Abstract
Objective
to examine the sensitivity and specificity of the Montreal Cognitive Assessment (MoCA), a brief cognitive screening measure previously validated for use in Parkinson disease (PD), and Alzheimer’s Disease-8 (AD8), an eight-item informant report used to screen for dementia, but not yet validated for use in PD, to identify cognitive impairment in a sample of 111 patients with PD.
Methods
cognitive impairment was determined based on a battery of neuropsychological measures, excluding the MoCA and AD8. Classification rates of both the MoCA and AD8 in identifying cognitive impairment were examined using logistic regression and receiver operator characteristic (ROC) analysis. Optimal cutoff scores were determined to maximize sensitivity and specificity.
Results
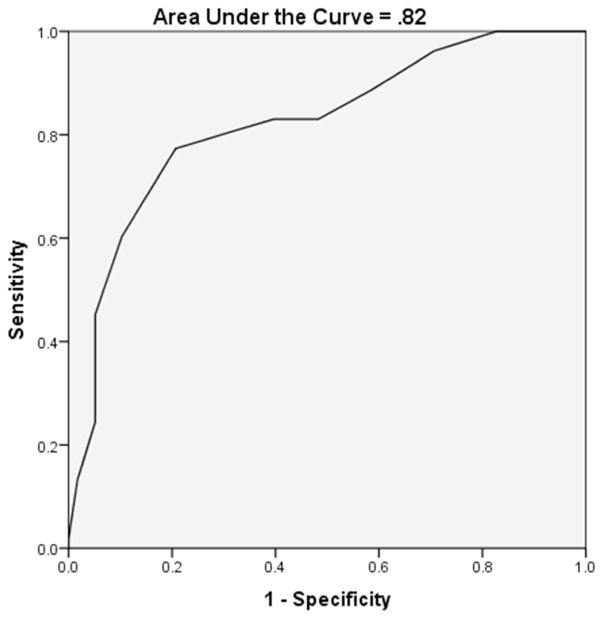
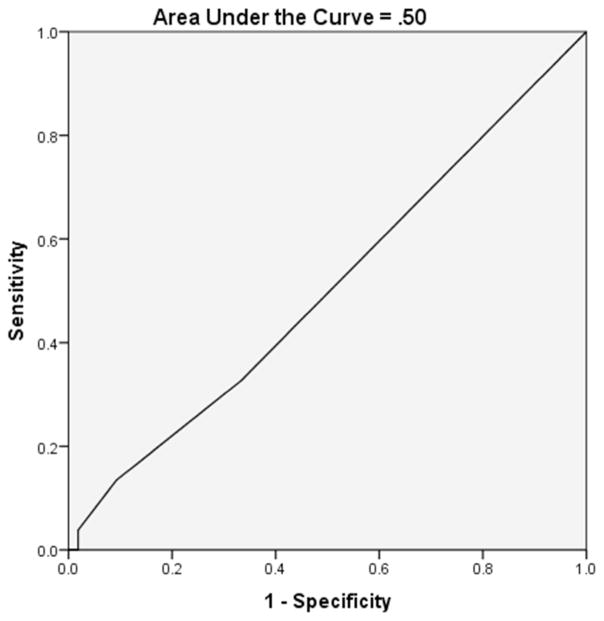
the MoCA correctly classified 78.4% of participants (p<.001) and ROC analysis yielded an area under the curve (AUC) of .82. A MoCA cutoff score of <25 yielded optimal sensitivity (.77) and specificity (.79) for identifying PD patients with cognitive impairment. Similar analyses for the AD8 were statistically nonsignificant, although the classification rate was 70.5%, with an AUC of .50.
Conclusions
these results provide additional support for the MoCA, but not the AD8, in identifying cognitive impairment in patients with PD.
Keywords: Parkinson disease, Montreal Cognitive Assessment, Alzheimer’s Disease-8, brief screening measures, cognitive impairment, neuropsychological assessment
Introduction
Cognitive impairment is common in individuals with Parkinson disease (PD), although studies of prevalence rates vary widely with ranges between 22% and 93% (Aarsland et al., 2001, 2010; Dubois and Pillon, 1997; Muslimović et al., 2005; Pirozzolo et al., 1982; Verbaan et al., 2007). Therefore, the identification of cognitive impairment in this population is imperative to help determine the specific level and type of care patients might require. To assist in this identification, there is a need for brief and valid screening measures for cognitive impairment in PD that can be incorporated as part of routine clinical care. Once validated for clinical use in this population, clinicians could then use the results of such screeners to determine if recommending a comprehensive neuropsychological evaluation for diagnostic clarification is indicated.
