Abstract
Objective
Incomplete lupus erythematosus (ILE) involves clinical and/or serologic manifestations consistent with but insufficient for SLE classification. Because the nature of ILE is poorly understood and no treatment recommendations exist, we examined clinical manifestations, medication history, and immunologic features in a diverse collection of ILE and SLE patients.
Methods
Medical records of subjects enrolled to the Lupus Family Registry and Repository were reviewed for medication history and American College of Rheumatology (ACR) classification criteria to identify ILE patients (3 ACR criteria; n=440) and SLE patients (≥4 ACR criteria; n=3,397). Participants completed the connective tissue disease screening questionnaire (CSQ). Anti-cardiolipin and plasma BLyS were measured by ELISA, anti-nuclear antibodies by indirect immunofluorescence, and 13 autoantibodies by bead-based assays.
Results
ILE patients were older than SLE patients (46.2 vs. 42.0 y, P<0.0001), and fewer ILE patients were African American (23.9% vs. 32.2%, P<0.001). ILE patients exhibited fewer autoantibody specificities (1.3 vs. 2.6, P<0.0001) than SLE patients and were less likely to have ANA titers ≥1:1080 (10.5% vs. 19.5%,P<0.0001). BLyS levels were intermediate in ILE patients (controls<ILE, P=0.016; ILE<SLE, P=0.008). Pericarditis, renal, or neurologic manifestations occurred in 12.5% of ILE patients and associated with non-European American race/ethnicity (P=0.012). Hydroxychloroquine use increased over time, but was less frequent in ILE than SLE patients (65.2% vs. 83.1%, P<0.0001).
Conclusion
Although usually characterized by milder symptoms, ILE manifestations may require immunomodulatory treatments. Longitudinal studies are necessary to understand how ILE impacts organ damage and future SLE risk, and to delineate molecular pathways unique to ILE.
Keywords: incomplete lupus erythematosus (ILE), systemic lupus erythematosus, undifferentiated connective tissue disease, preclinical lupus, autoantibodies, soluble mediators
Patients with incomplete lupus erythematosus (ILE) exhibit heterogeneous clinical and serologic manifestations consistent with aspects of systemic lupus, yet insufficient for SLE classification.(1–4) Many ILE patients follow a relatively mild disease course.(5) However, approximately 20% of ILE patients transition to classified SLE,(5–8) indicating that in some patients, ILE may represent an early stage of SLE.(9, 10) Moreover, even without reaching SLE classification, ILE patients may accrue irreversible tissue damage(11, 12) and lupus-associated complications resulting in hospitalization.(13) The use of immunosuppressants such as azathioprine, cyclophosphamide, and mycophenolate mofetil in ILE cohorts(7, 13) further suggests that some ILE patients have more serious clinical manifestations and are at risk for permanent organ damage. Improved understanding of ILE might help optimize treatment for patients at higher risk of major clinical disease or transition to SLE while also avoiding unnecessary treatment toxicities in patients who are likely to continue with a stable, mild condition.(9, 14)
No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE. Several single-center cohort studies have established ANA positivity, arthritis, hematologic involvement, and immunologic involvement as common features of ILE.(7, 10–13) Less commonly, ILE may involve clinical manifestations associated with permanent organ damage.(7, 10–13) However, because of their limited racial/ethnic composition, these cohorts may not fully reflect the clinical presentation of ILE in more diverse patient populations.
Insights into the immunopathology of ILE would support the establishment of evidence-based treatment regimens for ILE patients. Patterns of immune dysregulation in ILE are suspected to coincide with those observed in SLE.(15) For example, lupus-associated autoantibodies(16–18) and soluble mediator dysregulation(19, 20) accumulate prior to clinical SLE onset; these autoantibodies are also observed in ILE,(5, 7, 8, 11–13, 21) along with some evidence of soluble mediator dysregulation.(22, 23) On the other hand, unique immunologic features that distinguish SLE patients from ILE patients or define subsets of ILE patients may influence their disease course. Therefore, focused studies are needed to understand the relationships between autoantibody positivity, soluble mediators, and clinical disease in ILE patients and ILE patient subsets.
The goals of this study were to characterize the clinical and serological features of a large collection of geographically and racially/ethnically diverse ILE patients, to assess current treatment strategies for ILE, and to evaluate serologic characteristics of ILE patients compared to SLE patients and healthy controls.
PATIENTS AND METHODS
Study Cohort and Characterization
This study was performed in accordance with the Helsinki Declaration and approved by the OMRF Institutional Review Board. Study participants included patients and healthy controls who were previously enrolled to the Lupus Family Registry and Repository (LFRR; 1995–2012) and provided written informed consent.(24) Healthy controls did not have a family member with lupus and were recruited from the community. Study participants were recruited from a wide geographic range, covering all 50 states in the US, as well as the District of Columbia, Puerto Rico, the British Virgin Islands, the US Virgin Islands, and six other countries.
Each participant completed the SLE portion of the connective tissue disease screening questionnaire (SLE-CSQ),(25, 26) as well as questionnaires providing detailed clinical, demographic, and therapeutic information. Medical records were reviewed by a rheumatologist or rheumatology-trained nurse for medication use and the 1997 American College of Rheumatology SLE classification criteria(2). Records were later re-reviewed for novel aspects of the 2012 SLICC SLE criteria(27). Participants were classified with SLE if they met four or more 1997 ACR SLE classification criteria, or with ILE for this study if they met three ACR classification criteria.(2) Because ACR criteria were the standard for SLE classification throughout the recruitment period (1995–2012), none of the ILE patients reached SLE classification at the time their medical records were generated. BLyS levels were determined in a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls cohort matched to ILE and SLE patients by race/ethnicity, gender, and age (±5 y).
