Abstract
Background
Patients with primary sclerosing cholangitis (PSC) may be at higher of risk of malignancy after liver transplantation (LT) compared to other LT recipients. We aimed to determine the cumulative incidence of/risk factors for and long-term cancer-related mortality in patients with PSC after LT.
Methods
all adult patients who underwent LT for PSC without cholangiocarcinoma from 1984–2012, with follow-up through June 2015. We estimated cumulative incidence, risk factors and mortality from de novo malignancies after LT
Results
293 patients were identified (mean age, 47±12 years; 63.3% males; 2.4% smoking at LT). Over a median of 11.5 years (range, 6.4–18.6), 64 patients (21.8%) developed 73 nonskin cancers, including 46 solid-organ cancers (11 renal, 11 colorectal, 7 prostate, 5 breast, 5 pancreas, 3 ovarian/endometrial/vulvar, 4 de novo cholangiocarcinoma). Twenty-two patients developed hematological malignancies (18 posttransplant lymphoproliferative diseases [PTLD], 2 Hodgkin’s disease, 2 myelodysplastic syndrome). Five patients developed melanoma. The 1-, 5-, 10- and 20-year cumulative incidences of cancer were 2.1%, 8.6%, 18.7%, and 27%, respectively. Mortality of PSC patients who developed cancer was higher than for PSC patients without cancer (HR 2.2, p<0.01). On multivariate analysis, recipient age and elevated pre-LT INR were associated with increased risk of de novo (nonskin) malignancy.
Conclusion
The 10-year cumulative risk of cancer after LT for advanced stage PSC was 18.7%, with PTLD, colorectal and renal cell cancers being the most common. Post-LT de novo nonskin cancer decreased overall posttransplant survival. Only recipient age and elevated INR at LT were associated with increased nonskin cancer risk.
INTRODUCTION
Liver transplant (LT) recipients are known to have an increased risk of developing de novo malignancies when compared to an age-matched general population1–6. De novo cancer is an important leading cause of late death post-LT7–10. Previous studies have estimated that the incidence of de novo cancers after LT ranged from 2.6% to 21.7%3, 4, 8–17; with variation based on the demographics of the transplant population, duration of follow-up, differences in indications for LT, immunosuppression regimens, and the era of transplantation. Malignancies associated with environmental factors such viruses, alcohol and tobacco use are common after LT 18–22. Other factors such as age of the recipient, history of cigarette smoking, alcoholic liver disease, primary sclerosing cholangitis (PSC), and duration and intensity of immunosuppression increased the overall risk of developing malignancies 8, 9, 22–25.
Cancer is 1 of the leading causes of mortality in nontransplant patients with PSC 26. The risk for hepatobiliary, colorectal, and pancreatic cancer is increased in patients with PSC due to the presence of chronic inflammation 26. Knowledge of how LT modifies cancer risk in PSC patients is poor. In this study, we sought to examine the cumulative incidence of and risk factors for de novo malignancies in patients with PSC and their impact on long-term mortality after LT.
MATERIALS AND METHODS
The Mayo Clinic Institutional Review Board approved this study for patients who had consented to the use of their medical records for research.
Patients
Through a prospectively maintained solid organ transplant registry, we identified 373 adult patients (>18 years) with PSC who underwent LT at Mayo Clinic Rochester between January 1984 and December 2012. From this cohort, we included patients with PSC, with or without associated IBD, who underwent LT for advanced disease in our study. We excluded patients who underwent LT for cholangiocarcinoma, since these patients are inherently at higher risk of recurrent as well as de novo malignancy after LT.
Data on baseline demographics (age, sex, race), clinical variables (prior malignancy, alcohol dependence, cigarette smoking status, comorbidities), transplant-related (allograft failure, type of donor, recurrent PSC, biliary strictures after LT, cytomegalovirus [CMV] infection, Epstein-Barr virus [EBV] infection, CMV mismatch, EBV mismatch, ABO blood type mismatch, gender mismatch, human leukocyte antigen [HLA] mismatch, episodes of acute cellular rejection, chronic rejection, era of transplantation and re-transplantation), associated IBD-related (subtype of IBD, IBD disease activity status at time and after LT, IBD treatment before and after transplant, and intact colon at time of LT), laboratory-related (electrolyte/renal function, liver function, and complete blood count with differential at time of LT) and immune suppression-related variables (use of mycophenolate mofetil, azathioprine, prednisone, tacrolimus-, cyclosporine-, and sirolimus- based regimen) were abstracted systematically. At our center, immunosuppression protocols varied from cyclosporine, prednisone, and azathioprine before 1995, to a gradual shift to tacrolimus, prednisone and mycophenolate mofetil, after 1995. Patients who developed biopsy-proven acute cellular rejection were treated with 3 intravenous boluses of methylprednisolone (1000 mg).
Outcomes
The primary outcome of our study was to estimate the cumulative incidence of all de novo cancers (excluding nonmelanoma skin cancers [NMSC]) in patients with PSC post-LT, and the impact of these cancers on overall survival in these patients. Demographic, clinical, PSC-, transplant-, associated IBD, immunosuppression- and laboratory-related risk factors associated with development of de novo malignancy after LT for PSC were identified.
