Abstract
The nuclear envelope safeguards the genetic material inside the nucleus by separating it from the cytoplasm. Until recently, it was assumed that nuclear envelope breakdown occurs only in a highly controlled fashion during mitosis when the chromatin is condensed and divided between the daughter cells. However, recent studies have demonstrated that adherent and migrating cells exhibit transient nuclear envelope rupture during interphase caused by compression from cytoskeletal or external forces. Nuclear envelope rupture results in uncontrolled exchange between the nuclear interior and cytoplasm and leads to DNA damage. In this review, we discuss the causes and consequences of nuclear envelope rupture, and how nuclear envelope rupture could contribute to genomic instability.
Keywords: nuclear envelope rupture, lamin, confined migration, cancer, genomic instability
Genomic instability in cancer
Genomic instability, defined as an increased rate of alteration in the genome of cells, is one of the hallmarks of cancer and is thought to contribute to cancer progression and resistance to treatment [1, 2]. The most common forms of genomic instability in cancer include chromosomal instability (i.e., changes in chromosome number and structure) and genetic mutations/deletions. These changes can lead to inactivation of tumor suppressors or hyperactivation of oncogenes, thereby driving uncontrolled proliferation of cells and tumorigenesis [2]. Genomic instability typically arises from defects in DNA damage repair pathways or in DNA replication and segregation during mitosis that prevent high fidelity propagation of the genetic material to the progeny cells [3]. However, recent findings have pointed to a novel source of genomic instability. Nuclear envelope (NE) integrity was identified to play an important role in protecting the cell’s genome, with (transient) loss of nuclear envelope integrity resulting in DNA damage and severe DNA rearrangements known as chromothripsis.
The NE, which separates the nuclear contents from the cytoplasm, consists of the inner and outer nuclear membranes, nuclear membrane proteins, nuclear pores, and the nuclear lamina (see Box 1). These components work together to protect the cell’s genetic material during interphase by creating a tightly controlled nuclear compartment. The importance of the NE in maintaining genomic integrity is particularly apparent in micronuclei, which are chromosomes or chromosome fragments that have become separated from the primary nucleus during mitosis due to mis-segregation, and that have acquired their own NE [4]. Micronuclei are a common feature of cancer cells and a major contributor to genomic instability [4, 5]. Two recent reports have provided insight into how micronuclei can add to genomic instability: irreversible breakdown of the nuclear lamina in micronuclei during interphase results in loss of NE integrity and causes massive double strand breaks (DSBs) and chromothripsis, likely by allowing access of cytoplasmic content to the DNA in the micronucleus [4, 5]. The altered micronucleus DNA is then re-integrated into the nucleus during subsequent cell division, contributing to further genomic instability [5]. Intriguingly, transient NE rupture also occurs in the primary nucleus itself. Cells exhibit spontaneous loss of NE integrity at a rate of 0.1% to 10% of cells over a 24 hour period in unconfined, two-dimensional (2-D) cell culture conditions [6–9]. The frequency of NE rupture increases dramatically when cells are migrating through confined environments with pore sizes smaller than the nuclear cross section [7, 10, 11]. In these 3-D conditions, which are found in vivo in the interstitial space of tissues, and which can be recapitulated in vitro using collagen matrices or microfluidic devices, upwards of 70% of migrating cells incur transient NE rupture, with the specific percentage depending on the cell type and degree of confinement [7, 10]. Interestingly, the vast majority of cells survive these transient NE ruptures [7, 10]. Paralleling the findings in micronuclei, NE rupture of the primary nucleus also results in double stranded breaks [7, 10, 12], suggesting that transient loss of NE integrity could provide a novel mechanism that contributes to the genomic instability of cells, and that this effect might be particularly prevalent in invasive cancer cells. In this review, we summarize the causes and consequences of NE rupture, including how cells overcome NE rupture, and the implications for genomic instability in the context of cancer progression. Lastly, we discuss how insights gained from these recent studies could hint at novel therapeutic anti-cancer strategies targeting these mechanisms.
Box 1. The nuclear envelope: Separating the genome from the cytoplasm.
The NE forms a physical barrier between the nuclear interior and the cytoplasm, which is crucial to maintain the biochemical and physical integrity of the genome and to prevent DNA damage from cytoplasmic proteins or mechanical force [4, 5, 55]. The NE is composed of two lipid bilayers and their associated nuclear membrane proteins, nuclear pore complexes, and the nuclear lamina, an intermediate filament network surrounding the nuclear DNA. The inner and outer nuclear membranes fuse at the sites of nuclear pore complexes, which tightly control nuclear import and export.
In somatic cells, the nuclear lamina is primarily composed of two types of lamin proteins: A-type lamins, which include lamin A and C as the major isoforms and result from alternative splicing of the LMNA gene, and B-type lamins. Somatic B-type lamins include lamin B1 and B2 and are encoded by two different genes, LMNB1 and LMNB2, respectively. In contrast to A-type lamins, B-type lamins remain farnesylated and thereby maintain strong membrane association. The nuclear lamina provides mechanical support to the nucleus and, through interaction with the LINC (Linker of nucleoskeleton and cytoskeleton) complex, couples the nuclear interior to cytoplasmic actin, microtubule and intermediate filaments [56, 57]. The NE also interacts with various transcription factors and plays important roles in DNA repair, chromatin organization, and transcriptional regulation [58–60].
