Abstract
Heart failure (HF) is one of the most frequent cause of hospitalization in elderly and often coexists with concurrent geriatric syndromes, like cognitive disturbances; various pathophysiological mechanisms are shared by HF and cognitive decline, notably a substrate of low-grade inflammation. We investigated whether SNPs in the purinergic receptor (P2X7R) and apolipoprotein (APO) E genes, both involved in a series of inflammatory responses, are associated to HF or cognitive impairment and are able to predict post-discharge mortality in the elderly. We prospectively analyzed 198 patients (age 85 ± 8 years, predominantly females) admitted to a Geriatric unit for acute HF, whose diagnosis was based on clinical signs, brain natriuretic peptide (BNP) values and ecocardiography in uncertain diagnosis (BNP values between 100 and 400 pg/mL); cognitive performance was assesed by Short Portable Mental Status Questionnaire (SPMSQ). In all the participants, SNPs rs208294 and rs3751143 for P2X7R gene and rs429558 and rs7412 for APOE gene were assessed. Information on all-cause mortality was adjudicated by medical records review 36 months after discharge. We found no relationship between P2X7R and APOE polymorphisms and 36-month post-discharge mortality; a better outcome for overall survival was observed in patients with BNP values below the median (281 pg/mL) (p=0.002) persisting after adjustment for renal function and age, and in those with cognitive impairment (p<0.001). Patients harboring APOE-ε4 genotype showed higher BNP concentrations than noncarriers (1289.9 ± 226.9 vs 580.5 ± 90.2 pg/mL respectively,p=0.004), whereas none of the studied SNPs were associated to impairment in cognitive performance. In conclusion, neither P2X7R or APOE genotype seem to predict long-term mortality in elderly patients. Interestingly, APOE-ε4 genotype was associated to higher BNP values, suggesting a putative interaction between genetic and biochemical markers in identifying people at risk for HF.
Keywords: heart failure, brain natriuretic peptide, P2X7 receptor, apolipoprotein E, polymorphisms, long-term mortality
Acute cardiac diseases, including heart failure (HF), are among the main determinants of mortality in aging individuals; their prevalence dramatically rises in frail individuals with concomitant geriatric syndromes, in particular cognitive disorders, associated with increases in mortality risk, hospital stay, and costs [1, 2]. In aging subjects, HF and cognitive decline, even being fully diverse clinical conditions, might share common mechanisms, with low-grade inflammation playing a role in the pathogenesis of both these diseases [3, 4].
The extracellular signalling molecule (eATP) via specific P2 ionotropic (P2X) and metabotropic (P2Y) subtypes receptors is a major mediator of inflammatory responses [5]. The purinergic system is involved in the pathogenesis of cognitive decline, mediating the neuroinflammatory response; a main role is attributed to the P2X7 receptor (P2X7R), which promotes neuronal death through an activation of microglia-derived IL-1β release [6]. This receptor is highly polymorphic, but not many variants influence its function; the most studied SNPs are 489C>T (H155Y) and 1513A>C (E496A), linked to a receptor gain-of-function and a loss-of function, respectively [7, 8].
The involvement of purinergic system in myocardial fibrosis and remodeling is well documented [9]; moreover, in ischaemia-reperfusion injury, increased caspase-activity and cell apoptosis are associated to an increased P2X7R expression, and P2X7R silencing through siRNA, or prevention of the inflammasome assembly by pharmacologic P2X7R inhibition, reduce the infarct size in a murine model [10]. In the remodeling process occurring in HF, inflammatory reaction and fibrogenesis are markedly stimulated by IL-1β, whose release depends upon the P2X7R-NLRP3 activated inflammasome through the recruitment of pro-inflammatory leukocytes and myocardial fibroblasts [11, 12].
Elevated B-type natriuretic peptide (BNP) levels directly correlate with prognosis, NYHA score, intra-ventricular pressure, pulmonary pressure, and inversely to cardiac output [13], thus representing the most common biomarker of HF, widely used in the clinical practice. It is interesting to point out as BNP promotes myocardial cell apoptosis via caspase-1/IL-1β cascade [14]. IL-18 is another cytokine whose release is strictly depending upon P2X7R-inflammasome [15]; in subjects carrying HF, IL-18 concentrations are significantly higher compared to healthy controls, although no association was found with functional polymorphisms in P2X7R gene [16].