The Movement Disorder Society (MDS) Task Force for creating diagnostic procedures for Parkinson disease with dementia (PD-D) originally recommended the use of the Mini Mental State Examination (MMSE; Folstein et al., 1975) with a total score cutoff of <26 as the most appropriate standard objective assessment of global cognitive functioning (Dubois et al., 2007). Subsequent research evaluated the sensitivity and specificity of the MMSE to screen for cognitive impairment in PD, with comparisons made to other brief neuropsychological measures, such as the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005). Hanna-Pladdy et al. (2010) found that the MoCA was more sensitive than the MMSE in detecting cognitive decline in PD, but noted concerns that the MoCA may over-identify cognitive impairment. Also, Dalrymple-Alford et al. (2010) found that the MoCA, with a cutoff total score of <21 for PD-D and <26 for Parkinson disease with mild cognitive impairment (PD-MCI), had superior discrimination properties over both the MMSE and Scales for Outcomes in Parkinson disease-Cognition (SCOPA-COG; Marinus et al., 2003). Similarly, Zadikoff et al. (2007) found that the MoCA, with a cutoff total score of <26, was more sensitive than the MMSE in discriminating between PD without cognitive impairment and PD-MCI. These investigators pointed specifically to the issue of ceiling effects with the MMSE, which were less likely with the MoCA. Several other studies have also shown support for the MoCA as a screening measure in the PD population (Chou et al., 2010; Gill et al., 2008; Hoops et al., 2009; Nazem et al., 2009).
When the MDS task force revised their diagnostic criteria in 2012, they replaced the MMSE with the MoCA to assess global cognitive function (Litvan et al., 2012). However, it should be noted that a recent longitudinal comparison of the MoCA and MMSE in PD populations found that while the MoCA was more sensitive to the detection of early cognitive impairment, the MMSE was more useful at tracking cognitive change over time (Lessig et al., 2012).
In addition to brief neuropsychological tests, a potentially conjunctive technique to screen for cognitive impairment is the use of informant-based reports of cognitive function. Using informant-based report measures in conjunction with cognitive screening measures (e.g., MoCA) may increase the likelihood of detecting early stages of cognitive decline, particularly in higher functioning individuals. Informant-based reports may be especially useful in identifying cognitive change when there is no prior neurocognitive assessment with which to compare. A useful informant-based measure is the Alzheimer’s Disease-8 (AD8; Galvin et al., 2005), which is a brief eight-item questionnaire that can be administered in under three minutes, with good psychometric properties that was designed to differentiate between individuals with or without dementia. Items include questions that ask about memory, orientation, judgment, and function (Galvin et al., 2005). The AD8 has been validated as a self-report measure (Galvin et al., 2007a) and was found to have superior discrimination properties when used in combination with other brief objective measures (e.g., word recall list; Galvin et al., 2006). Also, Galvin et al. (2010) found that the AD8 had better sensitivity for the screening of early-stage dementia relative to the MMSE, and it discriminated between individuals with and without abnormal Pittsburgh compound B (PIB) binding, a biomarker of Alzheimer’s disease (AD).
Both the MoCA and AD8 appeared in the Journal of the American Board of Family Medicine’s recent Practical Guidelines for the Recognition and Diagnosis of Dementia (Galvin and Sadowsky, 2012). Also, the utility of the MoCA and AD8 has been compared in specific populations with known associations with cognitive disorders, including human immunodeficiency virus (HIV; Overton et al., 2013). That study found the AD8 successfully discriminated between participants with and without cognitive impairment, with 61% sensitivity and 51% specificity, which slightly underperformed relative to the MoCA (with sensitivity and specificity rates of 63% and 71%, respectively). To our knowledge, there has been no examination of the utility of the AD8 to screen cognitive functions in PD. If the AD8 was found to be useful in identification of cognitive impairment in PD, the measure could become a more standard part of clinical assessments for quick and easy early detection of cognitive issues. Also, there has been no comparison of the AD8 and the MoCA in the identification of cognitive impairment in PD. Thus, the purpose of the present study was to assess the utility of both the MoCA and AD8 as brief screening measures for identifying cognitive impairment in PD. We hypothesized that both the MoCA and AD8 would significantly differentiate cognitively impaired from cognitively unimpaired PD patients. Additionally, we hypothesized that the combined use of the MoCA and AD8 would successfully discriminate cognitively impaired from cognitively unimpaired PD patients above and beyond using either measure alone.