NE organization and function can become disturbed both by changes in lamin expression levels and by mutations in lamins or LINC complex proteins [61]. Lamin levels, especially those of lamin A/C, determine the stiffness of the nucleus and its susceptibility to mechanical strain [24, 37, 40]. Many cancers cells have substantial alterations in lamin A/C levels [43, 44]. Mutations in the lamin genes, particularly LMNA, cause a broad spectrum of diseases called laminopathies. These include Emery-Dreifuss muscular dystrophy, dilated cardiomyopathy, and the segmental aging disease Hutchinson-Gilford progeria syndrome [62, 63]. While lamin levels are altered in many cancers [44], most laminopathies do not correlate with increased cancer incidence or enhanced metastatic potential. This finding could be due to the severe cardiovascular disease and premature death associated with many laminopathies that likely preempts cancer progression, as well as the postulated role of lamins in cancer progression, rather than tumorigenesis.
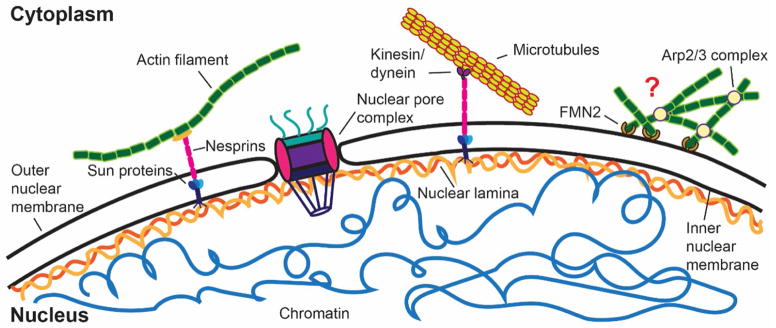
Box Figure.
Schematic overview of the NE and associated structures. The nuclear lamina interacts with inner nuclear membrane proteins, including chromatin and components of the LINC complex. The LINC complex, consisting of nesprins and SUN domain proteins, physically connects the nuclear interior with various cytoskeletal elements, including actin filaments, microtubules, and intermediate filaments (not shown). Perinuclear actin networks, mediated by FMN2 and Arp2/3, mediate nuclear deformation during confined migration, but their precise organization and composition remains to be determined.
Controlled nuclear envelope breakdown during mitosis and interphase
Nuclear envelope breakdown has been studied in extensive detail during mitosis, where the nuclear lamina disassembles in late prophase (prometaphase) in a highly regulated process [13]. In mitosis, disassembly of the NE is mediated by phosphorylation of nuclear pore proteins, lamins, and other NE proteins by protein kinase C (PKC) and cyclin dependent kinase 1 (Cdk1) [14, 15]. At this stage, the chromosomes are highly condensed, which protects the DNA upon exposure to the cytoplasm. Towards the end of mitosis, in late anaphase, the NE reassembles around the decondensing chromatin. Components of the endosomal sorting complex required for transport (ESCRT) machinery, ESCRT-III proteins CHMP2A, CHMP4B, and CHMP7 mediate the final resealing of the NE [16, 17].
Local nuclear envelope breakdown can also occur during interphase, using the same molecular mechanisms as employed during mitosis (reviewed in [15]). This was first demonstrated for HIV-infected cells, in which the viral Vpr protein triggers local disassembly of the nuclear lamina, resulting in NE herniation and rupture [18]. Similar loss of NE integrity is also seen when parvoviruses enter the nucleus by generating holes in both the nuclear membranes and the lamina, requiring Cdk2, caspase-3 and PKC-alpha activation for phosphorylation mediated lamina disassembly [19, 20]. Likewise, for the transport of large ribonucleoproteins that cannot proceed through the nuclear pores cells utilize similar local NE breakdown and budding of vesicles from the NE after PKC-mediated phosphorylation and disassembly of the nuclear lamina [21, 22].
Mechanically induced nuclear envelope rupture
Recent studies have started to look into the causes that lead to loss of NE integrity in interphase cells apart from the controlled, biochemically mediated NE breakdown events discussed above. These studies demonstrated that physical forces that deform the nucleus can lead to transient NE rupture at sites of local defects in the nuclear envelope. NE ruptures can be visualized by the escape of GFP tagged with a nuclear localization signal (NLS-GFP) from the nucleus into the cytoplasm, or entry of GFP with a nuclear export signal (NES-GFP) from the cytoplasm into the nucleus [6–10, 23]. Nucleo-cytoplasmic compartmentalization is typically restored within 10–90 minutes, although some cells exhibit permanent mislocalization of nuclear bodies to the cytoplasm or cytoplasmic organelles to the nucleus [6, 8]. Stunningly, most cells remain viable, even after repeated NE rupture, indicating efficient NE repair [7–10, 23].