Autoantibody and BLyS Detection
Serum and plasma samples were procured at the time of enrollment to the LFRR and were stored at −20°C; assays were performed on freshly thawed samples. Serum autoantibody positivity was assessed by the CAP (College of American Pathologists)-certified OMRF Clinical Immunology Laboratory. Anti-cardiolipin levels (IgG and IgM) were determined by enzyme-linked immunosorbent assay (ELISA) as previously described.(28) Indirect immunofluorescence to determine the presence of ANA and anti-dsDNA, and immunodiffusion to detect extractable nuclear antibodies were performed as previously described.(18)
Autoantibody specificities were also compared between ILE and SLE patients using an FDA-approved, multiplexed, bead-based assay system (BioPlex 2200, Bio-Rad, Hercules, CA).(29, 30) This system simultaneously detects autoantibodies against dsDNA, chromatin, ribosomal P, Ro/SSA (60kD and 52kD), La/SSB, Sm, SmRNP complex, RNP, centromere B, Scl-70, and Jo-1.(29) Anti-dsDNA was reported in IU/mL with a positive cut-off of 10 IU/mL as specified by the manufacturer. Other autoantibodies are reported as an Antibody Index (AI) value (range 0–8) based on the fluorescence intensity of each of the other autoantibody specificities, with a manufacturer-recommended positive cutoff of AI=1.(29) Serum BLyS levels were determined by ELISA (R&D Systems, Minneapolis, MN) per manufacturer’s protocol, with a positive cutoff of 812.7 pg/mL determined by receiver-operator characteristic (ROC) curves maximizing the Youden Index (J).(31)
Statistical Analyses
For comparisons between ILE and SLE, categorical variables were compared by Chi-square test or by Fisher test when fewer than ten events were expected; means were compared by unpaired t-test and medians by Mann-Whitney test. Logistic regression was performed to compare autoantibody specificities between ILE and SLE or between ILE patients with and without major clinical manifestations while accounting for race/ethnicity, and to compare CSQ responses between ILE and SLE, ILE and controls, and SLE and controls. BLyS levels were compared between SLE, ILE, and controls by Kruskal-Wallis test with Dunn’s multiple comparisons in GraphPad Prism version 6.04. All other tests were performed in R version 3.3.0 (R Foundation, http://www.r-project.org/).
RESULTS
Demographics of ILE and SLE patients
Medical record review and autoantibody testing were performed to define a group of patients with lupus manifestations insufficient for lupus classification, regardless of clinical diagnosis. Four hundred forty subjects were classified with ILE based on the presence of three ACR classification criteria. Another 3,397 individuals in the LFRR were classified with SLE based on the presence of ≥4 ACR classification criteria. Both groups were predominantly female (Table 1). ILE patients were on average older than SLE patients, and a lower percentage of ILE patients was African American (Table 1).
Table 1.
Demographics of SLE and ILE cohorts defined by the 1997 ACR SLE classification criteria.
| All SLE | All ILE | P* | ILE with major clinical features+ |
Other ILE | P* | |
|---|---|---|---|---|---|---|
| n | 3397 | 440 | 86 | 354 | ||
| Sex | ||||||
| Female, n (%) | 3050 (89.8) | 386 (87.7) | 0.213 | 72 (83.7) | 314 (88.7) | 0.280 |
| Age, y | ||||||
| Average (Range) | 42.0 (8–82) | 46.1 (9–81) | <0.001 | 42.8 (11–72) | 47.0 (9–81) | 0.010 |
|
Race/ethnicity, n (%) |
||||||
| European | 1518 (44.7) | 241 (54.8) | <0.001 | 36 (41.9) | 205 (57.9) | 0.010 |
| American | ||||||
| African American | 1093 (32.2) | 105 (23.9) | <0.001 | 27 (31.4) | 78 (22.0) | 0.092 |
| Hispanic | 244 (7.2) | 21 (4.8) | 0.076 | 6 (7.0) | 15 (4.2) | 0.269‡ |
| Asian American | 130 (3.8) | 18 (4.1) | 0.889 | 6 (7.0) | 12 (3.4) | 0.136‡ |
| American Indian | 104 (3.1) | 12 (2.7) | 0.812 | 2 (2.3) | 10 (2.8) | 1.0 |
| Pacific Islander | 5 (0.1) | 1 (0.2) | 0.519‡ | 0 (0.0) | 1 (0.3) | 1.0‡ |
| Mixed | 283 (8.3) | 40 (9.1) | 0.653 | 8 (9.3) | 32 (9.0) | 1.0 |
| Unknown | 20 (0.6) | 2 (0.5) | 1.0‡ | 1 (1.2) | 1 (0.3) | 0.353‡ |
Bold P-values are significant (P<0.05).
Pericarditis, renal disease, neurologic disease, hemolytic anemia, or thrombocytopenia.
Comparisons performed by Fisher’s test; all other categorical variables were compared by Chi-square and age by t-test.