Methods
Participants
The sample included 111 participants diagnosed with PD by a movement disorder specialist at the University of Texas Southwestern Medical Center who were part of a larger institutional review board-approved study that examined the psychometric properties of the National Institutes of Health Toolbox. In brief, participants completed clinical, sensory, neurologic, and neurocognitive assessments at three time points. The assessments included objective measures administered by trained clinical neuropsychometricians, and informant-based measures. The data used for this study was collected at the first study visit (baseline).
Participants in the study who were males and females between the ages of 39 and 89 years, diagnosed with PD (asymmetric features including bradykinesia plus resting tremor and/or rigidity), treated and responsive to dopaminergic therapy (dopamine agonists or levodopa) for at least 30 days, able to complete assessments, had English as a first language, and were willing and able to give informed consent and commit to three testing sessions. We excluded those participants who had other known or suspected cause of parkinsonism, or any significant features suggestive of a diagnosis of atypical parkinsonism, lifetime neurological diagnosis other than PD, lifetime Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision, Axis I psychiatric diagnosis other than major depressive disorder, current alcohol and/or substance abuse or dependence within the prior 6-months, and/or any unstable or clinically significant condition that could have interfered with completion of the study assessments.
Materials and Procedure
The AD8 (Galvin et al., 2005) was administered to a family member as part of the screening process into the parent study, and the MoCA (Nasreddine et al., 2005) was administered to participants as part of a larger neuropsychological battery that included the Rey Auditory Verbal Learning Test (RAVLT; Schmidt, 1996), Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997), Trail Making Test (Reitan and Wolfson, 1995), three subtests from the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Letter-Number Sequencing, Digit Symbol Coding, and Symbol Search subtests; Wechsler, 1997), and the Peabody Picture Vocabulary Test-Fourth Edition (PPVT-4; Dunn and Dunn, 2007). Neuropsychological variables of interest included MoCA and AD8 raw scores; the RAVLT trials 1–5 total and delayed recall T scores; BVMT-R total recall and delayed recall T scores; Trail Making Test part B total time T score; WAIS-III Letter-Number Sequencing, Digit Symbol Coding, and Symbol Search scaled scores; and PPVT-4 standard score. Normative data for the RAVLT, BVMT-R, WAIS-III subtests and PPVT-4 were derived from the test manuals (referenced above). Normative data for the Trail Making Test part B were derived from Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Norms for African American and Caucasian Adults (Heaton et al., 2004). These measures were selected from the larger battery to represent cognitive domains commonly assessed in PD: learning and memory (RAVLT and BVMT-R), complex visual scanning (WAIS-III Symbol Search), cognitive flexibility (Trail Making Test part B and WAIS-III Letter-Number Sequencing), attention (WAIS-III Digit Symbol Coding), and vocabulary (PPVT-4; Litvan et al., 2011). The AD8 was administered within 90 (median=39) days of formal cognitive assessment and included a subsequent review of each item by a neurologist and the informant together to ensure accuracy of the information. Specifically, the neurologist verified that items endorsed did in fact appear to be a function of cognitive impairment rather than other causes such as motor impairment. The Unified Parkinson’s Disease Rating Scale Part III—Motor Examination (UPDRS; Goetz et al., 2008) was administered (at the same visit the AD8 was given), to obtain a measure of motor function. The M.I.N.I. International Neuropsychiatric Interview Plus 6.0, Module A: Major Depressive Episode (MINI; Lecrubier et al., 1997) was administered with the neuropsychological battery, to obtain a measure of whether each participant qualifies for a diagnosis of Major Depressive Disorder. Following the Level I recommendations by Litvan et al. (2012), participants were classified as cognitively impaired if they had at least two of nine demographically-adjusted test scores (excluding the MoCA and the AD8), from two or more different tests in the battery, greater than or equal to one standard deviation below the mean. It should be noted that full Litvan criteria for mild cognitive impairment was not applied (e.g., requiring the presence of a cognitive complaint) as it was not our intention to make a formal diagnosis of mild cognitive impairment, but rather to identify subjects who had cognitive impairment on more detailed testing.