Biomechanics of nuclear envelope ruptures
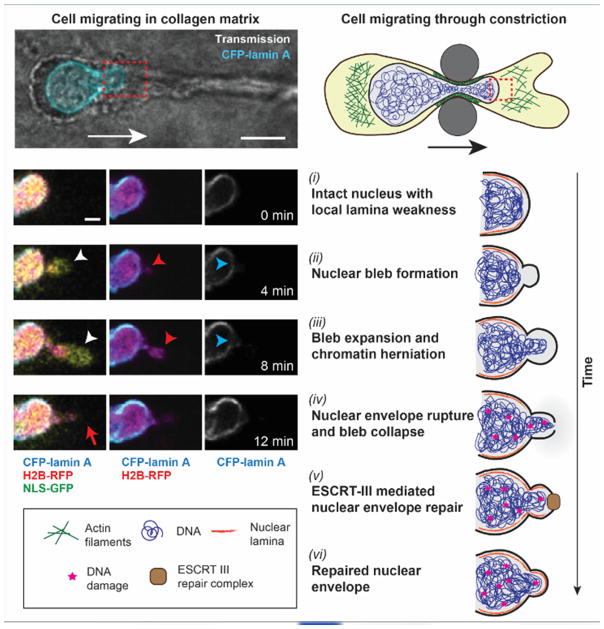
NE rupture, regardless of occurring spontaneously, induced by external compression, or migration through confined spaces, typically follows a sequence of events (Fig. 1): First, the nuclear membranes locally detach from the underlying nuclear lamina, resulting in the formation of a nuclear membrane ‘bleb’. These blebs are devoid of nuclear pores and many other NE proteins and commonly form at sites of local lamina weakness [6, 7, 24, 25]. In some cases, chromatin can protrude through the nuclear lamina into the blebs, forming ‘chromatin herniations’. Under continued nuclear pressure, the nuclear membrane bleb expands. Finally, the nuclear membrane in the bleb ruptures as it exceeds the critical areal strain for lipid membranes [7, 10], allowing uncontrolled exchange of soluble contents between the nucleus and cytoplasm through the membrane opening. In severe cases, the nucleus can fragment, with pieces of chromatin separating from the primary nucleus [7], resembling the formation of micronuclei.
Figure 1. Sequence of events during NE rupture and repair.
Example of a cell experiencing NE rupture and repair during confined migration. Left, time-lapse sequence of cell migrating through collagen matrix, exhibiting nuclear bleb formation (white arrowhead), chromatin protrusion (red arrowhead) through a defect in the nuclear lamina (blue arrowhead), and NE rupture (red arrow). Images reproduced from [7]. Right, schematic representation of events. Cytoskeletal forces mediated by contractile/branching actin filaments (green) compress the nucleus and mediate its transit through the constriction. Red dashed boxes indicate regions depicted in image sequence. (i) Nucleus before it enters the constriction. (ii) Compression of the nucleus by the cytoskeleton and external confinement increases intranuclear pressure, resulting in detachment of the nuclear membranes from the underlying nuclear lamina and nuclear bleb formation at sites of local defects in the lamina (orange lining) and high nuclear curvature. (iii) Continuous nuclear compression results in expansion of the nuclear membrane bleb. Chromatin can protrude through the lamina defect into the bleb. (iv) Rupture of the nuclear membrane in the bleb allows uncontrolled exchange between the nuclear and cytoplasmic compartments. Nuclear deformation and NE rupture result in DNA damage (red stars). (v) Nuclear membrane repair by the ESCRT-III machinery (brown rectangle). (vi) DNA damage persists even after the nucleus has passed through the constriction and NE integrity has been restored. Please note that while the figure depicts NE rupture during confined migration, similar events occur during external compression of cells, and by compression of the nucleus through perinuclear actomyosin networks in adherent, but otherwise unconfined cells in 2D culture.
In cells migrating through tight constrictions, the nuclear blebs and NE rupture most often occur at the leading edge of the deformed nucleus, which also has the highest membrane curvature [7, 10]. The formation of nuclear blebs, which closely resembles the formation of plasma membrane blebs during amoeboid migration [26], and the observation that nuclear blebs immediately collapse upon NE rupture [7] suggest that these events are driven by an increase in intranuclear pressure. Although the NE contains thousands of nuclear pores, measurements in living cells demonstrate that cells can sustain a pressure gradient across the NE [27, 28].
A number of studies point to a converging picture of mechanical compression of the nucleus as a major factor leading to NE rupture. External physical compression of cells below 3.5 μm using microindentors or microfluidic devices had been shown previously to induce chromatin protrusion and NE rupture [24, 29]. Recent findings indicate that cytoskeletal forces can similarly compress the nucleus in adherent cells on 2-D substrates as well as during confined migration [7, 9, 10, 23, 30]. During transmigration through tight spaces, perinuclear contractile actomyosin networks that depend on non-muscle myosin IIA and IIB assist in moving the cell nucleus through the constriction [31–33]. During this process, the nucleus is squeezed substantially, putting enormous stress on the nucleus and NE [12, 34]. In adherent cells, perinuclear actomyosin structures and stress fibers spanning the nucleus may impose similar confinement as cells encounter during 3D migration [9]. In both cases, inhibition of actomyosin contractility and actin fiber formation can reduce or abolish NE ruptures [7, 9]. It remains unclear whether similar mechanical stress also contributes to the spontaneous NE rupture in micronuclei.