Apolipoprotein E (ApoE) - initially described for its role in lipid homeostasis -is now emerging as a significant risk factor for both cardiovascular and neurologic pathologies [17]. The conformational change typically caused by two SNPs in APOE genes (rs429558 and rs7412) comes out in protein isoforms with critical modifications in protein functionality. These changes can result in variants with broad deleterious effects, like increased cholesterol and triglycerides levels, contributing to a pro-inflammatory milieu and to alter cell signaling pathways [18]. APOE-ε4 has been linked with about 65-75% of sporadic Alzheimer’s disease, but such association is also described for up to 20% of other types of dementia [19]; on the other hand, the link between APOE-ε4 and HF is not strictly consistent, depending firstly on the severity of cardiac disease or the selected population study (low or high risk subjects) [20, 21].
To investigate whether P2X7R and APOE polymorphisms impact on long-term mortality in a cohort of older patients with acute HF, and to evaluate their association with concurrent morbidities worsening their prognosis, like cognitive impairment, we designed the present study.
MATERIAL AND METHODS
Study population
Two hundred forty-four aging patients were recruited among those consecutively admitted to the Geriatric Unit of University Hospital of Pisa in 2012-2013. Inclusion criteria were age ≥65 years and diagnostic suspect of HF, according to the clinical evaluation in the emergency room; patients evolving into a terminal status within the first 24 hours after admission, or lacking a confirmed diagnosis of HF were then excluded. The study procedures were approved by the Institutional Ethics Committee of Pisa University (n. 3641/2012).
The diagnosis of HF was based on clinical data (personal history of cardiovascular diseases, suggestive signs and symptoms, NYHA score [22]) and the presence of BNP value higher than 100 pg/mL. An electrocardiogram was performed to exclude an acute ischaemic heart disease, while chest radiography allowed differentiating pulmonary affections. In patients falling within an intermediate zone (BNP 100-400 pg/mL), an ultrasonographic measurement of ejection fraction was performed to confirm the diagnosis of systolic dysfunction [23, 24]. According to this diagnostic algorithm, a total of 198 patients participated in the study.
At the admission, a physician specialist in geriatric medicine recorded all the relevant information related to the patient’s acute clinical conditions, medical history, and functional, mental, and sociodemographic status through personal interviews and medical record review. Comorbidity was measured with the Charlson comorbidity index. Baseline functional status was evaluated as the ability to perform six basic activities of daily living (ADLs)-bathing, dressing, transferring, toileting, continence, and feeding-2 weeks before their admission. Cognitive decline was evaluated using the Short Portable Mental Status Questionnaire (SPMSQ) [25], whose score is based on the total number of errors in answering to 10 questions. A diagnosis of cognitive impairment was established when the number of errors was ≥3 (cut-off adjusted by patient’s education level).
Diagnostic tests
Blood samples were collected from an antecubital vein to extract genomic DNA and to determine blood count and routine analysis. BNP was measured by immunoassay using the ADVIA Centaur System (Bayer Health Care, Tarrytown, NY,USA).
Genotyping analyses
Blood samples (3 ml) were collected at admission in EDTA tubes and stored at -80°C. DNA extraction was performed using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). Allelic discrimination of genes was performed using an Eco Real-Time System (Illumina Inc., San Diego, CA, USA) according to the standard protocol and with validated TaqMan® SNP genotyping assays (Applied Biosystems Carlsbad, CA, USA). PCR reactions were carried out according to the manufacturer’s protocol. The following polymorphisms were determined: rs7412 and rs429358 for APOE gene (APOE) and rs208294 and rs3751143 for P2X7R gene (P2X7R).
SNP selection
The SNPs included in our study were selected on the basis of the following reasons: i) they modulate gene expression of multifunctional proteins involved in inflammatory response, thus playing a potential role in the pro-atherosclerotic process [26-28] associated with an increased cardiac or neurologic risk profile; ii) rs208294 and rs3751143 represent the most common gain and loss of function SNPs of P2X7R gene, respectively; iii) allelic variations in APOE are mainly defined by these two SNPs rs429358 and rs7412; participants with 1 or more copies of ε4 allele (ε2/4 excluded) were considered APOE-ε4 carriers.