Statistical Analyses
Statistical analyses were accomplished using Statistical Analysis System (SAS) version 9.3 (SAS Institute, Cary, NC), and the Statistical Package for Social Sciences, version 22 (SPSS, Armonk, NY). Exploratory analyses were conducted to reveal relevant demographic information, including means, standard deviations and ranges of age and education, as well as percentages for gender and ethnicity. All relevant assumptions were tested. Classification rates of both the MoCA and AD8 in identifying cognitive impairment were examined using logistic regression and receiver operator characteristic (ROC) analysis. Optimal cutoff scores were determined to maximize sensitivity and specificity.
Results
Participants (N=111) had a mean age of 65.19 years (SD=9.65, range: 39–91 years) and mean education of 15.56 years (SD=1.88, range: 8–18 years). The majority of subjects were male (72.1%) and Caucasian (88.3%). Ninety-one (82.0%) participants were on a levodopa medication, 71 (64.0%) were on a dopamine agonist, 74 (66.7%) were on an MAO-B inhibitor, and 28 (25.2%) were on entacapone. Also, seven (6.3%) participants were on either modafinil or armodafinil, seven (6.3%) were on an acetylcholinesterase inhibitor, two (1.8%) were on memantine, and one (0.9%) was on Adderall; medications were unavailable for one participant. Table 1 provides additional sociodemographic and clinical characteristics of the sample.
Table 1.
demographic information
| Variable | Cognitively Impaired | Cognitively Unimpaired | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | range | n | M (SD) | range | n | M (SD) | range | |
| Age (years) | 58 | 67.43 (10.06) | 39–91 | 53 | 62.73** (8.61) | 47–81 | 111 | 65.19 (9.65) | 39–91 |
| Education (years) | 58 | 15.17 (2.15) | 8–18 | 51 | 15.98* (1.43) | 13–18 | 111 | 15.56 (1.88) | 8–18 |
| Disease duration (years) | 57 | 7.25 (4.89) | 0–22 | 53 | 5.72 (4.77) | 1–18 | 110 | 6.51 (4.87) | 0–22 |
| UPDRS-Part III | 55 | 13.20 (9.45) | 0–44 | 53 | 8.89** (6.48) | 0–28 | 108 | 11.22 (8.43) | 0–44 |
|
| |||||||||
| n | % | n | % | n | % | ||||
|
| |||||||||
| Gender (n male; %) | 46 | 79.3% | 34 | 64.2% | 80 | 72.1% | |||
| Ethnicity | |||||||||
| Caucasian | 47 | 81.0% | 51 | 96.2% | 98 | 88.3% | |||
| African-American | 4 | 6.9% | 0 | 0.0% | 4 | 3.6% | |||
| Latino | 5 | 8.6% | 2 | 3.8% | 7 | 6.3% | |||
| Asian | 1 | 1.7% | 0 | 0.0% | 1 | 0.9% | |||
| Unknown | 1 | 1.7% | 0 | 0.0% | 1 | 0.9% | |||
| Major Depression† (n yes; %) | 3 | 5.2% | 2 | 3.8% | 5 | 4.5% | |||
UPDRS = Unified Parkinson’s Disease Rating Scale Part III—Motor Examination;
p < .05,
p < .01;
whether participant qualifies for a Major Depressive Disorder diagnosis according to the M.I.N.I. International Neuropsychiatric Interview depression module
Using the criteria outlined above, 58 of the 111 participants (52.3%) were classified as having impaired cognitive function. The cognitively impaired group was significantly older [t (109)=2.63, p=.01], had slightly less years of education [t (100.15)=−2.35, p=.02], and had higher UPDRS total scores [t (95.89)=2.78, p=.01] relative to the unimpaired group. There were no differences between groups in terms of PD duration, gender, Major Depressive Disorder diagnosis qualification according to the MINI, or ethnicity (see Table 1). For the impaired group, performance on neurocognitive measures fell within the mildly impaired to low average range for most variables. For the unimpaired group, performance on neurocognitive measures fell within the average range, though there was a broad range of individual scores (see Table 2).