The full spectrum of ACR SLE classification criteria is observed in ILE patients
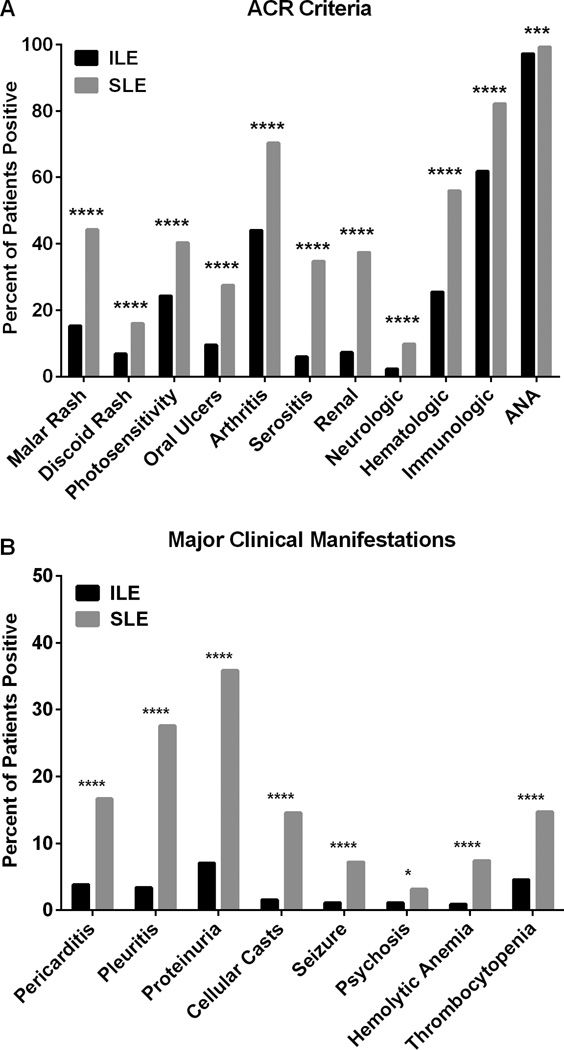
To identify clinical features of patients with ILE, we assessed ACR SLE classification criteria documented in the medical records. The most common ACR classification criteria observed in ILE patients were ANA positivity (97.3%), immunologic disorder (61.8%), arthritis (44.1%), and hematologic disorder (25.4%), but each ACR criterion was documented in at least some ILE patients (Figure 1A). Next, to investigate major clinical manifestations in ILE patients, we specifically examined clinical ACR criteria or sub-criteria that positively correlate with multiple indicators of disease severity.(32–34) These clinical criteria included serositis (pericarditis and pleuritis), renal (cellular casts and proteinuria), neurologic (seizures and psychosis), hemolytic anemia, and thrombocytopenia. Eighty-six ILE patients (19.5%) were affected by one or more of these manifestations (Figure 1B). European Americans were slightly under-represented among ILE patients with major clinical manifestations (41.9% European American) compared to ILE patients without major clinical manifestations (57.9% European American, P=0.0104). ILE patients with major clinical manifestations were younger than other ILE patients (42.8 y vs. 47.0 y, P=0.0101; Table 1). Approximately 7% of ILE patients (n=32) had documented findings of either proteinuria or renal casts, and 1.4% (n=6) met the ACR renal classification criterion plus one non-mucocutaneous, non-articular manifestation, including psychosis (n=2), pericarditis (n=1), seizures (n=1), hemolytic anemia (n=1), or thrombocytopenia (n=1).
Figure 1. SLE classification criteria in ILE patients and SLE patients.
Medical records of 440 ILE patients and 3,397 SLE patients were reviewed for the 1997 American College of Rheumatology (ACR) SLE classification criteria (A), including the major clinical sub-criteria of pericarditis, pleuritis, proteinuria, cellular casts, seizure, psychosis, hemolytic anemia, and thrombocytopenia (B). *P=0.027, ***P<0.001, ****P<0.0001
ILE patients and SLE patients report similar symptoms by questionnaire
To identify SLE symptoms self-reported by ILE patients, we evaluated their responses to the SLE-specific portion of the Connective Tissue Disease Screening Questionnaire (SLE-CSQ), a validated tool used to screen healthy populations for possible or probable lupus (3 or ≥4 positive responses, respectively).(25, 26) The SLE-CSQ defined 91.7% of ILE patients as possible or probable lupus cases, compared to 94.6% of SLE patients (OR 1.58 [95% CI 1.1–2.3], P=0.0195) and 12.1% of controls (OR 77.6 (53.8–112.3, P<0.0001). SLE patients reported slightly more symptoms than ILE patients (6.4 vs. 5.9, P<0.0001), and both ILE and SLE patients reported more SLE-related symptoms than healthy controls (Table 2). The number of reported symptoms did not differ between ILE patients with major clinical manifestations and ILE patients without major clinical manifestations (6.0 vs. 5.9, P=0.8582). Approximately half of ILE patients reported pleurisy (51.7%) and protein in urine (49.9%), and 14.3% of ILE patients reported seizure (Table 2). Symptoms self-reported through the SLE-CSQ corresponded to physician-documented ACR criteria in the medical records of 5.8% of ILE patients reporting pleurisy, 14.3% reporting protein in urine, and 4.8% reporting seizure. Similar results were obtained when African American and European American patients were analyzed separately (Supplementary Figure 1).
Table 2.
Percent of individuals with positive responses to questions in the SLE-specific portion of the Connective Tissue Disease Screening Questionnaire (SLE-CSQ).
| CSQ Response |
SLE, % n=3357 |
ILE, % n=432 |
HC, % n=1525 |
SLE vs. ILE OR (95%CI)P* |
ILE vs. HC OR (95%CI)P* |
SLE vs. HC OR (95%CI)P* |
|---|---|---|---|---|---|---|
| Cheek Rash | 55.6 | 51.2 | 4.0 | 1.2 (1.0–1.5) 0.080 |
25.1 (18.2–34.5) <0.0001 |
30.1 (23.0–39.2) <0.0001 |
| Discoid Lupus | 2.0 | 2.3 | 0.0 | 0.8 (0.4–1.6) 0.627 |
NC 0.970 |
NC 0.971 |
| Sun Sensitivity | 61.9 | 58.6 | 7.1 | 1.2 (0.9–1.4) 0.176 |
18.4 (14.0–24.1) <0.0001 |
21.1 (17.2–26.0) <0.0001 |
| Mouth Sores | 52.1 | 50.0 | 6.2 | 1.1 (0.9–1.3) 0.411 |
15.1 (11.4–20.0) <0.0001 |
16.4 (13.2–20.4) <0.0001 |
| Arthritis | 78.8 | 78.2 | 19.0 | 1.0 (0.8–1.3) 0.793 |
15.4 (11.8–20.0) <0.0001 |
15.9 (13.6–18.5) <0.0001 |
| Pleurisy | 58.2 | 51.9 | 9.0 | 1.3 (1.0–1.6) 0.012 |
10.9 (8.4–14.1) <0.0001 |
14.1 (11.7–17.0) <0.0001 |
| Protein in Urine | 65.1 | 50.0 | 9.0 | 1.9 (1.5–2.3) <0.0001 |
10.1 (7.8–13.1) <0.0001 |
18.9 (15.6–22.8) <0.0001 |
| Seizure | 19.4 | 14.4 | 2.8 | 1.4 (1.1–1.9) 0.012 |
5.9 (3.9–8.9) <0.0001 |
8.5 (6.2–11.6) <0.0001 |
| Low Blood Counts |
76.6 |
66.0 |
23.5 |
1.7 (1.4–2.1) <0.0001 |
6.3 (5.0–8.0) <0.0001 |
10.7 (9.3–12.3) <0.0001 |
| Positive ANA | 62.6 | 67.6 | 1.0 | 0.8 (0.6–1.0) 0.044 |
210 (122–363) <0.0001 |
169 (101–282) <0.0001 |
| Cold Sensitivity | 58.5 | 57.4 | 9.2 | 1.0 (0.8–1.3) 0.655 |
13.3 (10.3–17.3) <0.0001 |
14.0 (11.6–16.8) <0.0001 |
| Rapid Hair Loss |
52.2 |
47.2 |
5.7 |
1.2 (1.0–1.5) 0.049 |
14.8 (11.1–19.7) <0.0001 |
18.1 (14.4–22.7) <0.0001 |
Bold P-values significant (P<0.05) by logistic regression.