Follow up
Information on all-cause mortality was checked and completed by medical records review and official information from the national health system 36 months after hospital discharge.
Statistical analysis
Data were given as mean±SD or SEM, or median [interquartile range]. All polymorphisms were analyzed for deviation from the HWE by Χ2test. Clinical parameters among the genotypes were evaluated with a one-way analysis of variance (ANOVA) or multivariate analysis of variance (MANOVA) test for continuous variables. Survival curves were calculated by the Kaplan Meier method. Cox Regression was used to obtain an adjusted model. Statistics were performed using JMP?7.0; a p value≤0.05 was considered statistically significant.
RESULTS
Baseline
Clinical and biochemical characteristics of the whole study group, consisting of 198 patients, are reported in Table 1A and Table 1B, respectively. Mean age was 85 ± 8 years and 61 (32%) were male. Cognitive impairment was strongly represented, being observed in half the cohort; patients suffered of numerous comorbidities and were severely functionally impaired. The majority showed a moderate grade of clinical symptoms (dyspnea) and admission BNP values were nearly three times above the established cut-off for the diagnosis (100 pg/mL).
Table 1A.
Baseline characteristics of the study group (n=198).
| Age (years) | 85 ± 8 |
| Sex (M/F) | 61/137 |
| BMI (kg/m2) | 24.7 ± 4.5 |
| SBP (mmHg) | 136.7 ± 21.7 |
| DBP (mmHg) | 74.8 ± 12.4 |
| Comorbidity (Charlson Index) | 7.8 ± 2.0 |
| Severe dependence (ADL) | 121 (61.2) |
| Cognitive impairment (SPMSQ) | 100 (50.6) |
| NYHA functional class | |
| I | 53 (27) |
| II | 89 (45) |
| III | 54 (27) |
| IV | 2 (1) |
| Medications, n (%) | |
| RAAS inhibitors | 109 (55) |
| Beta blockers | 75 (38) |
| Diuretics | 48 (24) |
| Anti-platelets | 46 (23) |
Data are mean±SD or n (%)
Table 1B.
Biochemical variables of the study group (n=198).
| Red blood cell count (x106/mmc) | 3.93 ± 0.92 |
| Hemoglobin (g/dL) | 11.1 ± 3.0 |
| White blood cell count (x103/mmc) | 7.8 ± 1.6 |
| Platelets (x103/mmc) | 232 (130) |
| Fasting plasma glucose (mg/dL) | 115.7 ± 23.6 |
| Total cholesterol (mg/dL) | 163.7 ± 13.7 |
| Triglycerides (mg/dL) | 124.7 ± 33.5 |
| Fibrinogen (mg/dL) | 319 (178) |
| Creatinine (mg/dL) | 1.15 ± 0.63 |
| Estimated glomerular filtration rate (by MDRD, mL/min/1.73m2) | 58 ± 37 |
| Hs-PCR (mg/dL) | 4.66 (8.63) |
| BNP (pg/mL) | 281 (689) |
Data are mean±SD or median (interquartile range)
Suppl Table A shows as none of the genotypes of the examined polymorphisms significantly deviated from HWE distribution. APOE-ε4 genotype was present in 18% of the cohort (all carriers were heterozygous).
When we analysed whether or not the P2X7R gain-of-function (His155Tyr) or loss-of-function (Glu496Ala) polymorphisms were related with plasma BNP levels, no significant difference emerged between carriers and non carriers of the mutant allele for both polymorphisms. Conversely, APOE-ε4 carriers showed a significantly higher BNP concentrations than noncarriers (Table 2A). In a multivariate model, controlling for age, sex, Charlson comorbidity index and e-GFR, plasma BNP levels were still significantly associated with the presence of APOE-ε4 carriers (p=0.03).
Table 2.