Table 2.
mean, standard deviation and range of neuropsychological test scores in cognitively impaired and unimpaired groups
| Variable | Cognitively Impaired (n = 58) | Cognitively Unimpaired (n = 53) | ||
|---|---|---|---|---|
| M (SD) | range | M (SD) | range | |
| RAVLT Total (T score) | 40.17 (11.35) | 14–79 | 53.57* (10.37) | 31–77 |
| RAVLT Delayed Recall (T score) | 41.05 (12.02) | 19–76 | 53.04* (8.83) | 27–71 |
| BVMT-R Total (T score) | 36.88 (10.03) | 20–62 | 51.47* (9.68) | 25–72 |
| BVMT-R Delayed Recall (T score) | 41.10 (11.65) | 20–64 | 55.06* (8.58) | 32–67 |
| Trail Making Test B† (T score) | 39.18 (12.65) | 10–73 | 52.00* (10.64) | 26–88 |
| WAIS-IV Letter-Number Sequencing (scaled score) | 7.28 (2.35) | 1–11 | 10.02* (2.27) | 7–17 |
| WAIS-IV Digit Symbol Coding†† (scaled score) | 7.39 (2.62) | 1–13 | 10.40* (2.38) | 4–16 |
| WAIS-IV Symbol Search (scaled score) | 7.45 (2.58) | 1–13 | 10.83* (2.01) | 7–15 |
| PPVT-4 (standard score) | 103.38 (11.18) | 73–121 | 110.07* (10.07) | 93–142 |
|
| ||||
| MoCA (raw score) | 21.90 (3.55) | 12–29 | 25.74* (2.57) | 20–30 |
| AD8††† (raw score) | .50 (.99) | 0–6 | .54 (.98) | 0–5 |
RAVLT = Rey Auditory Verbal Learning Test; BVMT-R = Brief Visuospatial Memory Test-Revised; WAIS-IV = Wechsler Adult Intelligence Scale-IV; PPVT-4 = Peabody Picture Vocabulary Test4;
p < .001;
n = 56 for impaired group;
n = 57 for impaired group;
n = 54 for impaired and 52 for unimpaired group
Participants in the cognitively impaired group demonstrated lower MoCA scores [M=21.90 (SD=3.55), range: 12–29] compared to the unimpaired group [M=25.74 (SD=2.57), range: 20–30]. Contrary to prediction, there was no significant difference between groups with regard to the AD8 total score [t (104)=−.20, p=.84], with a slightly higher mean total AD8 score in the unimpaired group [n=52; M=.54 (SD=.98), range: 0–5] compared to the impaired group [n=54; M=.50 (SD=.99), range: 0–6], although the standard deviations were large.
The MoCA correctly classified 78.4% of participants (p<.001) using logistic regression. Neither age, gender, ethnicity, Major Depressive Disorder diagnosis qualification according to the MINI, education, disease duration, nor the UPDRS total score significantly (α=.05) or marginally (α=.15) contributed to the regression analysis. As such, those variables were excluded from the final model. Receiver operator characteristic analysis yielded an area under the curve (AUC) of .82 (see Figure 1) for the MoCA. Using a MoCA cutoff score of <25 yielded optimal sensitivity (.77) and specificity (.79) for identifying PD patients with cognitive impairment (p<.001). The sensitivity and specificity values for each cutoff score are provided in Table 3.
Figure 1.
Montreal Cognitive Assessment Receiver Operator Characteristic Curve
Table 3.
Montreal Cognitive Assessment sensitivity and specificity
| Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 11.0 | 100 | 0 |
| 12.5 | 100 | 2 |
| 14.0 | 100 | 13 |
| 15.5 | 100 | 15 |
| 16.5 | 100 | 10 |
| 17.5 | 100 | 12 |
| 19.0 | 100 | 17 |
| 20.5 | 96 | 29 |
| 21.5 | 89 | 41 |
| 22.5 | 83 | 52 |
| 23.5 | 83 | 60 |
| 24.5 | 77 | 79 |
| 25.5 | 60 | 90 |
| 26.5 | 45 | 95 |
| 27.5 | 25 | 95 |
| 28.5 | 13 | 98 |
| 29.5 | 2 | 100 |
| 31.0* | 0 | 100 |
A score of 31 reflects a one point correction for ≤ 12 years of education
The AD8, in combination with age, education, gender and disease duration, correctly classified 70.5% of participants, but did not significantly contribute to the regression model (p=.41). Age, education and gender significantly contributed (p=.01, .04 and .03 respectively), and disease duration marginally contributed (p=.11) to the regression analysis. Thus, those variables were included in the final model. Receiver operator characteristic analysis yielded an AUC of .50 for the AD8 (see Figure 2). We initially planned to assess the combined utility of the MoCA and AD8 in order to compare it to the independent efficacy of each. However, because the AD8 did not significantly differentiate our impaired and unimpaired groups, we did not analyze the combination of both instruments.