Perinuclear actin polymerization during confined migration can contribute to nuclear deformation but also help protect NE integrity, with cell-type dependent differences. In fast moving dendritic cells, Arp2/3 mediated actin nucleation around the nucleus facilitates migration through confined spaces in vitro and in vivo by increasing nuclear deformation [35]. In melanoma cells, the membrane-associated formin FMN2 helps in the formation of perinuclear actin structures that help move the nucleus through tight constrictions [11]. These FMN2-induced actin structures also protect the nucleus from catastrophic NE rupture and DNA damage during confined migration, thereby promoting cell survival and cancer metastasis [11]. The reason why nuclear envelope rupture in FMN2 depleted cells is permanent and lethal, whereas nuclear envelope rupture in most other cases is transient and non-lethal, remains unclear. Possible explanations include a still unknown role of FMN2 in NE repair, or NE damage in FMN2-deficient cells that is so extensive that it cannot be sufficiently repaired.
The role of nuclear envelope proteins in NE rupture
Besides the degree of nuclear confinement, the likelihood of NE rupture is determined by the strength and structure of the NE. The major component in regulating the structure and integrity of the NE are lamins [24, 36], with reduced levels of lamin A/C resulting in more deformable nuclei that are more prone to NE rupture [7, 10, 24, 36–38]. While the B-type lamins, lamins B1 and B2, have a less pronounced effect on nuclear stiffness [36, 39], loss of B-type lamins increases the likelihood of nuclear bleb formation and NE rupture [6, 7, 9, 40]. Even in cells expressing B-type lamins, nuclear membrane blebs form at sites with openings in the lamin B-network [7, 8]. These effects may be attributed at least in part to the membrane-tethering role of B-type lamins, which contain a farnesyl group at their C-terminus that anchors them to the inner nuclear membrane. Consequently, at sites lacking B-type lamins, the nuclear membrane could be more prone to detachment and bleb formation.
Apart from lamins, LINC complex proteins, which physically connect the nucleus and cytoskeleton (Box 1), can also affect NE rupture [9]. LINC complex proteins are not only crucial for force transmission from the cytoskeleton to the nucleus, but also in recruiting nuclear and cytoskeletal proteins to the NE (reviewed in [41]). Disrupting LINC complex function reduces the frequency of NE rupture in cells on 2-D substrates by disrupting perinuclear actin organization and thereby reducing nuclear confinement [9], further supporting a pressure-driven model of NE rupture.
Changes of NE composition in cancer cells
Abnormal nuclear morphology has been recognized as a tell-tale sign of cancer cells since the early 1800s, and continues to serve as an important diagnostic tool [42]. More recently, it has emerged that many cancers also have altered expression of lamins that can correlate with clinical outcome. For example, skin and ovarian cancer often have higher expression of lamins A/C, whereas leukemia, lymphoma, breast cancer, colon cancer, gastric carcinoma, and some ovarian carcinoma have lower expression levels (reviewed in [42–44]). Reduced lamin-A/C levels may not only promote invasion through small interstitial spaces by increasing nuclear deformability [38, 45, 46], but also increase the rate of spontaneous NE rupture in cancer cells on 2-D substrates and during confined 3-D migration [6, 7, 9, 12, 23]. In addition to their importance in providing structural support to the cell nucleus, lamin expression can also affect the formation and stability of micronuclei. Disorganization of the nuclear lamina, specifically lamin B1 deregulation, leads to NE rupture in micronuclei [4]. Lamin B1 levels are also low in the NE surrounding chromatin bridges and leads to rupture of the primary nucleus in those cells [47].