HC: Healthy Controls.
NC: Not calculable due to 0 value.
Use of hydroxychloroquine and steroids is common among ILE patients
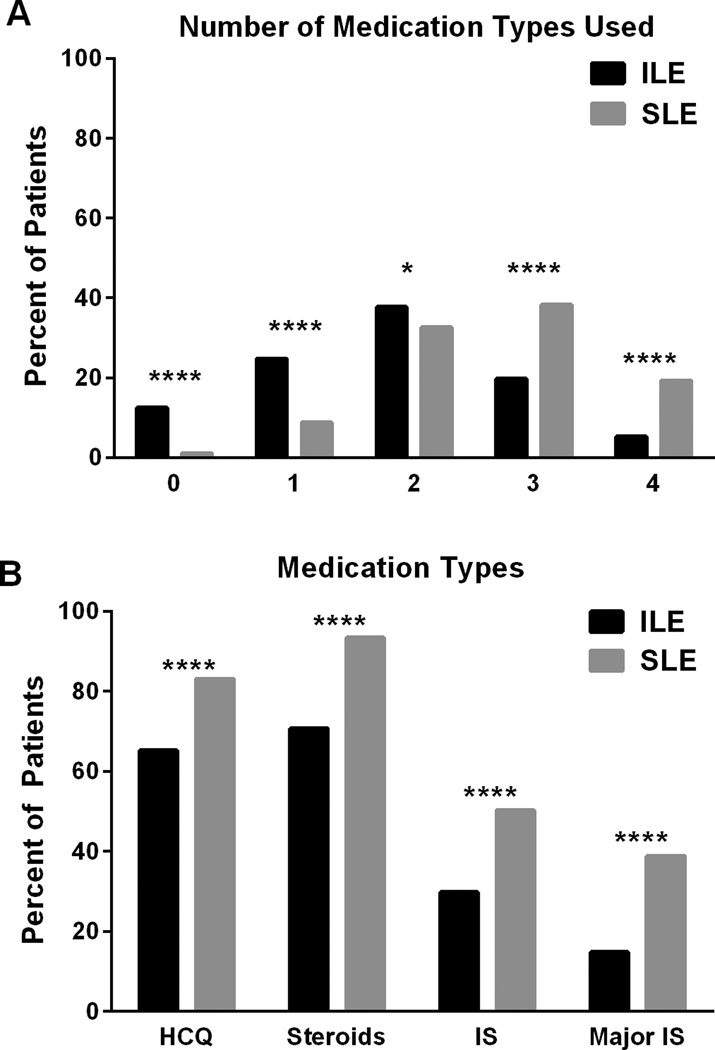
To better understand treatment of ILE patients in clinical practice, we examined medical records for use of hydroxychloroquine, steroids, immunosuppressants (methotrexate, azathioprine, and sulfasalazine), and major immunosuppressants (mycophenolate mofetil, cyclophosphamide). Compared to SLE patients, ILE patients used fewer medication types (1.8 vs. 2.6, P<0.0001; Figure 2B) and were less likely to have used each type of medication (P<0.0001; Figure 2A), despite increases in hydroxychloroquine use in both ILE patients and SLE patients from 1992–1996 to 2008–2012 (Supplementary Figure 2). Nonetheless, a large majority of ILE patients had been treated with hydroxychloroquine (65.2%) and/or steroids (70.7%). In addition, 29.8% of ILE patients had used immunosuppressants, and 14.8% had used major immunosuppressants (Figure 2A).
Figure 2. Use of anti-malarials, steroids, immunosuppressants, and major immunosuppressants in ILE patients and SLE patients.
Medical records were reviewed for use of hydroxychloroquine (HCQ), steroids, immunosuppressants (IS; methotrexate, azathioprine, and sulfasalazine), and major IS (mycophenolate mofetil, cyclophosphamide). ILE and SLE patients were compared for the types (A) and numbers (B) of medications used. *P=0.034, ****P<0.0001.
ILE patients with a major clinical manifestation (serositis, renal disease, neurologic disease, hemolytic anemia, or thrombocytopenia) had used more medication types than other ILE patients (2.16 vs. 1.73, P=0.004), with increased use of major immunosuppressants (42.8% vs. 8.8%, OR 7.8 [95% CI 4.3–13.8], P<0.0001). These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants.
Autoantibody prevalence and concentration are lower in ILE patients
Finally, we tested whether immunologic features associated with the underlying pathology of SLE were also present in ILE patients. Although nearly all ILE patients were ANA positive (97.2%) by indirect immunofluorescence, the prevalence of ANA positivity was significantly reduced in ILE patients compared to SLE patients (99.3%, P=0.0006; see Figure 1). Fewer ILE patients had ANA titers ≥1:1080 (10.5%, 46/439) compared to SLE patients (19.5%, 661/3391; P<0.0001, OR 2.03[1.6–2.5]). ILE patients were also less likely than SLE patients to show anti-dsDNA (9.1%, 40/439 vs. 27.0%, 918/3,391; P<0.0001, OR 3.7[2.6–5.3]) and other lupus-associated autoantibodies by indirect immunofluorescence (Supplementary Table 1). Approximately 42% of ILE patients had anti-cardiolipin, similar to SLE patients (Table 3).
Table 3.