Figure 2.
Alzheimer’s Disease-8 Receiver Operator Characteristic Curve
As a follow-up analysis, we examined the logistical regression models with cognitive impairment defined as demographically-adjusted total score on two different neurocognitive test variables less than or equal to 1.5 standard deviations, as well as less than or equal to two standard deviations below the mean. Although the outcomes of the logistic regression and ROC AUC analyses were not significantly different, the cell sizes between groups became increasingly more discrepant. For this reason, results are reported only for impairment defined using the original one standard deviation criterion.
Discussion
These results provide additional support for use of the MoCA in identifying cognitive impairment in patients with PD, but not the AD8. Previous studies have shown similar ROC AUC values (.79–.90) when they assessed the utility of the MoCA in the PD population (Dalrymple-Alford et al., 2010; Gill et al., 2008; Hoops et al., 2009). However, the optimal MoCA cutoff score of <25 in this sample was slightly lower than previous studies have suggested (<26), which may be a function of the differences in criteria used to define cognitive impairment across investigations (e.g., Dalrymple-Alford et al., 2010; Hoops et al., 2009). Hoops et al. (2009) used a more conservative criteria of at least ≤1.5 (versus ≤ 1 in the present study) standard deviations below the mean on neuropsychological tests in any cognitive domain to define impairment, and emphasized sensitivity (.90) at the expense of specificity (.53) in an effort to identify the optimal “screening cutoff.” Dalrymple-Alford et al. (2010) used an even more conservative criterion of ≤ 1.5 standard deviations below the mean on any two neuropsychological tests in their battery, and also found an optimal total score cutoff of <26. However, only PD-MCI patients were included in that group and the authors reported no optimal cutoff score for their PD-MCI and PD-D participants combined, whereas our study did not distinguish between the two groups. It is worth noting that our cutoff was derived based on how well the MoCA was able to identify cognitive impairment on a larger cognitive battery rather than a specific diagnosis of mild cognitive impairment, which might explain our lower cutoff. Clinically, determining an optimal cutoff score in this population will depend on whether the clinician wants to optimize sensitivity or specificity as well as considering other factors that could impact test performance (e.g., level of motor impairment, demographic factors).
Although none of the demographic covariates we incorporated significantly contributed to the regression model, the optimal MoCA cutoff score might still differ as a function of such variables. This may be especially true for education, as it has previously been found to be significantly associated with MoCA score (Bernstein et al., 2011; Malek-Ahmadi et al., 2015). Our sample size was too small to explore this possibility. However, future studies should examine whether sensitivity/specificity results from ROC analyses differ by education and/or other demographic factors.
The failure of the AD8 to yield utility in our sample may be due to several reasons. The AD8 scores were low in both groups and surprisingly, slightly higher in the unimpaired group, which contributed to the poor discriminability in identifying cognitive impairment. Few scores in both groups were above 2. In fact, 62.1% in the impaired group and 66.0% in the unimpaired group had scores of 0. Our results were similar to those of Overton et al. (2013) who found the AD8 AUC to be .56, when discriminating cognitively abnormal versus normal individuals (as determined by neuropsychological test performance falling below a z-score of -1 in at least two domains) diagnosed with HIV. This suggests the applicability of the AD8 may be limited in diverse neurological samples and/or those with less severe impairment.
The AD8 AUC of the present study was much lower than those reported in other studies that assessed the utility of the AD8 in discriminating cognitively normal from demented (including “very mild” and “uncertain dementia”) participants (AUC=.77 – .94) which may relate to the greater degree of deficits in their impaired groups, compared to our mildly impaired PD sample (Galvin et al., 2010; Galvin et al., 2005; Galvin, Roe, Coats, & Morris, 2007a; Galvin, Roe, & Morris, 2007b; Galvin et al., 2006). In addition, Carpenter et al. (2011) found the AD8 yielded an AUC of .82 for discriminating cognitively impaired from cognitively intact individuals over age 65 presenting to the emergency room. In contrast to the aforementioned studies, and more similar to the present study, the AD8 scores in the present study were relatively equal across groups. This may suggest that the utility of this informant-based measure in a mildly impaired population is limited, particularly in the context of a neurological disease that can also affect areas of functioning (i.e., motor ability). In such instances, the AD8 may be less sensitive to cognitive problems than the MoCA, which is not surprising, given the variability of subjective reports. Thus, objective neuropsychological assessment, including brief screening measures, may detect cognitive decline before it is functionally manifest and observable to others. This might be expected, as the AD8 was not designed to detect MCI. However, the AD8 may have increased utility in more impaired PD populations, such as PD-D.