Statistical Analysis
The data are reported as mean (±SD), median (interquartile range, IQR), and categorical variables by counts and percentages as appropriate. We recorded person-years at risk from the date of transplantation until the date of first cancer diagnosis (except NMSC), death, emigration or end of study period (June 30, 2015) for each individual in the cohort. We included only incident cancers occurring at least 30 days after LT to minimize the risk of detection bias and misclassification; patients with a history of cancer diagnosed prior to LT were still considered at risk of other organ cancers after LT. Cumulative incidence of and mortality from cancer was calculated using Kaplan-Meier survival analysis. To identify risk factors (present at time of LT) associated with development of cancer, we performed univariate time-to-event analysis using log-rank test. Variables which were significant (p<0.10) on univariate analysis were then included in a multivariate Cox proportional hazard analysis to identify independent risk factors associated with malignancy. All statistical analyses were conducted using JMP version 10 for Windows (SAS Institute Inc., Cary, NC) and EZR (Easy R) version 1.33. P-values <0.05 were considered statistically significant. EZR (Easy R) was used to calculate cumulative incidence of competing events.
RESULTS
Patient Eligibility and Demographics
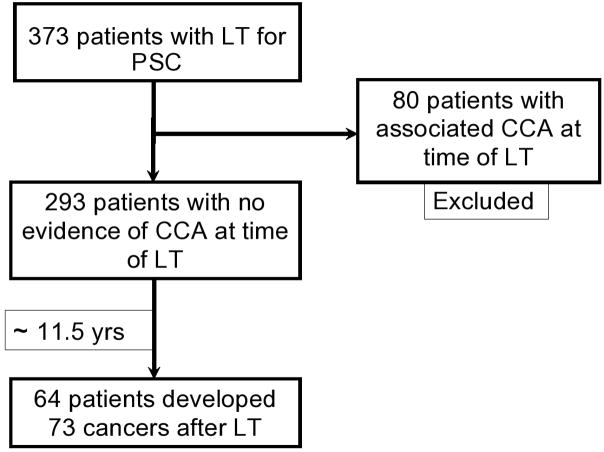
Of 373 patients who underwent LT for PSC in the study period, 293 were included in the current analysis; 80 patients who underwent LT for cholangiocarcinoma were excluded. Sixty-four patients (21.8%) developed 73 malignancies (excluding NMSC) (Figure 1). Only 1 developed cancer (PTLD) within the first 3 months. The baseline clinical and demographic characteristics for PSC patients who developed these de novo malignancies and PSC controls who did not are shown in Table 1. The mean age at the time of liver transplant was 48.2 ± 11.3 years for patients who developed de novo cancers and 47.0 ± 12.2 years for patients who did not. There were 67.2% men among cases, and 62.0% men among controls. The primary immunosuppressive regimen was not significantly different between the cases and controls. Lower rates of allograft failure were observed in the cancer group. Most of the anastomoses (92.8%) were Roux-en-Y hepaticojejunostomy and the rest were choledochal duodenostomy.
Figure 1.
Selection of patients included in the determination of de novo malignancies after LT for PSC
Table 1.
Comparison of clinical and demographic characteristics between cases who developed de novo cancers and controls who did not develop de novo cancers.
| Characteristics | N (%) (cases = 64) | N (%) (controls = 229) | P-value |
|---|---|---|---|
| Demographics | |||
| Recipient age at LT, years (mean ± SD) | 48.2 ± 11.3 | 47.0 ± 12.2 | 0.24 |
| Male sex, n (%) | 43 (67.2%) | 142 (62.0%) | 0.44 |
| History of smoking, (%) | 9 (14.1%) | 30 (13.1%) | 0.89 |
| Follow-up in years (median, IQR) | 14.7 (9.0–20.9) | 11.2 (6.2–18.0) | 0.01* |
| Deceased type of donor, n (%) | 56 (87.5%) | 182 (79.5%) | 0.11 |
| Donor age at LT, years (mean ± SD) | 31.8 ± 16.6 | 36.8 ± 16.8 | 0.04* |
| Male donor gender, n (%) | 38 (59.4%) | 118 (51.5%) | 0.12 |
| Gender mismatch, n (%) | 18 (28.1%) | 76 (33.2%) | 0.53 |
| Positive CMV recipient status, n (%) | 31 (48.4%) | 111 (48.5%) | 0.93 |
| Positive CMV donor status, n (%) | 32 (50.0%) | 106 (46.3%) | 0.52 |
| CMV mismatch, n (%) | 15 (23.4%) | 62 (27.1%) | 0.35 |
| EBV mismatch, n (%) | 11 (17.2%) | 50 (21.8%) | 0.65 |
| Transplant related variables, n (%) | |||
| Cirrhosis at time of LT ( stage 4 PSC) | 56 (87.5%) | 188 (82.1%) | 0.36 |
| Allograft failure | 10 (15.6%) | 55 (24.0%) | 0.12 |
| Recurrent PSC | 21 (32.8%) | 73 (31.9%) | 0.96 |
| CMV infection | 14 (21.9%) | 52 (22.7%) | 0.89 |
| Re-transplantation | 10 (15.6%) | 40 (17.5%) | 0.68 |
| ERA of transplantation (before1995) | 36 (56.2%) | 96 (41.9%) | 0.35 |
| Roux-en-Y hepaticojejunostomy anastomosis | 61 (95.3%) | 211 (92.14%) | 0.36 |
| Immunosuppression, n (%) | |||
| Mycophenolate mofetil after LT * | 26 (40.6%) | 119 (52.0%) | 0.10 |
| Azathioprine after LT* | 44 (68.7%) | 122 (54.9%) | 0.07 |
| Prolonged prednisone (>6 months) * | 41 (64.1%) | 138 (60.3%) | 0.75 |