Consequences of nuclear envelope rupture
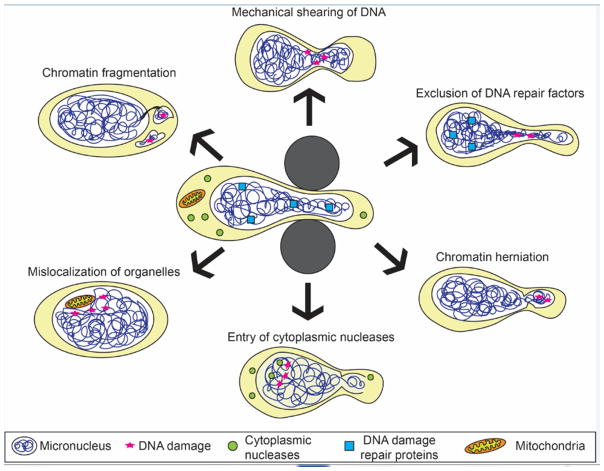
Nuclear deformation and NE rupture present severe challenges to the integrity of genomic DNA (Fig. 2). NE rupture caused by migration through confined environments results in DNA double strand breaks, which can be detected by staining for the DNA damage repair markers gamma-H2AX, or by monitoring accumulation of fluorescently labeled DNA damage marker 53BP1 [7, 10, 12]. Live cell imaging reveals 53BP1 accumulation within minutes of NE rupture, both near the NE rupture site but also within the nuclear interior [7, 10, 12]. Chromatin herniation and fragmentation can further contribute to genomic instability, as the exposed chromatin is particularly prone to DNA damage [6, 7, 29]. These results mirror the frequent DNA damage seen in micronuclei after permanent loss of their NE integrity during S and G2 phase, including double strand DNA breaks and chromothripsis [4, 5]. Further support that NE rupture can result in DNA damage comes from gene expression studies that compared cells subjected to mild compression, which did not induce NE rupture, to cells subjected to more extensive compression. Cells that underwent more severe compression and exhibited abundant NE ruptures displayed increased expression of genes involved in DNA damage response [29].
Figure 2. Factors contributing to mechanically induced genomic instability.
Compression of the nucleus, for example during migration through confined spaces (center), results in severe nuclear deformation and often leads to NE rupture. Depicted are some proposed mechanisms by which nuclear deformation and NE rupture can contribute to DNA damage (red star) and promote genomic instability. Mechanical shearing of DNA could result in DNA damage even in the absence of NE rupture. Squeezing of the nucleus through tight spaces can also lead to exclusion of mobile DNA repair proteins from the confined areas, which can delay DNA damage repair and add to genomic instability. Chromatin protrusion across the nuclear lamina can expose DNA to the cytoplasm, resulting in DNA damage. In severe cases, chromatin can become fragmented and separated from the main nucleus. NE rupture allows uncontrolled exchange between the nucleus and the cytoplasm, exposing DNA to cytoplasmic nucleases that can damage the DNA. In some instances, cytoplasmic organelles such as mitochondria can also enter the nucleus and cause DNA damage by producing reactive oxygen species. Mislocalization of organelles and chromatin fragmentation can persist even after cells have passed through the confined space and NE integrity has been restored. Note that the proposed mechanisms are not mutually exclusive and may have synergistic or additive effects on genomic instability.
While short-term DNA damage caused by NE rupture is well documented, the long-term consequences remain to be investigated. Non-homologous end-joining of double strand DNA breaks during G1 phase is likely to result in genomic deletions or even genomic translocation. The fraction of cancer cells with fragmented nuclei remains elevated even after transit through microfluidic constrictions, suggesting persistent fragmentation [7]. Furthermore, repeated transit of cancer cells through narrow (3 μm diameter) pores results in increased DNA mutations and chromosome copy number alterations not seen in cells that had passed through larger pores [48]. These findings suggest that migration through tight spaces and NE rupture can promote genomic instability in cancer cells.
The precise mechanisms leading to DNA damage in these conditions, however, remains incompletely understood. One possible explanation is that loss of nucleo-cytoplasmic compartmentalization allows entry of cytoplasmic proteins into the nucleus. In particular, access of cytoplasmic nucleases, which typically protect the cell from foreign DNA, could result in creation of double strand breaks in genomic DNA. Unlike NE breakdown during mitosis, when chromatin is highly condensed, much of interphase chromatin is accessible to nucleases [49]. Rapid accumulation of a fluorescently labeled cytoplasmic DNA-binding protein at the site of NE rupture confirms that genomic DNA becomes accessible to the cytoplasm following NE rupture [7, 10]. At the same time, it remains unclear which cytoplasmic nucleases may contribute to the DNA damage observed after NE rupture. The nuclease TREX1 has previously been reported to create single stranded DNA following NE rupture caused by chromatin bridges [47]. Furthermore, the DNA fragments created by TREX1 rearrange themselves randomly, leading to chromothripsis, which can possibly add to the genomic instability of cancer cells [47]. Other, yet to be identified nucleases could have similar effects on nuclear DNA following NE rupture. In severe cases, mitochondria can mislocalize into the nucleus following NE rupture [6]. If the mitochondria remain functional, their presence could further contribute to DNA damage by producing reactive oxygen species (ROS), which are known DNA damage agents. This effect could be exacerbated in lamin A/C-deficient cells, which have an increased sensitivity to ROS [50, 51].
While DNA damage typically follows NE rupture [7, 10], extensive nuclear deformation during confined migration alone may be sufficient to cause DNA damage in some cells, even without NE rupture [7, 12]. It is unlikely that intranuclear forces can directly snap DNA strands. Instead, the shearing of DNA during nuclear deformation could make the DNA more prone to damage, or result in stalling of replication forks. These effects could be particularly prominent in lamin A/C-deficient cells, which have more deformable nuclei [36], are more prone to NE rupture [7, 8], and are more sensitive to DNA damage and replication stress [49]. Furthermore, compression of the nucleus during transit through tight spaces squeezes out soluble DNA repair proteins from the confined area, including BRCA1, 53BP1, the MRN complex, and KU proteins, which are some of the first proteins complexes that detect and bind to DSBs to mediate repair [12]. The exclusion limits access to the DNA within the compressed region and delays DNA damage repair [12].