Even though the AD8 asks the participant to indicate a “change in the last several years caused by cognitive (thinking and memory) problems,” informants may over-estimate other determinants (e.g., motor symptoms) of functional difficulty, thereby minimizing the decline in cognitive abilities. Indeed, it may be difficult for an informant to accurately identify mild cognitive problems when completing the AD8 in the PD population. For example, question four asks if the informant has noticed a change in the patient’s ability of “learning how to use a tool, appliance, or gadget (e.g., VCR, computer, microwave, remote control).” In this case, an informant may be unable to accurately discern the cause of the problem and inadvertently indicate “NO, No change” (in cognitive problems). The findings of this study, in combination with the prior research of the poor utility of the AD8 in patients with HIV, suggest that the AD8 may be more useful in populations with cortical rather than subcortical neuropathology. However, additional research examining the AD8 in a more impaired PD population is needed to fully assess its utility in this population.
It is important to reiterate that while the MoCA was developed as a screening measure for both MCI and dementia, the AD8 was designed only to detect dementia. Furthermore, the cognitively impaired group in this study was very mildly impaired (mean scores falling in the low average to mildly impaired ranges), which may have made it more difficult for the AD8 to identify such subtle impairment.
Conclusion
The present study serves as an initial exploration of the utility of the AD8 to identify cognitive impairment in the PD population. These results support the MoCA, but not the AD8, as a valid, brief screening tool to detect cognitive impairment in PD. Thus, clinical practice may benefit from the inclusion of the MoCA to screen for cognitive impairment in PD populations. Including such a screening measure would help to advance integrated healthcare by identifying which patients may require additional neurocognitive examination. Overall, these results highlight the importance of objective (versus subjective) assessment for cognitive screening in PD and provide support for the utility of the MoCA for this purpose.
Key points.
there is a need for brief and valid measures for the identification of cognitive impairment in individuals with Parkinson disease (PD).
Results provide additional support for use of the Montreal Cognitive Assessment (a brief cognitive screening measure previously validated for use in PD), but not the Alzheimer’s Disease-8 (an eight-item informant report), in identifying cognitive impairment in patients with PD.
Acknowledgments
Funding
This research was supported by grants from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (RC1 NS68983, PI: Mustafa Husain), National Institute of Aging (P30 AG12300, PI: Roger Rosenberg).
Enisa Arslanagic, MD, MPH, CCRC, served as the coordinator of the parent study.
Footnotes
Ethics
Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and regional) and with the Helsinki Declaration of 1975, as revised in 1983.
Potential Conflicts of Interest
Daniel Brown, and Drs. Ira Bernstein and C. Munro Cullum report no conflicts that might bias their work. Dr. Shawn McClintock reports research support from the National Institutes of Health. Dr. Richard Dewey, Jr. reports serving as a consultant for Teva Pharmaceuticals, US WorldMeds, Lundbeck, Acadia, Merz, Xenoport, Impax, and GE Healthcare, and receiving speakers fees from Teva Pharmaceuticals, US WorldMeds, Lundbeck and UCB. Dr. Mustafa Husain reports research support from NIH/NIMH, NINDS, NIA, NARSD, Stanley Medical Foundation, Neuronetics (past), St. Jude Medical (ANS), MagStim (equip only), Brainsway, Neosync, and Alkamers Pharmaceutical. Dr. Husain also reports sitting on the Cerbain Biotech advisory board. Dr. Laura Lacritz reports she is a consultant for Teva Pharmceuticals. No supporting sources had any direct involvement in the study, including the study design, collection, analysis and interpretation of data, writing of the report, or decision to submit the report for publication.