| Tacrolimus-based immunosuppression* | 33 (51.6%) | 139 (60.1%) | 0.22 |
| Cyclosporine-based immunosuppression* | 30 (46.9%) | 81 (36.4%) | 0.31 |
| Sirolimus –based immunosuppression * | 0 (0.00%) | 3 (1.3%) | NA |
| IBD-related variables, n (%) | |||
| Associated IBD | 55 (86.0%) | 191 (83.4%) | 0.58 |
| Quiescent IBD after LT | 28 (43.75%) | 84 (36.7%) | 0.22 |
| Colectomy post LT | 20 (31.25%) | 24 (10.5%) | 0.13 |
| Colectomy prior to LT | 10 (15.6%) | 62 (27.1%) | 0.09 |
| Laboratory variables, mean ± SD | |||
| MELD score | 11.8 ± 3.4 | 11.2 ± 3.4 | 0.31 |
| White-cell count(per mm3) | 6.2 ± 3.3 | 6.6 ± 3.9 | 0.60 |
| Neutrophils (×10(9)/L) | 4.5 ± 2.7 | 4.2 ± 2.8 | 0.66 |
| Lymphocytes (×10(9)/L) | 0.85 ± 0.58 | 1.0 ± 0.9 | 0.27 |
| Neutrophil to lymphocyte ratio (NLR) | 7.5 ± 6.9 | 5.3±4.7 | 0.049* |
| Hemoglobin(g/dl) | 10.5 ± 1.5 | 10.9 ± 1.5 | 0.18 |
| Platelet count (per mm3) | 126 900 ± 88 700 | 159 400 ± 121 200 | 0.15 |
| Prothrombin time-international normalized ratio | 1.49 ± 0.53 | 1.40 ± 0.4 | 0.12 |
| Albumin(g/dl) | 3.1 ± 0.5 | 3.2 ± 0.5 | 0.13 |
| Alkaline phosphatase (U/liter) | 689.8 ± 511.8 | 747.6 ± 649.2 | 0.63 |
| Aspartate aminotransferase (U/liter) | 146.4 ± 79.7 | 159.0 ± 112.2 | 0.54 |
| Alanine aminotransferase (U/liter) | 94.9 ± 53.4 | 108.4 ± 79.9 | 0.35 |
| Bilirubin (mg/dl) | 12.7 ± 10.5 | 13.3 ± 12.5 | 0.80 |
| Creatinine (mg/dl) | 1.2 ± 0.6 | 1.1 ± 1.0 | 0.97 |
LT, liver transplantation; SD, standard deviation; IQR, interquartile range; CMV, cytomegalovirus; EBV, Epstein-Barr virus; PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; MELD, Model for End-Stage Liver Disease.
Any time after LT
Types of de novo malignancies that developed after LT in patients with PSC are depicted in Table 2. Renal and colorectal cancers were the most common solid organ tumor, and posttransplant lymphoproliferative disorder (PTLD) was the most common hematological malignancy. Table 3 demonstrates those with multiple de novo malignancies after LT.
Table 2.
Seventy-three de novo malignancies diagnosed in 64 patients after liver transplantation for primary sclerosing cholangitis (excluding nonmelanoma skin cancer)
| Organ | N (%) | Tumor type | Time to cancer in years Median(range) |
Outcome |
|---|---|---|---|---|
| Skin (8.2%) | 5 (6.8%) | Melanoma | 4.88 (4.16–13.8) | All survived |
| Hematology (30.1%) | 18 (24.7%) | Lymphoproliferative disease (PTLD) | 5.24 (0.19–30) | 3 died of refractory/recurrent disease 6 were positive for EBV, 9 were negative and 3 were unknown 1 developed CNS lymphoma |
| 2 (2.7%) | Myelodysplastic syndrome (MDS) | 13.25 (9.80–16.70) | 2 died of acute myeloid leukemia (AML) on MDS | |
| 2 (2.7%) | Hodgkin’s disease | 5.34 (0.57–10.10) | both died of cancer and complications | |
| Solid-organ (63.0%) | 11 (15.1%) | Renal Cell Cancer | 11.7 (3.54–26.7) | All survived |
| 11 (15.1%) | Colorectal Cancer | 6.17 (0.31–22.9) | 9 colon cancer and 2 rectal cancer 5 died of metastatic disease |
|
| 7 (9.6%) | Prostate adenocarcinoma | 6.24 (1.93–22.5) | All survived | |
| 5 (6.8%) | Pancreatic adenocarcinoma | 7.72 (5.22–10.0) | 5 died of metastatic disease | |
| 5 (6.8%) | Breast Cancer | 3.93 (2.12–12.8) | 4/5 survived (1 who died from Hodgkin’s disease) | |
| 4 (5.5%) | Intrahepatic cholangiocarcinoma (de novo) | 18.05 (5.17–28.5) | All had Roux-en-Y hepaticojejunostomy anastomosis PSC recurred in all patients All died of metastatic disease |
|
| 1 (1.4%) | Ovarian Cancer | 22.4 | died of metastatic disease | |
| 1 (1.4%) | Endometrial Cancer | 5.65 | died of metastatic disease | |
| 1 (1.4%) | Vulvar Cancer | 22.1 | survived |
Table 3.
Types of consecutive malignancies in 9 patients who developed multiple de novo malignancies after LT
| N | 1st Cancer | Time to cancer(yrs) | 2nd Cancer | Time to cancer(yrs) |
|---|---|---|---|---|
| 1 | Breast | 2.1 | Hodgkin’s disease | 10.1 |
| 2 | Colon | 6.2 | RCC | 15.5 |
| 3 | RCC | 6.0 | Prostate | 19.0 |
| 4 | Prostate | 4.0 | RCC | 17.3 |
| 5 | Prostate | 1.9 | AML | 9.8 |
| 6 | RCC | 3.5 | Prostate | 6.2 |
| 7 | RCC | 5.2 | CCA | 9.4 |
| 8 | PTLD | 4.6 | AML | 16.7 |
| 9 | PTLD | 6.9 | CCA | 20.5 |
RCC, renal cell cancer; AML, acute myeloid leukemia; CCA, cholangiocarcinoma; PTLD, posttransplant proliferative disorder.