Association between genotypes and BNP value (A) or percentage of cognitive impairment (B) in the study population.
| A | |||
|---|---|---|---|
| SNPs | BNP (pg/mL) | p | |
| P2X7R rs3751143 | A carriers C carriers | 702.0 ± 115.0 674.3 ± 167.0 | 0.892 |
|
P2X7R rs208294 |
C carriers T carriers |
626.2 ± 162.6 721.6 ± 103.4 |
0.621 |
| APOE rs429358 rs7412 | ε4 carriers ε4 noncarriers | 1289.9 ± 226.9 580.5 ± 90.2 | 0.004 |
| B | |||
|---|---|---|---|
| SNPs | Cognitive impairment (% of patients) | p | |
| P2X7R rs3751143 | A carriers C carriers | 49 49 | 0.892 |
|
P2X7R rs208294 |
C carriers T carriers |
46 50 |
0.621 |
|
APOE rs429358 rs7412 |
ε4 carriers ε4 noncarriers | 63 37 | 0.07 |
Data are mean±SEM; p values are from ANOVA.
Cognitive impairment, according to SPMSQ evaluation, was not significantly related to P2X7R genotypes, whilst it trended to be positively linked to the presence of APOE-ε4 genotype (p=0.07) (Table 2B). In the pool data, BNP values did not significantly differ in patients carrying or not cognitive impairment.
Follow-up
Thirty-six months after discharge from hospital, 93 (47%) patients were still alive. As expected, at the admission they were younger (83 ± 1 vs 87 ± 1 years, p<0.0001), presented a lower number of comorbidities (Charlson index 7.4 ± 0.3 vs 8.1 ± 0.2, p<0.05) and cognitive impairment was less represented (28 vs 13%, p<0.0001); no difference was observed according to gender.
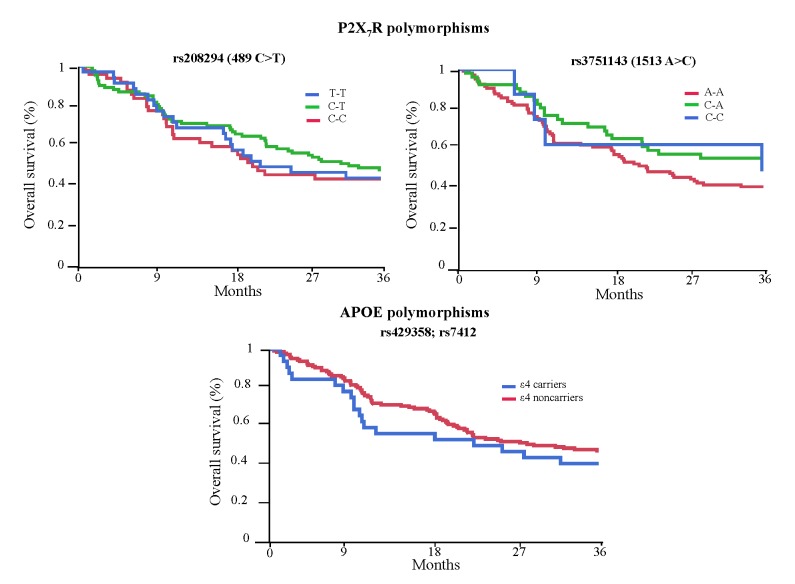
No significant association between polymorphisms of interest and overall survival (OS) was found (Fig. 1). This result was confirmed even when the analysis was run comparing carriers and non carriers of the mutant allele for P2X7R and APOE genes polymorphisms. However, when the whole cohort was divided according to BNP median value during hospitalization (281 pg/mL), a lower OS was observed in those having higher values (p=0.002). After running a proportional hazards regression analysis, the relationship between BNP values and reduced OS still remained significant (p=0.03) (Suppl Table B). Likewise, the presence of cognitive impairment was significantly associated with higher mortality rate (p<0.001).
Figure 1. Overall survival curves of all the patients according to genetic profiles of the studied polymorphisms.
Survival curves were calculated with the Kaplan Meier method.
DISCUSSION
The main finding of the present study is a negative one: even though P2X7R (rs208294 and rs3751143) and APOE (rs429358 and rs7412) polymorphisms have been recognized as potential determinants of unfavorable prognosis in several and heterogeneous morbid conditions [29, 30], they were not independently associated to three-year post-discharge mortality in a cohort of older patients, admitted at emergency room for HF. Instead, our observation that higher BNP values and impairment in cognitive status tracked the poorest outcome deserves attention. Lastly, whilst P2X7R polymorphisms were linked neither to HF and cognitive decline, APOE-ε4 carriers showed significantly higher BNP values, indicative of a cardiac dysfunction.