References
- Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson’s disease: A community-based, prospective study. Neurology. 2001;56(6):730–736. doi: 10.1212/WNL.56.6.730. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test-Revised: Professional Manual. Psychological Assessment Resources; Lutz: 1997. [Google Scholar]
- Bernstein IH, Lacritz L, Barlow CE, Weiner MF, DeFina LF. Psychometric Evaluation of the Montreal Cognitive Assessment (MoCA) in Three Diverse Samples. Clin Neuropsychol. 2011;25(1):119–126. doi: 10.1080/13854046.2010.533196. [DOI] [PubMed] [Google Scholar]
- Carpenter CR, Bassett ER, Fischer GM, et al. Four Sensitive Screening Tools to Detect Cognitive Dysfunction in Geriatric Emergency Department Patients: Brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the Caregiver-completed AD8. Acad Emerg Med. 2011;18(4):374–384. doi: 10.1111/j.1553-2712.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Amick MM, Brandt J, et al. A Recommended Scale for Cognitive Screening in Clinical Trials of Parkinson’s Disease. Mov Disord. 2010;25(15):2501–2057. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, et al. Diagnostic Procedures for Parkinson’s Disease Dementia: Recommendations from the Movement Disorder Society Task Force. Mov Disord. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244(1):2–8. doi: 10.1007/PL00007723. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4) The Psychological Corporation; San Antonio: 2007. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Fagan AM, Holtzman DM, Mintun MA, Morris JC. Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain. 2010;133(11):3290–3300. doi: 10.1093/brain/awq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s Rating of Cognitive Ability: Using the AD8, a Brief Informant Interview, as a Self-rating Tool to Detect Dementia. Arch Neurol. 2007a;64(5):725–730. doi: 10.1001/archneur.64.5.725. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Morris JC. Evaluation of Cognitive Impairment in Older Adults: Combining Brief Informant and Performance Measures. Arch Neurol. 2007b;64(5):718–724. doi: 10.1001/archneur.64.5.718. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Sadowsky CH. Practical Guidelines for the Recognition and Diagnosis of Dementia. J Am Board Fam Med. 2012;25(3):367–382. doi: 10.3122/jabfm.2012.03.100181. [DOI] [PubMed] [Google Scholar]
- Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a Screening tool for Cognitive Impairment in Parkinson’s Disease. Mov Disord. 2008;23(7):1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Enslein A, Fray M, et al. Utility of the NeuroTrax Computerized Battery for Cognitive Screening in Parkinson’s Disease: Comparison with the MMSE and the MoCA. Int J Neurosci. 2010;120(8):538–543. doi: 10.3109/00207454.2010.496539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources; Florida: 2004. [Google Scholar]
- Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan D, Weiller E, et al. The M.I.N.I. International Neuropsychiatric Interview (M.I.N.I) A Short Diagnostic Structured Interview: Reliability and Validity According to the CIDI. European psychiatry. 1997;12(5):224–231. doi: 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on Brief Cognitive Instruments Over Time in Parkinson’s Disease. Mov Disord. 2012;27(9):1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on Mild Cognitive Impairment in Parkinson’s Disease: Critical Review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, et al. Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Powell JJ, Belden CM, et al. Age- and education-adjusted normative data for the Montreal Cognitive Assessment (MoCA) in older adults age 70–99. Aging Neuropsychol C. 2015 doi: 10.1080/13825585.2015.1041449. < http://www.tandfonline.com/doi/abs/10.1080/13825585.2015.1041449?journalCode=nanc20>. [DOI] [PubMed]
- Marinus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61(9):1222–1228. doi: 10.1212/01.WNL.0000091864.39702.1C. [DOI] [PubMed] [Google Scholar]
- Muslimović D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nazem S, Siderowf AD, Duda JE, et al. Montreal Cognitive Assessment Performance in Patients with Parkinson’s Disease with “Normal” Global Cognition According to Mini-Mental State Examination Score. J Am Geriatr Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton ET, Azad TD, Parker N, et al. The Alzheimer’s disease-8 and Montreal Cognitive Assessment as screening tools for neurocognitive impairment in HIV-infected persons. J Neurovirol. 2013;19(1):109–16. doi: 10.1007/s13365-012-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzolo FJ, Hansch EC, Mortimer JA, Webster DD, Kuskowski MA. Dementia in Parkinson Disease: A Neuropsychological Analysis. Brain Cogn. 1982;1(1):71–83. doi: 10.1016/0278-2626(82)90007-0. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. Category Test and Trail Making Test as measures of frontal lobe functions. Clin Neuropsychol. 1995;9(1):50–56. doi: 10.1080/1385404950840205. [DOI] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- Verbaan D, Marinus J, Visser M, et al. Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(11):1182–1187. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Third Edition (WAIS–III) The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Zadikoff C, Fox SH, Tang-Wai DF. A Comparison of the Mini Mental State Exam to the Montreal Cognitive Assessment in Identifying Cognitive Deficits in Parkinson’s Disease. Mov Disord. 2008;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]