The cumulative incidence for de novo cancer (excluding NMSC) in PSC patients after LT was 2.1%, 8.6%, 18.7%, and 27% at 1, 5, 10 and 20 years, respectively (figure 2). PSC patients had a 1-, 5-, and 10-year probability for developing a hematological malignancy of 0.3%, 3.4%, and 7.4%, respectively, compared to 0.3%, 3.4%, and 10.0% for developing a solid malignancy. The 10-year cumulative incidence of colorectal cancer in patients with an intact colon was 4.5% in patients with PSC. The 10-year cumulative incidence of colectomy for colonic dysplasia in PSC-IBD patients was 25%.
Figure 2.
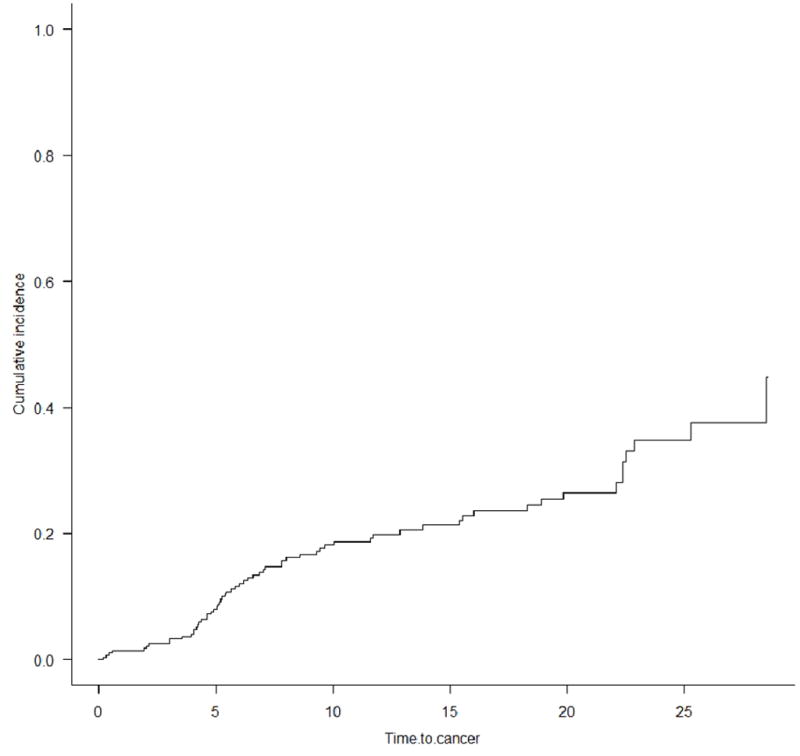
Cumulative incidence of cancer after liver transplantation for PSC (competing risk analysis)
Survival after LT with and without de novo cancer
The median follow up after OLT was 14.7 years (IQR, 9.0–20.9) for cases and 11.2 years (IQR 6.2–18.0) for controls. Among the 30 patients in the cancer group who died, de novo malignancy was the cause of death in 22 patients (73.3%), followed by PSC recurrence in 3 patients (10%), infection in 2 patients (6.7%), and other causes in 3 patients (cardiac arrest, respiratory failure, and mechanical fall). In general, patients with renal cell carcinomas and prostate adenocarcinomas responded well to cancer treatment, but some patients developed a second cancer after treatment (Table 3). Approximately 1/2 of patients with colorectal cancer and all patients with pancreatic cancer died of metastatic disease.
The noncancer related cumulative mortality rates were 2.1%, 8.6%, and 18.7% at 1, 5 and 10 years after OLT, respectively. The cancer-related cumulative mortality rates were 0.7%, 1.1%, and 4.9% at 1, 5 and 10 years after OLT, respectively (Figure 3). The cumulative probability of cancer-related death after the diagnosis of any de novo cancer (excluding NMSC) was 16.7% at 1 year, and 40.0% at 5 years. The cumulative probability of death after the diagnosis of a solid organ de novo malignancy was 12.5% at 1 year and 56.3% at 5 years, and 18.8% at 1 year and 18.8% after 5 years for hematological malignancies. At last follow-up, 47.7% of cases and 40.0% of controls had died. Patients diagnosed with de novo cancer experienced higher mortality compared to those who did not develop de novo cancer (hazard ratio [HR], 2.2; 95% CI, 1.4–3.5; P<0.01).
Figure 3.