Autoantibody specificities detected in SLE and ILE cohorts.
| Autoantibody n (%) |
SLE (n=2720) |
ILE (n=434) |
OR (95% CI)* |
Adj P+ |
ILE, maj clinical (n=86) |
Other ILE (n=348) |
OR (95% CI)* |
Adj P+ |
|---|---|---|---|---|---|---|---|---|
| Cardiolipin‡ | 1333 (39.6) | 179 (41.6) | 0.9 (0.7–1.1) |
0.322 | 36 (42.9) | 142 (41.2) | 1.0 (0.6–1.7) |
0.898 |
| dsDNA | 804 (29.6) | 48 (11.1) | 3.2 (2.4–4.4) |
<0.001 | 16 (18.6) | 32 (9.2) | 2.2 (1.1–4.3) |
0.102 |
| Chromatin | 1445 (53.1) | 103 (23.7) | 3.4 (2.7–4.4) |
<0.001 | 31 (36.0) | 72 (20.7) | 2.0 (1.2–3.3) |
0.102 |
| Ribosomal P | 357 (13.1) | 11 (2.5) | 5.3 (3.0–10.3) |
<0.001 | 1 (1.2) | 10 (2.9) | 0.4 (0.0–2.0) |
0.592 |
| Ro/SSA | 1065 (39.2) | 108 (24.9) | 1.8 (1.5–2.3) |
<0.001 | 21 (24.4) | 87 (25.0) | 0.9 (0.5–1.5) |
0.809 |
| La/SSB | 392 (14.4) | 39 (9.0) | 1.7 (1.2–2.5) |
0.004 | 9 (10.5) | 30 (8.6) | 1.2 (0.5–2.6) |
0.809 |
| Sm | 730 (26.8) | 39 (9.0) | 3.4 (2.4–4.8) |
<0.001 | 11 (12.8) | 28 (8.0) | 1.5 (0.7–3.0) |
0.592 |
| SmRNP | 1067 (39.2) | 76 (17.5) | 2.8 (2.1–3.7) |
<0.001 | 22 (25.6) | 54 (15.5) | 1.6 (0.8–2.9) |
0.437 |
| RNP | 966 (35.5) | 87 (20.0) | 1.9 (1.5–2.5) |
<0.001 | 24 (27.9) | 63 (18.1) | 1.5 (0.9–2.7) |
0.437 |
| Centromere B | 102 (3.8) | 25 (5.8) | 0.7 (0.4–1.0) |
0.087 | 3 (3.5) | 22 (6.3) | 0.6 (0.1–1.7) |
0.592 |
| Scl-70 | 74 (2.7) | 11 (2.5) | 1.0 (0.6–2.1) |
0.916 | 2 (2.3) | 9 (2.6) | 0.8 (0.1–3.2) |
0.829 |
| Jo-1 | 8 (0.3) | 2 (0.5) | 0.5 (0.1–3.7) |
0.489 | 1 (1.2) | 1 (0.3) | 2.9 (0.1–74.7) |
0.689 |
Calculated by logistic regression using race/ethnicity as a covariate.
Bold values are significant (P<0.05) after false discovery rate adjustment.
Tested by ELISA; n=3,363 SLE, 430 ILE, 75 ILE with major clinical, 355 other ILE.
Using a bead-based multiplex assay, 55.5% of ILE patient samples (241/434) had lupus-associated autoantibodies, with variation in the prevalence of individual autoantibody specificities by race/ethnicity. The most common lupus-associated autoantibody specificities in African American ILE patients were anti-SmRNP (37.9%), -RNP (36.9%), -chromatin (32.0%), and -Ro/SSA (26.2%), with 15.5% of African American ILE patients having anti-dsDNA (Supplementary Table 2). The most common specificities in European American ILE patients were anti-Ro/SSA (20.2%), -chromatin (18.5%), -RNP (12.6%), and –dsDNA (10.5%). Therefore, we accounted for race/ethnicity in analyses of autoantibody prevalence. Approximately 78% of SLE patient samples (2129/2720) had lupus-associated autoantibodies by the bead-based assay. Compared to SLE patients, ILE patients had fewer autoantibody specificities (1.3 vs. 2.6, P<0.0001), and were less likely to be positive for each lupus-associated autoantibody (Table 3). ILE patients with major clinical manifestations showed significantly more autoantibody specificities (median 2, IQR[1,3]) than other ILE patients (median 1, IQR[0,2], P=0.0137), with significantly greater frequencies of anti-chromatin and anti-SmRNP (Table 3), but still had fewer autoantibody specificities than SLE patients without major clinical manifestations (average of 2.0 vs. 2.6, P=0.0479).
