Abstract
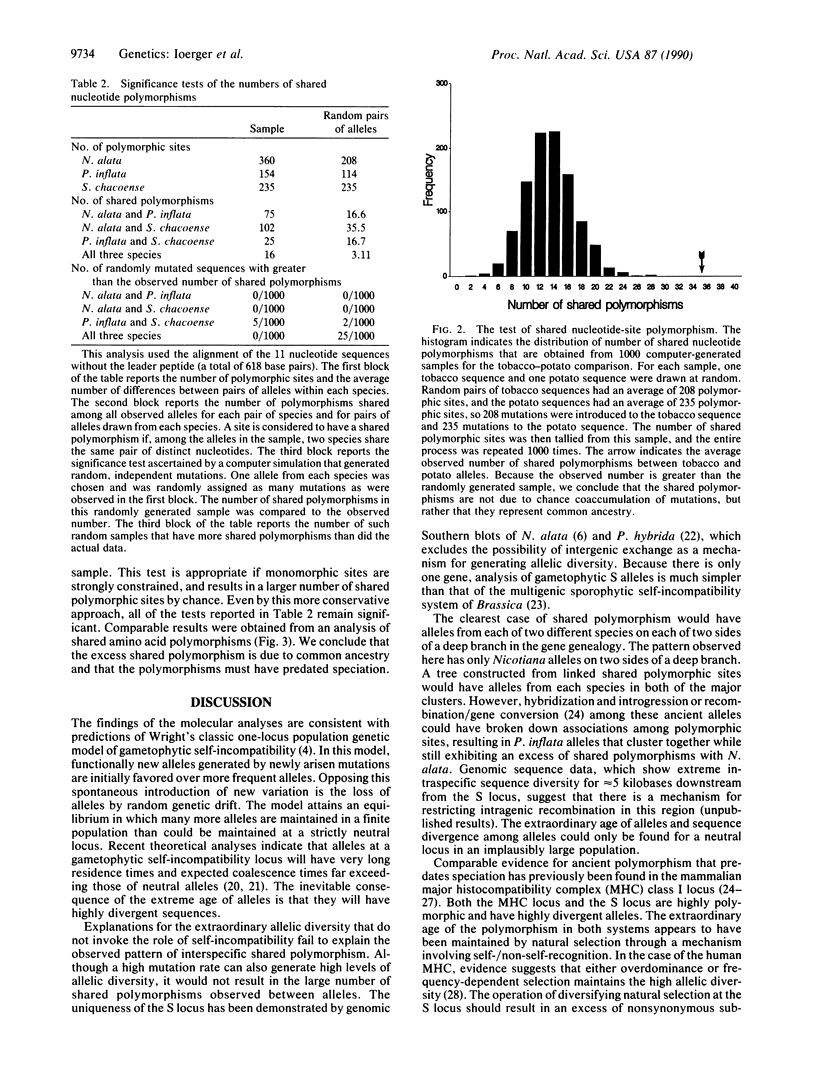
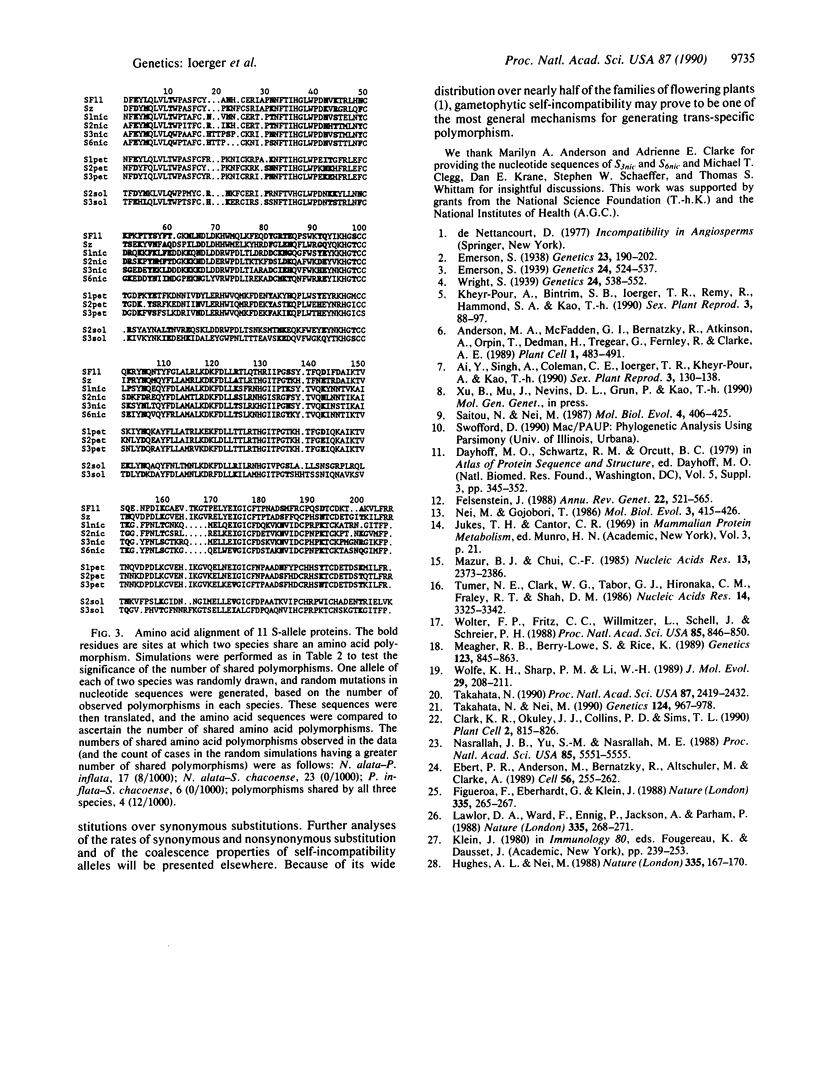
Sequences of 11 alleles of the gametophytic self-incompatibility locus (S locus) from three species of the Solanaceae family have recently been determined. Pairwise comparisons of these alleles reveal two unexpected observations: (i) amino acid sequence similarity can be as low as 40% within species and (ii) some interspecific similarities are higher than intraspecific similarities. The gene genealogy clearly illustrates this unusual pattern of relationships. The data suggest that some of the polymorphism at the S locus existed prior to the divergence of these species and has been maintained to the present. In support of this hypothesis, the number of shared polymorphic sites was found to exceed the number found in simulations with independent accumulation of mutations. Strictly neutral evolution is exceedingly unlikely to maintain the polymorphism for such a long time. The allele multiplicity and extreme age of the alleles is consistent with Wright's classic one-locus population genetic model of gametophytic self-incompatibility. Similarities between the plant S locus and the mammalian major histocompatibility complex are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., McFadden G. I., Bernatzky R., Atkinson A., Orpin T., Dedman H., Tregear G., Fernley R., Clarke A. E. Sequence variability of three alleles of the self-incompatibility gene of Nicotiana alata. Plant Cell. 1989 May;1(5):483–491. doi: 10.1105/tpc.1.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. R., Okuley J. J., Collins P. D., Sims T. L. Sequence variability and developmental expression of S-alleles in self-incompatible and pseudo-self-compatible petunia. Plant Cell. 1990 Aug;2(8):815–826. doi: 10.1105/tpc.2.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. R., Anderson M. A., Bernatzky R., Altschuler M., Clarke A. E. Genetic polymorphism of self-incompatibility in flowering plants. Cell. 1989 Jan 27;56(2):255–262. doi: 10.1016/0092-8674(89)90899-4. [DOI] [PubMed] [Google Scholar]