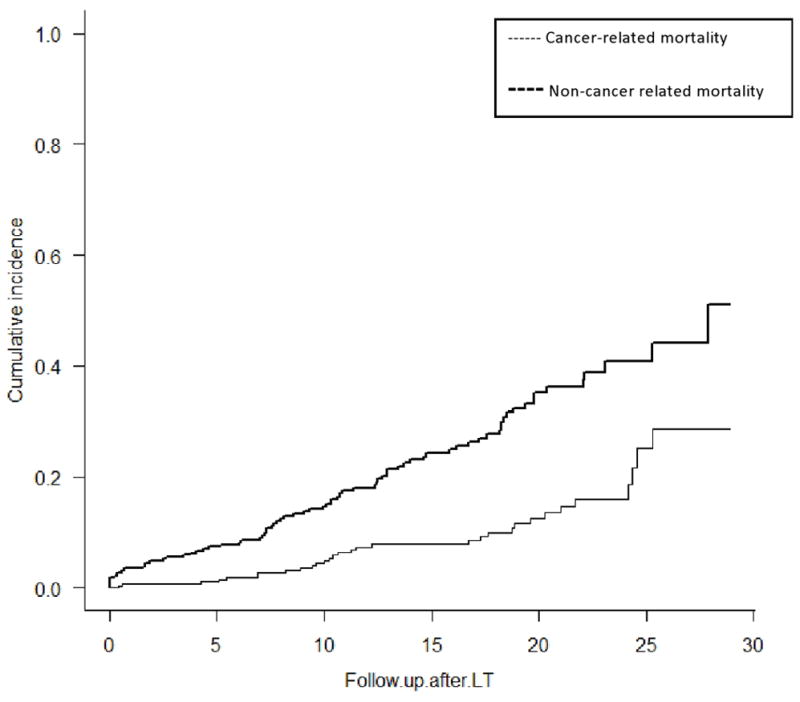
Cumulative incidence of death after liver transplantation for PSC
Risk factors associated with development of de novo malignancy
By univariate analysis, recipient age (per year) at time of LT (HR, 1.03; 95% CI, 1.004–1.052; P = .019), high neutrophil to lymphocyte ratio (NLR >4.27) at time of LT (HR, 1.10; 95% CI, 1.01–1.18; P = .023), and elevated INR at time of LT (HR, 1.09; 95% CI, 1.005–1.17; P = .039) were significant factors associated with de novo malignancy development. In a multivariate model that included age at LT, high INR, and high NLR, only recipient age at time of LT (HR per year, 1.05; 95% CI, 1.02–1.10; P <.01) and elevated INR at time of LT (HR, 1.12; 95% CI, 1.02–1.21; P =0.02) were associated with increased risk. IBD status at time of and after LT, the presence or absence of colon, and the era of transplantation were not significantly associated with time to malignancy (Table 4).
Table 4.
Risk factors for cancers after LT for PSC*
| Risk factors | Univariate analysis Hazard Ratio (95% CI) | P | multivariate analysis Hazard Ratio (95% CI) | P |
|---|---|---|---|---|
| Recipient age at LT (per yr) | 1.03 ( 1.004–1.052) | 0.019* | 1.05 ( 1.02–1.10) | 0.002* |
| Donor age at LT (per Yr) | 0.992 ( 0.975–1.007) | 0.348 | - | - |
| Recipient gender (M:F) | 1.56 ( 0.93–2.69) | 0.091 | - | - |
| Smoking at time of LT (Yes: No) | 2.93 (0.48–9.55) | 0.585 | - | - |
| Type of Donor (Deceased: Living) | 1.65 (0.60–6.80) | 0.644 | - | - |
| Donor gender (M:F) | 1.63 ( 0.931–2.976) | 0.088 | - | - |
| Gender mismatch (Yes: No) | 0.75 (0.42–1.31) | 0.317 | - | - |
| Recipient CMV status( Positive: Negative) | 0.82 (0.47–1.45) | 0.480 | - | - |
| Donor CMV status( Positive: Negative) | 1.13 (0.65–1.97) | 0.668 | - | - |
| CMV mismatch (Yes: No) | 0.90 (0.47–1.62) | 0.727 | - | - |
| Roux-en-Y hepaticojejunostomy (Yes:No) | 0.64 (0.23–2.66) | 0.49 | ||
| Recurrent PSC (Yes:No) | 0.76 (0.44–1.26) | 0.29 | ||
| Era of Transplantation ( after 1995: before 1995) | 0.85 (0.49–1.47) | 0.558 | - | - |
| Pre-LT IBD | 1.23 (0.62–2.82) | 0.768 | - | - |
| Intact colon at time of LT (Y:N) | 1.47 (0.79–2.98) | 0.229 | - | - |
| MELD score at time of LT (per unit) | 1.06 (0.96–1.17) | 0.221 | - | - |
| White-cell count at time of LT (per unit) | 0.97 (0.87–1.07) | 0.568 | - | - |
| Neutrophils at time of LT (per unit) | 1.02 (0.89–1.14) | 0.706 | - | - |
| Lymphocytes at time of LT (per unit) | 0.62 (0.30–1.05) | 0.086 | - | - |
| Neutrophil to lymphocyte ratio (NLR) (per 1 increase )** | 1.10 (1.01–1.18) | 0.023* | 1.06 (0.98–1.14) | 0.123 |
| Hemoglobin at time of LT (per unit) | 0.78 (0.59–1.01) | 0.061 | - | - |
| Platelet count at time of LT (per unit) | 0.997 (0.993–1.001) | 0.122 | - | - |
| Prothrombin time-international normalized ratio at time of LT (per 0.1 increase) | 1.09 (1.005–1.17) | 0.039* | 1.12 (1.02–1.21) | 0.017* |
Excluding nonmelanoma skin cancers
Excluding patients with infection or on steroids at time of LT
Posttransplant Lymphoproliferative Disease after Liver Transplantation for Primary Sclerosing Cholangitis
PTLD accounted for the majority (81.8%) of the hematological malignancies which developed after LT. The 1-, 5-, and 10-year cumulative incidences of PTLD were 0.7%, 2.9% and 6.0%, respectively.
Development of PTLD after LT was not associated with a decrease in survival (HR, 0.98; 95% CI, 0.36–2.68; P=0.97). On univariate Cox proportional hazard analysis, leukopenia at time of LT (WBC<3.500 per mm3) (HR, 4.63; 95% CI, 1.27–16.81; P=0.02) and high INR at time of LT (HR, 5.39; 95% CI, 1.38–18.10; P=0.02) were associated with increased risk of PTLD. NLR did not increase the risk of PTLD development after LT (HR, 1.04; 95% CI, 0.29–3.78; P=0.95).