Other groups have previously evaluated the impact of SNPs in P2X7R gene on clinical prognostic markers and survival in different clinical contexts from ours, like in oncological diseases, reporting a neutral effect, although significant interactions with other genes are reported [31, 32]. APOE polymorphisms are, instead, according to a vast literature, among the genes affecting life span; notably, APOE-ε4 allele is associated with an increased mortality risk during adulthood [33]. Controversy exists whether this relationship is still valid at the oldest ages; our results are in line with those supporting the notion of a reduced effect of the ‘risk’ ApoE-ε4 allele in elderly individuals [34]; however, in our population ApoE-ε4 homozygosity was not represented (likely for the limited size of our cohort) and several other causes could compete as mortality risks.
On the other hand, we confirmed the prognostic value of natriuretic peptides in elderly hospitalized patients for HF, in terms of post-discharge mortality [35]. We found also a consistent association between cognitive impairment and poor outcome; these data confirm those of other authors obtained in aging patients [36], even affected by HF [37].
An intriguing, novel observation is that APOE-ε4carriers present significantly higher BNP concentrations than non carriers. Previous reports suggested a positive association between systolic dysfunction and APOE-ε4 genotype, although it was more pronounced for homozygous than heterozygous carriers; odds ratio increased when the study population was restricted to subjects aged 65 years or older, and did not significantly change when controlling for different causes of ventricular dysfunction [38]. Moreover, Van der Cammen et al. showed that, in patients affected by Alzhemeir’s disease, the risk of having electrocardiographic abnormalities indicative of left ventricular dysfunction was over seven fold increased [39]. Our results endorse the hypothesis of an effect of APOE-ε4 genotype on ventricular dysfunction, at least as estimated by BNP values. Besides, it is well documented that BNP concentrations have a prognostic role in the natural history of HF [40], as shown by the impact on mortality rate in our cohort.
Several limitations need to be highlighted in the present study. From a clinical/methodologic view point, echocardiographic data in the whole cohort would have better characterized the HF spectrum, as well as a complete multifunctional evaluation in the cognitive function would have allowed a more refined diagnosis of the cognitive decline and its causes. Besides, likely due to the limited number of patients, APOE-ε4 homozygous carriers were not represented, so that any recessive effect could be explored. The main strength is to have further investigated the role of SNPs potentially involved in the pathogenesis of HF and cognitive disorders, highly prevalent in a geriatric population, as determinants of all-cause mortality. Furthermore, our work shows for the first time a positive association between BNP values, surrogate/marker of ventricular dysfunction, and ApoE-ε4 genotype, which is broadly known to affect cardiovascular morbidity; such preliminary observation requires, obviously, to be confirmed by others.
In conclusion, in our cohort of frail elderly individuals common P2X7R and APOE polymorphisms do not influence long-term all-cause mortality. Further studies involving larger population will be able to answer the question whether major and impactful events could have blunted the effect of such polymorphisms on clinical outcome, as well as to better characterize the relationship between heart failure and APOE gene polymorphisms.
Suppl Table A.
Genotypes, allele frequencies and Hardy-Weinberg Equilibrium (HWE) of the studied polymorphisms
| SNP | Genotype | N | Allele | N | % | HWE (p) |
|---|---|---|---|---|---|---|
|
P2X7R rs208294 |
T-T C-T C-C |
38 100 52 |
C T |
152 138 |
0.90 0.10 |
0.42 |
|
P2X7R rs3751143 |
A-A C-A C-C |
111 55 8 |
A C |
166 63 |
0.72 0.28 |
0.72 |
|
APOE rs7412 |
C-C C-T |
181 17 |
C T |
198 17 |
0.92 0.08 |
0.53 |
|
APOE rs429358 |
T-T C-T |
157 32 |
C T |
32 189 |
0.14 0.86 |
0.20 |
Suppl Table B.
