisol suppression was not associated with fear extinction in individuals with PTSD in the current study. Previous studies indicate that baseline and post-dexamethasone concentrations of ACTH, but not cortisol, are correlated with FPS to danger signals in a fear discrimination task, but only in PTSD+ individuals (Jovanovic et al., 2010). Importantly, other HPA signals have been implicated in enhanced fear responses in animal models. Elevated CRH concentrations are associated with increased fear responses (Kalin and Takahashi, 1990) and anxiety (Sutton et al., 1982), including the startle response (Keen-Rhinehart et al., 2009) and enhanced fear conditioning (Roozendaal et al., 2002; Swerdlow et al., 1989) in rodents and in monkeys. CRH hypersensitivity has been particularly associated with survivors of childhood trauma (de Kloet et al., 2008a; Heim et al., 2008), as is very common in our cohort. Increased concentrations of CRH also occur in individuals with PTSD (Baker et al., 2005; de Kloet et al., 2008b) in tandem with augmented GR levels (Matic et al., 2013) and lower levels of the GR co-chaperone FKBP5 (Yehuda et al., 2009a) that can facilitate augmented glucocorticoid sensitivity in PTSD (Yehuda et al., 2004a). Our current findings suggest that dexamethasone-mediated stabilization of HPA hyperactivity at multiple levels may contribute to the decreased fear and enhanced extinction effects.
A separate but potentially important mechanism of stress-related fear and extinction deficits in PTSD is the immune system. Multiple lines of evidence from separate samples and studies indicate that PTSD is associated with increased inflammation (Michopoulos et al., 2015; Passos et al., 2015; Plantinga et al., 2013). Importantly, heightened immune activation in the form of elevated C-reactive protein (CRP) has also been associated with increased FPS and hyperarousal symptoms (Michopoulos et al., 2015; Passos et al., 2015). Because GR activity can directly modulate peripheral inflammation levels, dexamethasone administration may suppress the heightened immune activation associated with PTSD and fear responses. Future studies are necessary to characterize the effects of dexamethasone administration on pro-inflammatory markers such as CRP and cytokines, and how dexamethasone-induced suppression of the immune system is associated with dexamethasone’s facilitation of fear discrimination and extinction.
In the present study, dexamethasone suppressed cortisol levels similarly in those with and without PTSD. This result does not replicate previous reports of enhanced glucocorticoid negative feedback inhibition of the HPA axis in PTSD (Yehuda et al., 2004a). One reason for this may be due to the fact that we did not exclude participants with comorbid depression symptoms that have been shown to be associated with diminished glucocorticoid negative feedback inhibition of the HPA axis (McEwen, 2008; Yehuda et al., 2004b); however co-varying for depression symptoms did not change the lack of group difference. Another reason for a lack of a main effect of PTSD on dexamethasone suppression may be that the PTSD− group also reported high rates of trauma exposure, even though not as high as those that met PTSD diagnosis. Trauma exposure itself has been found to have similar effects as PTSD on cortisol suppression following dexamethasone administration in combat veterans compared to non-traumatized controls (de Kloet et al., 2007), suggesting that our research cohort may have a ‘ceiling effect’ in this phenotype due to level of total trauma exposure. Additionally, recent findings indicate that single nucleotide polymorphisms within the FKBP5 gene that have been associated with increased PTSD risk (Binder et al., 2008) may be direct contributing factors to the hypersensitivity of the HPA axis (Klengel et al., 2013). Thus, assessing the role of genetic variation within FKBP5 in the future may help disentangle the relationship between PTSD and glucocorticoid negative feedback.
Taken together, the results generated from our double-blind, placebo-controlled study indicate that dexamethasone facilitates fear extinction and safety discrimination in those with PTSD compared to placebo. Limitations of the current study include the use of self-report PSS to determine a probable PTSD diagnosis based upon DSM-IV criteria and the inclusion of both traumatized and non-traumatized controls in the PTSD− group. Additionally, the lack of full medical histories did not allow us to control for other psychiatric (i.e bipolar disorder) and medical (i.e. body mass index) co-morbidities that are known to influence HPA axis function and glucocorticoid sensitivity (Maripuu et al., 2014; Pasquali et al., 2002). Follow-up studies are necessary to replicate the current findings and expand the generalizability of the results to other populations of traumatized individuals as our urban study sample consisted primarily of African Americans of low socioeconomic status.
Importantly, our data suggest that dexamethasone may serve as a pharmacological agent with which to facilitate extinction-based interventions such as exposure therapy delivered during cognitive behavioral therapy (CBT) or virtual reality exposure therapy (VRE). The efficacy of such an approach has recently been highlighted by studies showing that D-cycloserine, an NMDA receptor partial agonist, facilitates fear extinction in rodent models of PTSD (Davis et al., 2006) and augments exposure therapy for PTSD (Rothbaum et al., 2014). A similar approach should be tested to examine whether dexamethasone enhances treatment of PTSD using fear-extinction based therapies exposure therapy. A recent study used hydrocortisone as an adjunct to prolonged exposure therapy and found greater treatment retention and decreased GR sensitivity in responders (Yehuda et al., 2015), emphasizing that agents targeting the glucocorticoid system have great potential in PTSD treatment. The current study points to improved fear regulation as a mechanism for these promising treatment approaches.
Research Highlights.