Colorectal Dysplasia after Liver Transplantation for Primary Sclerosing Cholangitis
Of 128 patients (mean age, 36.1 ± 17.8 years; 68.0% males; 1.6% smoking at time of LT) who had an intact colon at time of LT and were followed up for screening colonoscopy, 59 patients (46.1%) developed colorectal dysplasia of any type (39 developed low-grade dysplasia, 3 developed high-grade dysplasia, and 17 indefinite dysplasia) over a median follow-up of 7.8 years (IQR, 4.1–14.1). The 1-, 5-, and 10-year cumulative incidence probabilities of colorectal dysplasia of any type were 5.5 %, 22.8%, and 36.9%, respectively. On univariate Cox proportional hazard analysis, male gender (HR, 1.8; 95% CI, 1.02–3.57; P=0.04) was associated with increased risk of colonic dysplasia.
DISCUSSION
This study showed that de novo malignancy is a substantial cause of death in LT recipients with underlying PSC. Excluding NMSC, de novo cancer developed in 21.8% of the patients within 12 years of transplantation, and accounted for half of the deaths after LT. PTLD was the most common observed hematological malignancy, whereas renal cell cancer and colorectal cancer were the most commonly observed solid tumors after LT for PSC. Although not a cohort with advanced age, recipient age was still associated with increased cancer risk. Surprisingly, elevated INR at time of LT was also an independent risk for malignancy. This would likely reflect cirrhotic stage disease with liver failure as opposed to recurrent cholangitis as the indication for transplantation or could reflect qualitative changes in vitamin K producing bacteria in the gut, but how this confers higher risk for de novo malignancy posttransplant is unclear.
The overall frequency of de novo cancer was high when compared to previous studies including all liver transplant recipients 3, 4, 8–17, but this is somewhat expected since we studied a patient cohort known to be higher risk of certain malignancies such as colorectal cancer and cholangiocarcinoma. Reported frequencies of cancer can also fluctuate depending on factors such as colectomy rates, given patients with PSC and associated IBD are at higher risk for colorectal cancer 5, 11, 27. Furthermore, since patients with cholestatic liver disease have experienced the best long-term post-LT survival, these patients may have had longer follow-up time to develop malignancies compared to other transplant patients23. Our data found that the rates of graft failure were lower in the group of patients with cancer; this may be due to less time of being at risk for graft failure. On the other hand, PSC patients are thought to be exposed to more intense immunosuppression therapy, with coexisting IBD possibly reducing graft rejection 28. Unfortunately, we do not have detailed data on pretransplant IBD-related immunosuppression, nor levels of immunosuppression posttransplant to evaluate this risk factor further.
High NLR is a biomarker for chronic inflammation, which can predispose the individual to malignancy and can affect the host immune response 29. Although not an independent variable for all de novo malignancy in this study, further studies are needed to examine the prognostic utility of NLR with transplant outcomes. The increased risk of PTLD in subjects with leukopenia may be related to decreased immune surveillance. Elevated INR at time of LT was also found to be a risk factor for de novo cancer. Coagulopathy reflects the deterioration of liver function, which results in immune dysfunction30. Coagulopathy leading to thrombosis can also represent the earliest clinical manifestation of an occult cancer 31. Elevated INR could be caused by altered host-microbiota interactions and dysbiosis which have tumor-promoting effects 32. However, the mechanisms underlying this phenomenon and the true correlation between coagulopathy and future de novo malignancy remains unclear.
In this study, we found that PTLD was the most commonly reported de novo cancer after LT for PSC, which accounted for 24% of de novo cancers. PTLD is associated with Epstein-Bar virus ( EBV) infection in 90% of the cases, especially in the case of EBV seronegative recipients of organs from EBV seropositive donors33. The overall frequency of PTLD was 6.1% in all patients, which is higher than the reported frequency in the literature5,9. This supports the previous finding by our group in a multicenter study showing that PSC patients accounted for the highest fraction of hematological malignancies post-LT when compared to other indications for LT8. This increased risk could be secondary to the potency and duration of immunosuppression, but other factors are likely at play. Further investigations into genetic and possibly pharmacogenomics of these patients are warranted34, 35. The presence of leukopenia at the time of LT reflects a more advanced stage of cirrhosis, since the occurrence of leukopenia lags thrombocytopenia by almost 2.5 years. Almost all of our leukopenic patients (97.2%) had thrombocytopenia at time of LT, and this combination could predict increased morbidity and mortality36. The occurrence of PTLD in leukopenic patients could be attributed to an additional underlying immunodeficiency in such patients, or to impaired cytokine release and activity. Further investigation is warranted.
Our study found that colorectal cancer and renal cell cancer were the most common solid tumors after LT for PSC. We confirmed the results of a study by the NIDDK that most post-LT patients who developed colorectal cancer (10/11 patients) had ulcerative colitis (with intact colon at the time of LT)8. The 10-year cumulative incidence of colorectal cancer in our population was lower than what was reported previously8, 37. This low rate could be attributed to the exclusion of patients with colonic dysplasia and due to frequent colonoscopic surveillance and early colectomy in selected patients with longstanding severe bowel disease.
The increased risk of renal cell cancer among liver transplant patients has been reported in previous studies38. The risk increased up to 30-fold after LT in 1 study23. Chronic kidney disease, smoking, overweight, and hypertension are known risk factors for renal cell cancer after solid organ transplantation39, 40. In contrast to what was reported in the literature, patients with renal cell cancer had a good prognosis in our study2. This could be attributed to early detection on routine annual abdominal ultrasound performed per protocol.