Proportional hazards regression analysis showing BNP impact on reduced overall survival
| Β coefficient | p | |
|---|---|---|
| BNP | 0.0003 | 0.032 |
| Age | 0.046 | 0.084 |
| Sex (f) | -0.162 | 0.340 |
| Charlson comorbidity index | 0.079 | 0.257 |
Acknowledgements
This study has been supported by a grant from the University of Pisa (PRA 2015).
Footnotes
Conflict of Interest
The Authors have nothing to disclose
Contributor Information
Giuseppe Pasqualetti, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Marta Seghieri, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Eleonora Santini, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Chiara Rossi, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Edoardo Vitolo, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Livia Giannini, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Maria Giovanna Malatesta, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Valeria Calsolaro, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Fabio Monzani, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
Anna Solini, Department of Clinical and Experimental Medicine University of Pisa, Pisa, Italy.
References
- [1].Dharmarajan K, Dunlay SM (2016). Multimorbidity in older adults with heart failure. Clin Geriatr Med, 32(2): 277-89. [DOI] [PubMed] [Google Scholar]
- [2].van de Vorst IE, Koek HL, de Vries R, Bots ML, Reitsma JB, Vaartjes I (2016). Effect of vascular risk factors and diseases on mortality in individuals with dementia: a systematic review and meta-analysis. J Am Geriatr Soc, 64(1): 37-46. [DOI] [PubMed] [Google Scholar]
- [3].Briasoulis A, Androulakis E, Christophides T, Tousoulis D (2016). The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev, 21(2): 169-76. [DOI] [PubMed] [Google Scholar]
- [4].Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2016). Neuroinflammation pathways: a general review. Int J Neurosci, 13:1-29. [DOI] [PubMed] [Google Scholar]
- [5].Di Virgilio F (2015). P2X receptors and inflammation. Curr Med Chem, 22(7): 866-77. [DOI] [PubMed] [Google Scholar]
- [6].Rodrigues RJ, Tomé AR, Cunha RA (2015). ATP as a multi-target danger signal in the brain. Front Neurosci, 28(9): 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Portales-Cervantes L, Niño-Moreno P, Salgado-Bustamante M, García-Hernández MH, Baranda-Candido L, Reynaga-Hernández E et al. (2012). The His155Tyr (489C>T) single nucleotide polymorphism of P2RX7 gene confers an enhanced function of P2X7 receptor in immune cells from patients with rheumatoid arthritis. Cell Immunol, 276(1-2): 168-75. [DOI] [PubMed] [Google Scholar]
- [8].Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S et al. (2001). A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem, 276(14): 11135-42. [DOI] [PubMed] [Google Scholar]
- [9].Erlinge D, Burnstock G (2008). P2 receptors in cardiovascular regulation and disease. Purinergic Signal, 4(1): 1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN et al. (2001). The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA, 108(49):19725-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC Jr, Francis GS et al. ; BNP Consensus Panel (2004). A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail, 10(5 Suppl 3):1-30. [DOI] [PubMed] [Google Scholar]
- [12].Sandanger Ø, Ranheim T, Vinge LE, Bliksøen M, Alfsnes K, Finsen AV et al. (2013). The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res, 99(1):164-174. [DOI] [PubMed] [Google Scholar]
- [13].Butts B, Gary RA, Dunbar SB, Butler J (2015). The importance of NLRP3 inflammasome in heart failure. J Card Fail, 21(7): 586-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang X, Sha M, Yao Y, Da J, Jing D (2015). Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1β signaling pathway. Mol Med Rep, 12(5): 6761-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dubyak GR (2012). P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol, 14(11): 1697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eslick GD, Thampan BV, Nalos M, McLean AS, Sluyter R (2009). Circulating interleukin-18 concentrations and a loss-of-function P2X7 polymorphism in heart failure. Int J Cardiol, 11;137(1): 81-3. [DOI] [PubMed] [Google Scholar]
- [17].Lopez MF, Krastins B, Ning M (2009). The role of apolipoprotein E in neurodegeneration and cardiovascular disease. Expert Rev Proteomics,11(3): 371-81. [DOI] [PubMed] [Google Scholar]