- Emerson S. A Preliminary Survey of the Oenothera Organensis Population. Genetics. 1939 Jun;24(4):524–537. doi: 10.1093/genetics/24.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. The Genetics of Self-Incompatibility in Oenothera Organensis. Genetics. 1938 Mar;23(2):190–202. doi: 10.1093/genetics/23.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Figueroa F., Günther E., Klein J. MHC polymorphism pre-dating speciation. Nature. 1988 Sep 15;335(6187):265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988 Sep 8;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Berry-Lowe S., Rice K. Molecular evolution of the small subunit of ribulose bisphosphate carboxylase: nucleotide substitution and gene conversion. Genetics. 1989 Dec;123(4):845–863. doi: 10.1093/genetics/123.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah J. B., Yu S. M., Nasrallah M. E. Self-incompatibility genes of Brassica oleracea: Expression, isolation, and structure. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5551–5555. doi: 10.1073/pnas.85.15.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Takahata N. A simple genealogical structure of strongly balanced allelic lines and trans-species evolution of polymorphism. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2419–2423. doi: 10.1073/pnas.87.7.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N., Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990 Apr;124(4):967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. The Distribution of Self-Sterility Alleles in Populations. Genetics. 1939 Jun;24(4):538–552. doi: 10.1093/genetics/24.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]