Pancreatic cancer and de novo cholangiocarcinoma accounted for 8% and 5.4% of cancers, respectively. Pancreatic adenocarcinoma accounted for all of the pancreatic cancer cases, and all patients did poorly despite systemic treatment. One previous study found a significantly increased risk of pancreatic cancer after LT41. Similar to the general population, the 5-year relative survival rate for pancreatic cancer is low (8%) because more than 1/2 of patients are diagnosed at a distant late stage, which decreases the survival to 2%42. The retained intrapancreatic portion of the common bile duct is the likely source for the pancreatic cancers. Of note, all patients who developed pancreatic cancer were immunosuppressed with mycophenolate mofetil, prednisone, and tacrolimus, and none of them were exposed to azathioprine or cyclosporine. Whether such agents increased the risk or simply reflects a different era of transplant is difficult to determine, but this observation needs further evaluation in prospective studies. Notably, all patients with posttransplant cholangiocarcinoma, had a hepaticojejunostomy and recurrent PSC, thus truly a de novo bile duct cancer.
Common de novo solid tumors after LT such as head and neck which accounts for 17.0% of the solid tumors post-LT, esophageal (12.0%), and lung cancer (10.0%) were not frequent in our study43. The risk of these cancer development is inversely related to age and highly associated with excess alcohol consumption and cigarette smoking2, 25, 44, 45. The low proportion of smokers and the young age of patients in our study accounted for such results.
Intensive screening protocols were followed at our institution after liver transplantation for PSC. This was proved to promote early diagnosis and improved survival46. Most of renal, colon, and prostate cancers and some of the pancreatic, PTLD, and CCA were detected due to adherence to such protocols. The strategies which were followed included annual dermatological skin exam, annual abdominal ultrasound, chest and abdominal CT scan annually for the first 3 years post transplant, annual prostate-specific antigen and digital rectal examination, mammography every annually, colonoscopy every 5 years if no history of colonic dysplasia or IBD, every 3 to 5 years with history of neoplasia or advanced adenomas, and yearly in patients with IBD43, 47. All of the aforementioned screening tests are performed pretransplant to ensure the patient is cancer free prior to transplant.
While this is the only study to determine the cumulative incidence and risk factors for de novo malignancies after LT for PSC patients, there are some limitations. In addition to the retrospective nature of our study, we were not able to report the intensity of immunosuppression in all patients due to logistic reasons. In addition, other confounding factors may not have been accounted for. We follow transplant recipients for life with annual evaluations; thus, the capture rate of identified cancers is high in our medical record. However, it is possible that not all cancers in this population were accounted for.
In conclusion, de novo malignancy developed in 21.8 percent of PSC patients after LT. The estimated 10-year cumulative risk for de novo cancer was 18.7%%. The most common malignancies were PTLD, renal cell cancer and colorectal cancer. Mortality relating to de novo malignancies was high. Adherence to screening protocols is recommended to detect malignancies in early stages to increase the probability of survival.
Acknowledgments
We acknowledge support from NCI CA 170357 and the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567).
Abbreviation
- HLA
Human leukocyte antigen
- LT
liver transplantation
- NLR
Neutrophil to lymphocyte ratio
- NMSC
Nonmelanoma skin cancers
- PTLD
Posttransplant lymphoproliferative diseases
- PSC
Primary sclerosing cholangitis
Footnotes
Authors’ contributions:
M. Mouchli: reviewed medical records and data and participated in the writing of the paper
S. Singh: reviewed medical records and data and participated in the writing of the paper
EV. Loftus Jr.: edited the manuscript and participated in the writing of the paper
JA Talwalkar: edited the manuscript
JK. Heimbach: edited the manuscript
CB Rosen: edited the manuscript
RH. Wiesner: edited the manuscript
L Boardman: edited the manuscript
B Hasan: reviewed medical records and edited the manuscript
JJ. Poterucha: reviewed medical records and data and participated in the writing of the paper
KD. Watt: reviewed medical records and data and participated in the writing of the paper
Disclosures: The authors have no financial or personal relationships that could present a potential conflict of interest.
Dr. Loftus has consulted for Takeda, Janssen, UCB Pharma, AbbVie, Seres Therapeutics, Bristol-Myers Squibb, Amgen, Mesoblast, Eli Lilly, and Salix, and has received research support from Takeda, Janssen, UCB Pharma, AbbVie, Seres Therapeutics, Amgen, Pfizer, Receptos, Celgene, and Robarts Clinical Trials.