In addition to effects on genomic stability, nuclear deformation and NE rupture could also affect various other nuclear functions. NE rupture results in transient mislocalization or loss of transcription factors, DNA repair factors, and other soluble proteins from the nucleus [8, 12], while allowing uncontrolled influx of cytoplasmic molecules. Although transcription factors may require additional regulation to activate transcription, NE ruptures could alter transcriptional activity, epigenetic regulation, and chromatin organization, with both short- and long-term consequences. Future studies will be needed to assess the effects on cellular function, including migration, proliferation, and viability, and how cells differ in their response to NE rupture.
Nuclear envelope repair
Cells exhibiting NE rupture quickly restore nucleo-cytoskeletal compartmentalization and remain viable, suggesting that they can efficiently reseal the NE. Nuclear membrane repair requires the endosomal sorting complex required for transport (ESCRT) machinery, including the ESCRT-III proteins CHMP2B, CHMP4B, and CHMP7, as well as the ESCRT-associated AAA ATPase VPS4B [7, 10, 12]. These proteins, which are also involved in resealing the reforming NE in late anaphase [16, 17], are rapidly recruited to sites of NE rupture [7, 10], and inhibition of the ESCRT-III machinery significantly delays NE repair [7, 10, 12, 23]. Currently, it remains unclear which molecular events trigger accumulation of ESCRT-III proteins following NE repair, and whether additional mechanisms contribute to DNA repair. Interestingly, depletion of the formin FMN2 results in catastrophic, irreversible NE rupture, DNA damage, and cell death during confined migration [11], suggesting that either FMN2 is critical for NE repair, or that absence of FMN2 and the associated perinuclear actin network lead to such extensive NE damage that cannot be overcome. Analysis of plasma membrane repair suggests that ESCRT-III mediated membrane repair is limited to openings of <100 nm in diameter [52], consistent with the estimated size of transient NE ruptures in migrating cells [7]. The reported mislocalization of micron-sized organelles such as mitochondria [6] and PML bodies [8, 53], however, suggests that cells can overcome larger NE ruptures as well, but it remains unclear whether the repair of such large NE ruptures also involves the ESCRT machinery or other, still unknown mechanisms.
Given the importance of maintaining/restoring NE integrity, inhibiting NE repair could present a novel therapeutic strategy to target metastatic cancer cells. Intriguingly, inhibiting the ESCRT machinery, along with DNA damage repair pathways, dramatically reduced cell viability in migrating cancer cells after NE rupture, while inhibiting each pathway separately had no/limited effect [7]. Normal epithelial RPE-1 cells also exhibited a similar response to combined ESCRT-III/DNA damage repair inhibition [10]. Furthermore, since ESCRT-III proteins are also required for NE resealing during mitosis, more research will be required to identify potential targets specific to cancer cells.
Concluding remarks
Genomic instability has long been recognized as a driver of cancer progression and resistance to intervention, motivating research to better understand the underlying mechanisms. Recent findings suggest that transient loss of NE integrity, resulting from cytoskeletal forces acting on the nucleus, provides a novel mechanism that could contribute to the increased genomic instability of cancer cells. Metastasizing cancer cells that encounter tight interstitial spaces or narrow openings during intra- and extravasation would be particularly prone to NE rupture, but cancer cells also undergo spontaneous NE rupture, which could be further increased by elevated pressure in solid tumors [54]. The emerging role of NE rupture on genomic stability, along with increasing findings of altered lamin levels in cancers and the well-recognized power of abnormal nuclear morphology in cancer cells for diagnosis and prognosis, have turned nuclear envelope structure and dynamics into a burgeoning field of interest. Nonetheless, many open questions remain (see Outstanding Questions box). Although defects in nuclear lamina organization and nuclear compression by the actin cytoskeleton have been identified as some key factors underlying NE rupture, the role of other molecular components in promoting/preventing NE rupture needs to be elucidated further. Furthermore, the short-term and long-term consequences of NE rupture on the cells, including genomic integrity, gene expression, and other cellular functions, remain important areas for future studies, particularly in cancer cells. Identifying the molecular details of NE resealing by ESCRT proteins and other, yet to be identified NE repair mechanisms, will not only help improve our understanding of basic cell biology, but may also provide new avenues for therapy developments. Exploiting this new field of study to develop therapeutic targets for cancer, especially metastatic cancer cells, which shows higher signs of nuclear rupture, will be an exciting journey ahead for translational cancer research.
Outstanding Questions.
How does NE rupture cause DNA damage? Is it through the influx of cytoplasmic nucleases, escape of nuclear repair factors, mechanical shearing of DNA, or a combination of these mechanisms? And can nuclear deformation alone be sufficient to induce DNA damage?