BLyS levels in ILE patients are higher than in controls but lower than in SLE patients
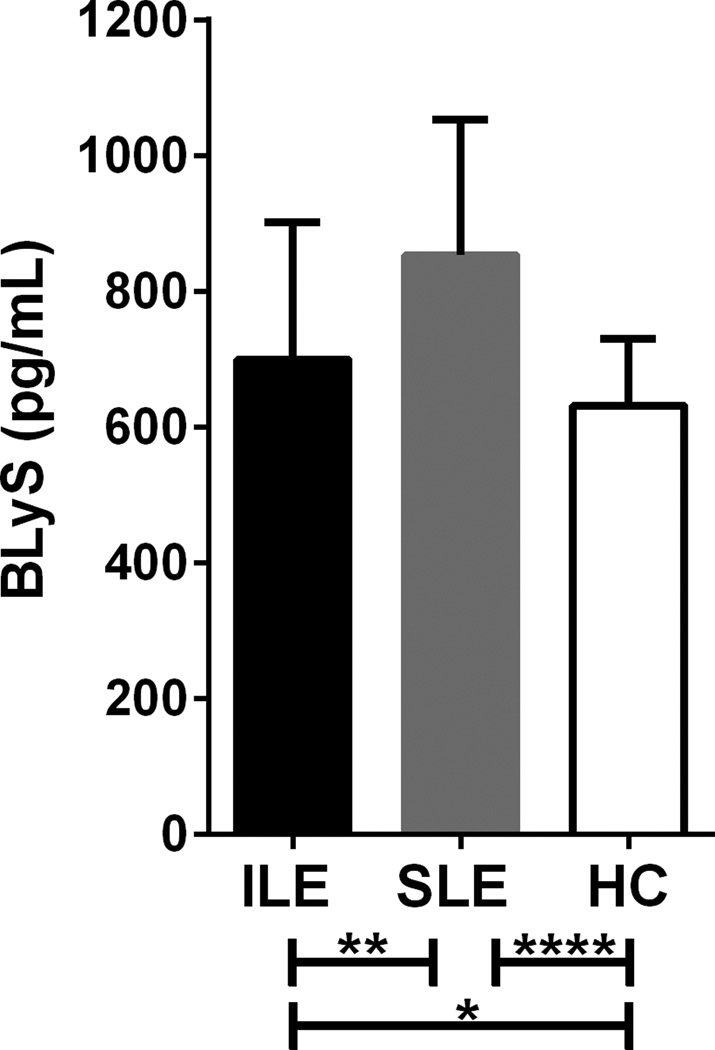
We hypothesized that the lower autoantibody levels and reduced number of autoantibody specificities in ILE patients might correlate with differences in BLyS, which is linked to autoantibody production in SLE.(35) Therefore, we measured plasma levels of BLyS in a subset of 72 ILE patients and 100 SLE patients demographically matched to 172 unrelated, unaffected healthy controls. Median BLyS levels in ILE patients (699.8 pg/mL) were significantly elevated compared to healthy controls (631.6 pg/mL, P=0.004), yet remained lower than BLyS levels in SLE patients (853.5 pg/mL, P=0.002; Figure 3). Similarly, BLyS levels exceeded the positive cutoff value (see receiver operating characteristic curve in Supplementary Figure 3) in 36% of ILE patients (26/72), compared to just 9% of healthy controls (14/164, P<0.0001) and 54% of SLE patients (54/100; P=0.030). Of note, BLyS levels correlated with the number of DNA/RNA-binding autoantibodies in ILE patients alone (r=0.247, P=0.037) and across ILE and SLE patients (r=0.255, P=0.001). Together, these results suggest that ILE patients may have similar patterns, but reduced levels of immune dysregulation compared to SLE patients.
Figure 3. BLyS levels are elevated in ILE patients, but remain lower than in SLE patients.
Plasma BLyS concentrations (pg/mL) were analyzed by ELISA for a subset of 72 ILE patients, 100 SLE patients, and 124 healthy controls (HC). Medians and inter-quartile ranges are shown. *P=0.016, **P=0.008, ****P<0.0001 by Kruskal-Wallis with Dunn’s multiple comparisons test.
DISCUSSION
ILE is clinically heterogeneous, encompassing both patients with relatively mild disease and patients who have major clinical manifestations but are not classified with SLE. Moreover, while the majority of ILE patients never reach SLE classification, a subset of ILE patients is at high risk of progressing to classified SLE.(5–10) These features present a challenge for the development of formal ILE classification guidelines. Although not defining diagnosis, classification criteria both inform diagnosis and influence patient access to treatments such as belimumab that were tested only in patients meeting SLE classification criteria.(36) Therefore, classification of patients as SLE or ILE has implications for clinical care. This study of 440 geographically and racially/ethnically diverse ILE patients provides new clinical and immunological insights on patients with incomplete lupus erythematosus.
ILE has perhaps been traditionally thought of as a ‘less severe’ version of SLE, and the clinical presentation is relatively mild in a majority of ILE patients.(3, 5, 7, 8, 10, 13) Although data to complete a validated SLE damage index were not available for this study, major clinical manifestations (including serositis, renal, neurologic, hemolytic anemia, and thrombocytopenia) affected nearly one in five ILE patients, similar to other large ILE cohorts.(7, 13) Renal involvement was more common in the current study (7.3%) compared to studies of ILE cohorts from the Spanish Rheumatology Society Lupus Registry (4.3%) and the Brigham and Women’s Hospital (4.5%).(7, 13) This difference may be partly attributed to the lower percentage of European American patients in our study (54.8% vs. 94% and 67%, respectively). Indeed, African American ILE patients exhibited a higher rate of major clinical manifestations (25.7%) compared to European Americans (14.9%). Given the association between renal disease and future transition to classified SLE,(7) these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus.
These results also provide additional information about the clinical treatment of patients with incomplete lupus classification. The prevalence of anti-malarial use among ILE patients (65.2%), along with the increased use of anti-malarials in recent years and data from other cohorts,(7, 13) suggests that anti-malarials are commonly used in ILE treatment. This is not surprising, given the evidence that early treatment with anti-malarials may limit organ damage,(37–39) delay SLE onset,(40) slow the accrual of autoantibodies,(40) and reduce the need for drugs with greater toxicity.(39, 41) In addition, hydroxychloroquine use was associated with reduced total medical costs and fewer disease flares in an observational cohort study of SLE patients diagnosed within the previous four years.(42) However, despite the low cost and relative safety of anti-malarials,(43, 44) their effectiveness for treating ILE symptoms, reducing autoantibody accrual, or delaying the transition to classified SLE has not yet been formally tested in longitudinal studies.
Medication usage was increased in ILE patients with more severe manifestations, suggesting that these patients may have received a clinical diagnosis of SLE without reaching SLE classification. It should also be noted that these patients were diagnosed and treated within the purview of ACR criteria, as the medical records in this analysis predate the 2012 SLICC criteria.(27) The SLICC criteria are more sensitive than the ACR criteria and may allow for earlier classification of certain lupus patients, potentially resulting in earlier diagnosis in some cases.(45, 46) Indeed, approximately one-third of the ILE patients in this study (149/440) reached SLE classification by SLICC criteria. However, even patients with incomplete lupus classification by both SLICC and ACR criteria may exhibit severe clinical manifestations and require aggressive treatment.(47) Further, some ILE patients at low risk of disease transition or major organ involvement may be over-treated, with increased risk of toxicity compared to the potential benefit. These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.