Posttraumatic stress disorder (PTSD) is associated with deficits in fear regulation
Placebo-controlled, double-blind study of dexamethasone effects on fear responses
Dexamethasone suppressed endogenous cortisol levels
Dexamethasone normalized fear extinction in those with PTSD
Dexamethasone facilitated fear discrimination in those with PTSD
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, the National Institute for Health (R01 MH094757, KJR; R21 MH092576 TJ), the National Institute of Child Health and Human Development (K12 HD085850, VM) and the Brain and Behavior Research Foundation. Support was also provided by the Emory and Grady Memorial Hospital General Clinical Research Center (GCRC), NIH National Centers for Research Resources (M01 RR00039), and the Burroughs Wellcome Fund. We thank Alex Rothbaum, Allen Graham, Angelo Brown and the nurses and staff of the Grady GCRC for their assistance with data collection and support. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors
Authors Jovanovic, Norrholm, Ressler and Rothbaum designed and organized the studies and wrote the protocols. Authors Michopoulos, Stevens and Glover collected the data. Authors Michopoulos and Jovanovic undertook the statistical analyses. Authors Michopoulos, Stevens, Glover, Jovanovic, Norrholm, Ressler and Rothbaum contributed to the analysis and interpretation of the data. Authors Michopoulos and Jovanovic wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Disclosure
Dr. Rothbaum owns equity in Virtually Better, Inc. that creates virtual reality products. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Dr. Rothbaum has funding from Wounded Warrior Project, Department of Defense Clinical Trial Grant No.W81XWH-10-1-1045, “Enhancing Exposure Therapy for PTSD: Virtual Reality and Imaginal Exposure with a Cognitive Enhancer” and McCormick Foundation “Brave Heart: MLB’s Welcome Back Veterans SouthEast Initiative.” Dr. Rothbaum receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received one advisory board payment from Genentech. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA, A.P.A. Diagnostic and Statistical Manual of Mental Disorders. 2000. [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD., Jr Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. The American journal of psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biological psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Kloet C, Vermetten E, Lentjes E, Geuze E, van Pelt J, Manuel R, Heijnen C, Westenberg H. Differences in the response to the combined DEX-CRH test between PTSD patients with and without co-morbid depressive disorder. Psychoneuroendocrinology. 2008a;33:313–320. doi: 10.1016/j.psyneuen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Progress in brain research. 2008b;167:287–291. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, Westenberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39:61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behaviour Therapist. 1993:161–161. [Google Scholar]
- Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Anal Bioanal Chem. 2011;401:1319–1330. doi: 10.1007/s00216-011-5164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Andero R, Sawamura T, Jovanovic T, Papini S, Ressler KJ, Norrholm SD. A cross species study of heterogeneity in fear extinction learning in relation to FKBP5 variation and expression: Implications for the acute treatment of posttraumatic stress disorder. Neuropharmacology. 2016;116:188–195. doi: 10.1016/j.neuropharm.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biological psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, Duncan E, Bradley B, Ressler K. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology. 2010;35:846–857. doi: 10.1016/j.psyneuen.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, Ressler KJ. Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36:1540–1552. doi: 10.1016/j.psyneuen.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. The American journal of psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain research. 1990;509:80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Molecular psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Koopmans RP, Braat MC, Oosterhuis B, van Boxtel CJ. Time-dependent effects of dexamethasone administration on the suppression of plasma hydrocortisone, assessed with a pharmacokinetic model. The Journal of pharmacology and experimental therapeutics. 1992;262:503–508. [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative hypo- and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: a cross-sectional study. PloS one. 2014;9:e98682. doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic G, Milutinovic DV, Nestorov J, Elakovic I, Jovanovic SM, Perisic T, Dunderski J, Damjanovic S, Knezevic G, Spiric Z, Vermetten E, Savic D. Lymphocyte glucocorticoid receptor expression level and hormone-binding properties differ between war trauma-exposed men with and without PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:238–245. doi: 10.1016/j.pnpbp.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Matic G, Vojnovic Milutinovic D, Nestorov J, Elakovic I, Manitasevic Jovanovic S, Elzaedi YM, Perisic T, Dunderski J, Damjanovic S, Knezevic G, Spiric Z, Vermetten E, Savic D. Mineralocorticoid receptor and heat shock protein expression levels in peripheral lymphocytes from war trauma-exposed men with and without PTSD. Psychiatry research. 2014;215:379–385. doi: 10.1016/j.psychres.2013.11.022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. The American journal of psychiatry. 2015;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of psychiatric research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, Ressler KJ, Jovanovic T. Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol. 2015;98:270–275. doi: 10.1016/j.ijpsycho.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. The Journal of clinical endocrinology and metabolism. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV, Kapczinski F, Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Progress in brain research. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A, Ressler KJ. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. The American journal of psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura T, Klengel T, Armario A, Jovanovic T, Norrholm SD, Ressler KJ, Andero R. Dexamethasone Treatment Leads to Enhanced Fear Extinction and Dynamic Fkbp5 Regulation in Amygdala. Neuropsychopharmacology. 2016;41:832–846. doi: 10.1038/npp.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41) Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Lawley M, Collin C, Bonne O, Agarwal R, Hadd K, Charney DS, Grillon C. Hydrocortisone impairs hippocampal-dependent trace eyeblink conditioning in post-traumatic stress disorder. Neuropsychopharmacology. 2006;31:182–188. doi: 10.1038/sj.npp.1300843. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, Flory JD, Makotkine I, Hildebrandt T. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–597. doi: 10.1016/j.psyneuen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biological psychiatry. 2009a;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological psychiatry. 2004a;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004b;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Harvey PD, Golier JA, Newmark RE, Bowie CR, Wohltmann JJ, Grossman RA, Schmeidler J, Hazlett EA, Buchsbaum MS. Changes in relative glucose metabolic rate following cortisol administration in aging veterans with posttraumatic stress disorder: an FDG-PET neuroimaging study. J Neuropsychiatry Clin Neurosci. 2009b;21:132–143. doi: 10.1176/jnp.2009.21.2.132. [DOI] [PubMed] [Google Scholar]