What are the long-term effects of NE rupture on genomic instability? Do the chromosomal fragments produced during NE rupture persist for long and are they divided unequally during mitosis? Can these fragments lead to persistent DNA alterations and/or aneuploidy?
Are there specific DNA repair pathways that become activated following NE rupture? Do these pathways affect cell viability and migration efficiency (for example, by recruitment of SUN domain proteins as part of the DNA damage response) during confined migration?
Can nuclear envelope rupture impact on nuclear functions such as transcriptional regulation, chromatin organization, and chromatin modifications? And if so, are such changes only short-term, or can they persist for longer times?
Other than changes in lamin levels and organization, are there specific structural features in the nuclear envelope or the surrounding cytoskeleton that make some cells particularly resistant or prone to NE rupture?
Have particularly migratory cells such as immune cells or invasive cancer cells developed specific mechanisms to deal with NE rupture?
How does the ESCRT machinery sense the NE rupture? What cellular pathway is followed to initiate NE repair? Is it similar to the one during mitosis?
Are there additional/alternative pathways that mediate NE repair? Can inhibiting NE repair present a viable approach to specifically target metastatic cancer cells?
Trends Box.
Cells exhibit transient NE rupture during migration through confined spaces and actomyosin-based compression, with the incidence of NE rupture increasing with nuclear confinement.
NE rupture is preceded by nuclear membrane blebbing from the nuclear lamina, similar to plasma membrane blebbing at the cell cortex.
Nuclear membrane blebbing and NE rupture are driven by intranuclear pressure, resulting from perinuclear actomyosin structures that compress the nucleus. At the same time, perinuclear actin structures associated with Arp2/3 and/or the formin FMN2 may promote nuclear transit through constrictions and prevent NE rupture and DNA damage.
NE rupture allows the uncontrolled exchange between nuclear and cytoplasmic content, which, together with mechanical deformation of the nucleus, can lead to chromatin protrusion/fragmentation and DNA damage that promote genomic instability.
ESCRT-III proteins mediate NE repair, and inhibiting this machinery along with DNA damage repair pathways reduces cell viability after NE rupture.
Acknowledgments
The authors thank Emily Bell and Philipp Isermann for helpful discussion. The authors apologize to all investigators whose work could not be cited due to space constraints. This work was supported by awards from the National Institutes of Health (R01 HL082792 and U54 CA210184, to J.L.), the Department of Defense Breast Cancer Research Program (Breakthrough Award BC150580, to J.L.), the National Science Foundation (CAREER Award CBET-1254846, to J.L.), and the Netherlands Science Organization (NWO-VIDI 917.10.364 to K.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Negrini S, et al. Genomic instability--an evolving hallmark of cancer. Nature reviews Molecular cell biology. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera A, Garcia-Muse T. Causes of genome instability. Annual review of genetics. 2013;47:1–32. doi: 10.1146/annurev-genet-111212-133232. [DOI] [PubMed] [Google Scholar]
- 4.Hatch EM, et al. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang CZ, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas JD, et al. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2012;3:88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denais CM, et al. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vos WH, et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Human molecular genetics. 2011;20:4175–4186. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- 9.Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. The Journal of cell biology. 2016;215:27–36. doi: 10.1083/jcb.201603053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raab M, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- 11.Skau CT, et al. FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis. Cell. 2016;167:1571–1585. e1518. doi: 10.1016/j.cell.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Irianto J, et al. Nuclear constriction segregates mobile nuclear proteins away from chromatin. Molecular biology of the cell. 2016;27:4011–4020. doi: 10.1091/mbc.E16-06-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smoyer CJ, Jaspersen SL. Breaking down the wall: the nuclear envelope during mitosis. Current opinion in cell biology. 2014;26:1–9. doi: 10.1016/j.ceb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Guttinger S, et al. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nature reviews Molecular cell biology. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 15.Hatch E, Hetzer M. Breaching the nuclear envelope in development and disease. The Journal of cell biology. 2014;205:133–141. doi: 10.1083/jcb.201402003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olmos Y, et al. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. doi: 10.1038/nature14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vietri M, et al. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [DOI] [PubMed] [Google Scholar]
- 18.de Noronha CM, et al. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294:1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- 19.Porwal M, et al. Parvoviruses cause nuclear envelope breakdown by activating key enzymes of mitosis. PLoS pathogens. 2013;9:e1003671. doi: 10.1371/journal.ppat.1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, et al. Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle. Journal of virology. 2011;85:4863–4874. doi: 10.1128/JVI.01999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunwald D, et al. Nuclear export dynamics of RNA-protein complexes. Nature. 2011;475:333–341. doi: 10.1038/nature10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speese SD, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robijns J, et al. In silico synchronization reveals regulators of nuclear ruptures in lamin A/C deficient model cells. Scientific reports. 2016;6:30325. doi: 10.1038/srep30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broers JL, et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Human molecular genetics. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 25.Bercht Pfleghaar K, et al. Gene-rich chromosomal regions are preferentially localized in the lamin B deficient nuclear blebs of atypical progeria cells. Nucleus. 2015;6:66–76. doi: 10.1080/19491034.2015.1004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergert M, et al. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14434–14439. doi: 10.1073/pnas.1207968109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrie RJ, et al. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science. 2014;345:1062–1065. doi: 10.1126/science.1256965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neelam S, et al. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5720–5725. doi: 10.1073/pnas.1502111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Berre M, et al. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integrative biology: quantitative biosciences from nano to macro. 2012;4:1406–1414. doi: 10.1039/c2ib20056b. [DOI] [PubMed] [Google Scholar]