ILE patients with major clinical manifestations showed fewer autoantibody specificities than SLE patients without major clinical manifestations, suggesting that the progression from ILE to SLE may involve changes in the regulation of autoantibody production. BLyS is linked to autoantibody production in established SLE through its roles in B cell differentiation and survival.(35) BLyS becomes elevated prior to SLE classification,(19) and a subset of ILE patients has been shown to display an interferon gene expression signature linked with the production of BLyS(22) and ANA(23). In this study, plasma levels of BLyS protein were higher in ILE patients than healthy controls, and a subset of ILE patients (36%) exhibited significantly elevated BLyS. While ANA-positive healthy individuals maintain normal or reduced levels of BLyS,(48) we have previously shown that BLyS levels increase in SLE patients shortly prior to SLE classification.(19) This raises the possibility that ILE patients with elevated levels of BLyS may be at increased risk of transitioning to SLE. Focused studies are needed to understand the relationships between autoantibody positivity, soluble mediator dysregulation, and clinical disease in ILE patients and ILE patient subsets.
In conclusion, this study suggests that ILE can potentially be defined through a rubric of clinical and immunologic phenotypes that distinguish ILE from SLE. Although ILE patients exhibit fewer autoantibody specificities and lower BLyS levels than SLE patients, patients with incomplete lupus classification report nearly as many SLE-related symptoms as patients with classified SLE. Moreover, subsets of ILE patients are at risk of permanent organ damage and potential transition to classified SLE. By establishing and characterizing a large, diverse collection of ILE patients, this study provides a foundation for future longitudinal studies to identify ILE patients at the highest risk of transition to SLE, and to address the impact of ILE on quality of life, organ damage, increased morbidity, and early mortality.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
This study characterized a diverse collection of 440 ILE patients who fulfilled three 1997 American College of Rheumatology (ACR) SLE classification criteria.
Compared to SLE patients, ILE patients exhibited fewer autoantibody specificities, lower antinuclear autoantibody titer, and lower levels of the soluble mediator BLyS, which is associated with autoantibody production.
Although usually presenting with milder symptoms, a subset of ILE patients develop more serious clinical manifestations.
This study provides a foundation for future investigations to characterize ILE and determine its impact on organ damage, quality of life, and future SLE risk, as well as molecular pathways unique to ILE.
Acknowledgments
The authors thank the personnel and participants of the Lupus Family Registry and Repository. The authors thank Cathy Velte, Camille Anderson, Sandy Long, Tim Gross, Bola Adebayo, and Joseph Kheir for technical assistance, as well as Miles Smith for editorial assistance. Research reported in this publication was supported by the US NIH through the National Institute of Allergy and Infectious Disease (U19AI082714, U01AI101934, and R37AI24717), Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences (P30GM103510 and U54GM104938), the National Institute of Arthritis, Musculoskeletal and Skin Diseases (P30AR053483, P30AR070549) the National Human Genome Research Institute (U01HG008666), the National Heart, Lung, and Blood Institute (R24HL105333), and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK107502). This work was also supported by the US Department of Veterans Affairs (I01BX001834). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States government.
NJO reports grants from Mallinckrodt Pharmaceuticals, Resolve Therapeutics, Horizon Pharmaceuticals, Roche/Genentech, and Aurinia Pharmaceuticals outside the submitted work.
Footnotes
All other authors declare no conflicts of interest.
REFERENCES
- 1.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 3.Greer JM, Panush RS. INcomplete lupus erythematosus. Arch Int Med. 1989;149(11):2473–2476. [PubMed] [Google Scholar]
- 4.Costenbader KH, Schur PH. We need better classification and terminology for “people at high risk of or in the process of developing lupus”. Arthritis Care Res (Hoboken) 2015;67(5):593–596. doi: 10.1002/acr.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vila L, Mayor A, Valent A, Garc M, Vila S. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus. 2000;9(2):110–115. doi: 10.1191/096120300678828073. [DOI] [PubMed] [Google Scholar]
- 6.Laustrup H, Voss A, Green A, Junker P. Occurrence of systemic lupus erythematosus in a Danish community: an 8-year prospective study. Scand J Rheumatol. 2009;38(2):128–132. doi: 10.1080/03009740802419073. [DOI] [PubMed] [Google Scholar]
- 7.Al Daabil M, Massarotti EM, Fine A, Tsao H, Ho P, Schur PH, et al. Development of SLE among “potential SLE” patients seen in consultation: long-term follow-up. Int J Clin Pract. 2014;68(12):1508–1513. doi: 10.1111/ijcp.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl Hallengren C, Nived O, Sturfelt G. Outcome of incomplete systemic lupus erythematosus after 10 years. Lupus. 2004;13(2):85–88. doi: 10.1191/0961203304lu477oa. [DOI] [PubMed] [Google Scholar]
- 9.Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol. 2014;10(4):212–228. doi: 10.1038/nrrheum.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swaak AJ, van de Brink H, Smeenk RJ, Manger K, Kalden JR, Tosi S, et al. Incomplete lupus erythematosus: results of a multicentre study under the supervision of the EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials (ESCISIT) Rheumatol. 2001;40(1):89–94. doi: 10.1093/rheumatology/40.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Olsen N, Yousif M, Mutwally A, Cory M, Elmagboul N, Karp D. Organ damage in high-risk patients with systemic and incomplete lupus syndromes. Rheumatol Int. 2013;33(10):2585–2590. doi: 10.1007/s00296-013-2783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Li M-T, Xu D, Leng X-M, Wang Q, Tian X-P, et al. Organ damage in patients with incomplete lupus syndromes: from a Chinese academic center. Clin Rheumatol. 2015:1–7. doi: 10.1007/s10067-015-2884-3. [DOI] [PubMed] [Google Scholar]