References
- 1.Collett D, Mumford L, Banner NR, et al. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 2.Jain AB, Yee LD, Nalesnik MA, et al. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998;66:1193–200. doi: 10.1097/00007890-199811150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Schrem H, Kurok M, Kaltenborn A, et al. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19:1252–61. doi: 10.1002/lt.23722. [DOI] [PubMed] [Google Scholar]
- 4.Aberg F, Pukkala E, Hockerstedt K, et al. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14:1428–36. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Villeneuve PJ, Fenton SS, et al. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14:1588–97. doi: 10.1002/lt.21554. [DOI] [PubMed] [Google Scholar]
- 6.Tjon AS, Sint Nicolaas J, Kwekkeboom J, et al. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl. 2010;16:837–46. doi: 10.1002/lt.22064. [DOI] [PubMed] [Google Scholar]
- 7.Na R, Grulich AE, Meagher NS, et al. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Transplant. 2013;13:1296–304. doi: 10.1111/ajt.12192. [DOI] [PubMed] [Google Scholar]
- 8.Watt KD, Pedersen RA, Kremers WK, et al. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–7. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung JJ, Jain A, Kwak EJ, et al. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7:S109–18. doi: 10.1053/jlts.2001.28645. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oo YH, Gunson BK, Lancashire RJ, et al. Incidence of cancers following orthotopic liver transplantation in a single center: comparison with national cancer incidence rates for England and Wales. Transplantation. 2005;80:759–64. doi: 10.1097/01.tp.0000173775.16579.18. [DOI] [PubMed] [Google Scholar]
- 12.Na R, Grulich AE, Meagher NS, et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13:174–83. doi: 10.1111/j.1600-6143.2012.04302.x. [DOI] [PubMed] [Google Scholar]
- 13.Galve ML, Cuervas-Mons V, Figueras J, et al. Incidence and outcome of de novo malignancies after liver transplantation. Transplant Proc. 1999;31:1275–7. doi: 10.1016/s0041-1345(98)01994-0. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez EQ, Marubashi S, Jung G, et al. De novo tumors after liver transplantation: a single-institution experience. Liver Transpl. 2002;8:285–91. doi: 10.1053/jlts.2002.29350. [DOI] [PubMed] [Google Scholar]
- 15.Saigal S, Norris S, Muiesan P, et al. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482–7. doi: 10.1053/jlts.2002.32977. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, Patil VP, Fung J. Incidence of de novo cancer and lymphoproliferative disorders after liver transplantation in relation to age and duration of follow-up. Liver Transpl. 2008;14:1406–11. doi: 10.1002/lt.21609. [DOI] [PubMed] [Google Scholar]
- 17.Yao FY, Gautam M, Palese C, et al. De novo malignancies following liver transplantation: a case-control study with long-term follow-up. Clin Transplant. 2006;20:617–23. doi: 10.1111/j.1399-0012.2006.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai R, Neuberger J. Donor transmitted and de novo cancer after liver transplantation. World J Gastroenterol. 2014;20:6170–9. doi: 10.3748/wjg.v20.i20.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettorre GM, Piselli P, Galatioto L, et al. De novo malignancies following liver transplantation: results from a multicentric study in central and southern Italy, 1990–2008. Transplant Proc. 2013;45:2729–32. doi: 10.1016/j.transproceed.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Aberg F, Isoniemi H, Hockerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- 21.Herrero JI. De novo malignancies following liver transplantation: impact and recommendations. Liver Transpl. 2009;15(Suppl 2):S90–4. doi: 10.1002/lt.21898. [DOI] [PubMed] [Google Scholar]
- 22.Carenco C, Faure S, Herrero A, et al. Incidence of solid organ cancers after liver transplantation: comparison with regional cancer incidence rates and risk factors. Liver Int. 2015;35:1748–55. doi: 10.1111/liv.12758. [DOI] [PubMed] [Google Scholar]
- 23.Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 24.Bakker NA, van Imhoff GW, Verschuuren EA, et al. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int. 2007;20:207–18. doi: 10.1111/j.1432-2277.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 25.Benlloch S, Berenguer M, Prieto M, et al. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant. 2004;4:596–604. doi: 10.1111/j.1600-6143.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- 26.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 27.Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31–41. doi: 10.1055/s-2006-933561. [DOI] [PubMed] [Google Scholar]
- 28.Graziadei IW, Wiesner RH, Batts KP, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–6. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 29.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipeki N, Antal-Szalmas P, Lakatos PL, et al. Immune dysfunction in cirrhosis. World J Gastroenterol. 2014;20:2564–77. doi: 10.3748/wjg.v20.i10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aderka D, Brown A, Zelikovski A, et al. Idiopathic deep vein thrombosis in an apparently healthy patient as a premonitory sign of occult cancer. Cancer. 1986;57:1846–9. doi: 10.1002/1097-0142(19860501)57:9<1846::aid-cncr2820570925>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalesnik MA, Starzl TE. Epstein-Barr virus, infectious mononucleosis, and posttransplant lymphoproliferative disorders. Transplant Sci. 1994;4:61–79. [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493–9. doi: 10.1097/00007890-199808270-00014. [DOI] [PubMed] [Google Scholar]
- 35.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–30. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 36.Qamar AA, Grace ND, Groszmann RJ, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–95. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S, Edakkanambeth Varayil J, Loftus EV, Jr, et al. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 2013;19:1361–9. doi: 10.1002/lt.23741. [DOI] [PubMed] [Google Scholar]
- 38.Jonas S, Rayes N, Neumann U, et al. De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or antithymocyte globulin. Cancer. 1997;80:1141–50. doi: 10.1002/(sici)1097-0142(19970915)80:6<1141::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Liew G, Mitchell P, Wong TY, et al. CKD increases the risk of age-related macular degeneration. J Am Soc Nephrol. 2008;19:806–11. doi: 10.1681/ASN.2007080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godley PA, Escobar MA. Renal cell carcinoma. Curr Opin Oncol. 1998;10:261–5. doi: 10.1097/00001622-199805000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko J, Sugawara Y, Tamura S, et al. De novo malignancies after adult-to-adult living-donor liver transplantation with a malignancy surveillance program: comparison with a Japanese population-based study. Transplantation. 2013;95:1142–7. doi: 10.1097/TP.0b013e318288ca83. [DOI] [PubMed] [Google Scholar]
- 42.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 43.Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol. 2015;21:8753–68. doi: 10.3748/wjg.v21.i29.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellamy CO, DiMartini AM, Ruppert K, et al. Liver transplantation for alcoholic cirrhosis: long term follow-up and impact of disease recurrence. Transplantation. 2001;72:619–26. doi: 10.1097/00007890-200108270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaughan GW, Vajdic CM. De novo malignant disease after liver transplantation? Risk and surveillance strategies. Liver Transpl. 2013;19(Suppl 2):S62–7. doi: 10.1002/lt.23738. [DOI] [PubMed] [Google Scholar]
- 46.Finkenstedt A, Graziadei IW, Oberaigner W, et al. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355–61. doi: 10.1111/j.1600-6143.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 47.Bhat M, Al-Busafi S, Deschenes M, et al. Care of the liver transplant patient. Can J Gastroenterol Hepatol. 2014;28:213–9. doi: 10.1155/2014/453875. [DOI] [PMC free article] [PubMed] [Google Scholar]