- 30.Lammerding J, Wolf K. Nuclear envelope rupture: Actin fibers are putting the squeeze on the nucleus. The Journal of cell biology. 2016;215:5–8. doi: 10.1083/jcb.201609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas DG, et al. Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. The Journal of cell biology. 2015;210:583–594. doi: 10.1083/jcb.201502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf K, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. The Journal of cell biology. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung WC, et al. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. The Journal of cell biology. 2013;202:807–824. doi: 10.1083/jcb.201302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao X, et al. A Chemomechanical Model for Nuclear Morphology and Stresses during Cell Transendothelial Migration. Biophysical journal. 2016;111:1541–1552. doi: 10.1016/j.bpj.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiam HR, et al. Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nature communications. 2016;7:10997. doi: 10.1038/ncomms10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammerding J, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. The Journal of clinical investigation. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada T, et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. The Journal of cell biology. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrera D, et al. Lamin B1 overexpression increases nuclear rigidity in autosomal dominant leukodystrophy fibroblasts. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:3906–3918. doi: 10.1096/fj.13-247635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lammerding J, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. The Journal of biological chemistry. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 41.Chang W, et al. Accessorizing and anchoring the LINC complex for multifunctionality. The Journal of cell biology. 2015;208:11–22. doi: 10.1083/jcb.201409047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Las Heras JI, Schirmer EC. The nuclear envelope and cancer: a diagnostic perspective and historical overview. Advances in experimental medicine and biology. 2014;773:5–26. doi: 10.1007/978-1-4899-8032-8_1. [DOI] [PubMed] [Google Scholar]
- 43.Irianto J, et al. Nuclear lamins in cancer. Cellular and molecular bioengineering. 2016;9:258–267. doi: 10.1007/s12195-016-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell ES, Lammerding J. Causes and consequences of nuclear envelope alterations in tumour progression. European journal of cell biology. 2016;95:449–464. doi: 10.1016/j.ejcb.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowat AC, et al. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. The Journal of biological chemistry. 2013;288:8610–8618. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson PM, et al. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cellular and molecular bioengineering. 2014;7:293–306. doi: 10.1007/s12195-014-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maciejowski J, et al. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irianto J, et al. DNA Damage Follows Repair Factor Depletion and Portends Genome Variation in Cancer Cells after Pore Migration. Current biology: CB. 2017;27:210–223. doi: 10.1016/j.cub.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cusanovich DA, et al. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348:910–914. doi: 10.1126/science.aab1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pekovic V, et al. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging cell. 2011;10:1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 51.Sieprath T, et al. Sustained accumulation of prelamin A and depletion of lamin A/C both cause oxidative stress and mitochondrial dysfunction but induce different cell fates. Nucleus. 2015;6:236–246. doi: 10.1080/19491034.2015.1050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 53.Houben F, et al. Cytoplasmic localization of PML particles in laminopathies. Histochemistry and cell biology. 2013;139:119–134. doi: 10.1007/s00418-012-1005-5. [DOI] [PubMed] [Google Scholar]
- 54.Jain RK, et al. The role of mechanical forces in tumor growth and therapy. Annual review of biomedical engineering. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burke B, Stewart CL. Functional architecture of the cell’s nucleus in development, aging, and disease. Current topics in developmental biology. 2014;109:1–52. doi: 10.1016/B978-0-12-397920-9.00006-8. [DOI] [PubMed] [Google Scholar]
- 56.Mejat A, Misteli T. LINC complexes in health and disease. Nucleus. 2010;1:40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alam S, et al. Nuclear forces and cell mechanosensing. Progress in molecular biology and translational science. 2014;126:205–215. doi: 10.1016/B978-0-12-394624-9.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma. 2013;122:13–31. doi: 10.1007/s00412-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund E, et al. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome research. 2013;23:1580–1589. doi: 10.1101/gr.159400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lottersberger F, et al. 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DNA Repair. Cell. 2015;163:880–893. doi: 10.1016/j.cell.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zwerger M, et al. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Human molecular genetics. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chojnowski A, et al. Nuclear lamina remodelling and its implications for human disease. Cell and tissue research. 2015;360:621–631. doi: 10.1007/s00441-014-2069-4. [DOI] [PubMed] [Google Scholar]
- 63.Broers JL, Ramaekers FC. The role of the nuclear lamina in cancer and apoptosis. Advances in experimental medicine and biology. 2014;773:27–48. doi: 10.1007/978-1-4899-8032-8_2. [DOI] [PubMed] [Google Scholar]