- 13.Rua-Figueroa I, Richi P, Lopez-Longo FJ, Galindo M, Calvo-Alen J, Olive-Marques A, et al. Comprehensive description of clinical characteristics of a large systemic lupus erythematosus cohort from the Spanish Rheumatology Society Lupus Registry (RELESSER) with emphasis on complete versus incomplete lupus differences. Medicine (Baltimore) 2015;94(1):e267. doi: 10.1097/MD.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugarte-Gil MF, Alarcon GS. Incomplete Systemic Lupus Erythematosus: Early Diagnosis or Overdiagnosis? Arthritis Care Res (Hoboken) 2016;68(3):285–287. doi: 10.1002/acr.22663. [DOI] [PubMed] [Google Scholar]
- 15.Nakken B, Bodolay E, Szodoray P. Cytokine Milieu in Undifferentiated Connective Tissue Disease: a Comprehensive Review. Clin Rev Allergy Immunol. 2015;49(2):152–162. doi: 10.1007/s12016-014-8452-9. [DOI] [PubMed] [Google Scholar]
- 16.Heinlen LD, McClain MT, Ritterhouse LL, Bruner BF, Edgerton CC, Keith MP, et al. 60 kD Ro and nRNP A frequently initiate human lupus autoimmunity. PLoS One. 2010;5(3):e9599. doi: 10.1371/journal.pone.0009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinlen LD, Ritterhouse LL, McClain MT, Keith MP, Neas BR, Harley JB, et al. Ribosomal P autoantibodies are present before SLE onset and are directed against non-C-terminal peptides. J Mol Med (Berl) 2010;88(7):719–727. doi: 10.1007/s00109-010-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56(7):2344–2351. doi: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 19.Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.06.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147(1):60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181(3):2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 23.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159(3):281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen A, Sevier S, Kelly JA, Glenn SB, Aberle T, Cooney CM, et al. The lupus family registry and repository. Rheumatol. 2011;50(1):47–59. doi: 10.1093/rheumatology/keq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walitt BT, Constantinescu F, Katz JD, Weinstein A, Wang H, Hernandez RK, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women’s Health Initiative. J Rheumatol. 2008;35(5):811–818. [PMC free article] [PubMed] [Google Scholar]
- 26.Karlson EW, Costenbader KH, McAlindon TE, Massarotti EM, Fitzgerald LM, Jajoo R, et al. High sensitivity, specificity and predictive value of the Connective Tissue Disease Screening Questionnaire among urban African-American women. Lupus. 2005;14(10):832–836. doi: 10.1191/0961203305lu2227oa. [DOI] [PubMed] [Google Scholar]
- 27.Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain MT, Arbuckle MR, Heinlen LD, Dennis GJ, Roebuck J, Rubertone MV, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50(4):1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 29.Bruner BF, Guthridge JM, Lu R, Vidal G, Kelly JA, Robertson JM, et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum. 2012;64(11):3677–3686. doi: 10.1002/art.34651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R, Robertson JM, Bruner BF, Guthridge JM, Neas BR, Nath SK, et al. Multiple Autoantibodies Display Association with Lymphopenia, Proteinuria, and Cellular Casts in a Large, Ethnically Diverse SLE Patient Cohort. Autoimmune Dis. 2012;2012:819634. doi: 10.1155/2012/819634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 32.Bello GA, Brown MA, Kelly JA, Thanou A, James JA, Montgomery CG. Development and validation of a simple lupus severity index using ACR criteria for classification of SLE. Lupus Sci Med. 2016;3(1):e000136. doi: 10.1136/lupus-2015-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziakas PD, Dafni UG, Giannouli S, Tzioufas AG, Voulgarelis M. Thrombocytopaenia in lupus as a marker of adverse outcome--seeking Ariadne’s thread. Rheumatol. 2006;45(10):1261–1265. doi: 10.1093/rheumatology/kel101. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez M, Alarcon GS, Apte M, Andrade RM, Vila LM, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic US cohort: XLIII. The significance of thrombocytopenia as a prognostic factor. Arthritis Rheum. 2007;56(2):614–621. doi: 10.1002/art.22376. [DOI] [PubMed] [Google Scholar]
- 35.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119(5):1066–1073. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken) 2015;67(7):891–897. doi: 10.1002/acr.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virdis A, Tani C, Duranti E, Vagnani S, Carli L, Kuhl AA, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res Ther. 2015;17:277. doi: 10.1186/s13075-015-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fessler BJ, Alarcon GS, McGwin G, Jr, Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52(5):1473–1480. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 39.Pons-Estel GJ, Alarcon GS, McGwin G, Jr, Danila MI, Zhang J, Bastian HM, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61(6):830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James J, Kim-Howard X, Bruner B, Jonsson M, McClain M, Arbuckle M, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16(6):401–409. doi: 10.1177/0961203307078579. [DOI] [PubMed] [Google Scholar]
- 41.Meinao IM, Sato EI, Andrade LE, Ferraz MB, Atra E. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus. 1996;5(3):237–241. doi: 10.1177/096120339600500313. [DOI] [PubMed] [Google Scholar]
- 42.Kan H, Nagar S, Patel J, Wallace DJ, Molta C, Chang DJ. Longitudinal Treatment Patterns and Associated Outcomes in Patients With Newly Diagnosed Systemic Lupus Erythematosus. Clin Ther. 2016;38(3):610–624. doi: 10.1016/j.clinthera.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol. 2011;7(12):718–729. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- 45.Ines L, Silva C, Galindo M, Lopez-Longo FJ, Terroso G, Romao VC, et al. Classification of Systemic lupus erythematosus: Systemic Lupus International Collaborating Clinics versus American College of Rheumatology criteria. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22539. [DOI] [PubMed] [Google Scholar]
- 46.Pons-Estel GJ, Wojdyla D, McGwin G, Jr, Magder LS, Petri MA, Pons-Estel BA, et al. The American College of Rheumatology and the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus in two multiethnic cohorts: a commentary. Lupus. 2014;23(1):3–9. doi: 10.1177/0961203313512883. [DOI] [PubMed] [Google Scholar]
- 47.Aberle T, Bourn RL, Chen H, Roberts VC, Guthridge JM, Bean KM, et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated ILE subjects. Lupus Sci Med. doi: 10.1136/lupus-2016-000176. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slight-Webb S, Lu R, Ritterhouse LL, Munroe ME, Maecker HT, Fathman CG, et al. Autoantibody-Positive Healthy Individuals Display Unique Immune Profiles That May Regulate Autoimmunity. Arthritis Rheumatol. 2016 doi: 10.1002/art.39706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.