- [18].Mahley RW (2016). Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl), 94(7): 739-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bu G (2009). Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci, 10: 333-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jurkovicova D, Goncalvesova E, Sedlakova B, Hudecova S, Fabian J, Krizanova O (2006). Is the ApoE polymorphism associated with dilated cardiomyopathy? Gen Physiol Biophys, 25(1): 3-10. [PubMed] [Google Scholar]
- [21].Economou-Petersen E, Aessopos A, Kladi A, Flevari P, Karabatsos F, Fragodimitri C et al. (1998). Apolipoprotein E epsilon4 allele as a genetic risk factor for left ventricular failure in homozygous beta-thalassemia. Blood, 92(9): 3455-9. [PubMed] [Google Scholar]
- [22].The Criteria Committee of the New York Heart Association (1994). Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, Mass: Little, Brown & Co; 253-256. [Google Scholar]
- [23].Iwaz JA, Maisel AS (2016). Recent advances in point-of-care testing for natriuretic peptides: potential impact on heart failure diagnosis and management. Expert Rev Mol Diagn, 16(6): 641-50. [DOI] [PubMed] [Google Scholar]
- [24].Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG et al. (2008). State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail, 10(9): 824-39. [DOI] [PubMed] [Google Scholar]
- [25].Pfeiffer E (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23(10): 433-41. [DOI] [PubMed] [Google Scholar]
- [26].Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong W,et al. (2015) P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med, 35(5): 1179-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Di Virgilio F, Solini A (2002). P2 receptors: new potential players in atherosclerosis. Br J Pharmacol, 135(4): 831-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holmes MV, Frikke-Schmidt R, Melis D, Luben R, Asselbergs FW, Boer JM,et al. (2014). A systematic review and meta-analysis of 130,000 individuals shows smoking does not modify the association of APOE genotype on risk of coronary heart disease. Atherosclerosis, 237(1): 5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thunberg U, Tobin G, Johnson A, Söderberg O, Padyukov L, Hultdin M et al. (2002). Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet, 360(9349): 1935-9. [DOI] [PubMed] [Google Scholar]
- [30].Tamam Y, Tasdemir N, Yalman M, Tamam B (2011). Association of apolipoprotein E genotypes with prognosis in multiple sclerosis. Eur Rev Med Pharmacol Sci, 15(10): 1122-30. [PubMed] [Google Scholar]
- [31].Paneesha S, Starczynski J, Pepper C, Delgado J, Hooper L, Fegan C et al. (2006). The P2X7 receptor gene polymorphism 1513 A-->C has no effect on clinical prognostic markers and survival in multiple myeloma. Leuk Lymphoma, 47(2): 281-4. [DOI] [PubMed] [Google Scholar]
- [32].Solini A, Simeon V, Derosa L, Orlandi P, Rossi C, Fontana A et al. (2015). Genetic interaction of P2X7 receptor and VEGFR-2 polymorphisms identifies a favorable prognostic profile in prostate cancer patients. Oncotarget, 6(30): 28743-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Christensen K, Johnson TE, Vaupel JW (2006). The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet, 7(6): 436-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ewbank DC (2007). Differences in the association between apolipoprotein E genotype and mortality across populations. J Gerontol A Biol Sci Med Sci, 62: 899-907. [DOI] [PubMed] [Google Scholar]
- [35].Leto L, Testa M, Feola M (2015). Correlation between B-type natriuretic peptide and functional/cognitive parameters in discharged congestive heart failure patients. Int J Endocrinol, 2015: 239136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yaffe K, Peltz CB, Ewing SK, McCulloch CE, Cummings SR, Cauley JA et al. (2016). Long-term cognitive trajectories and mortality in older women. J Gerontol A Biol Sci Med Sci, 71(8): 1074-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rodriguez-Pascual C, Paredes-Galan E, Vilches-Moraga A, Ferrero-Martinez AI, Torrente-Carballido M, Rodriguez-Artalejo F (2014). Comprehensive geriatric assessment and 2-year mortality in elderly patients hospitalized for heart failure. Circ Cardiovasc Qual Outcomes, 7(2): 251-8. [DOI] [PubMed] [Google Scholar]
- [38].Bleumink GS, van Duijn CM, Kingma JH, Witteman JC, Hofman A, Stricker BH (2004). Apolipoprotein E epsilon4 allele is associated with left ventricular systolic dysfunction. Am Heart J, 147(4): 685-9. [DOI] [PubMed] [Google Scholar]
- [39].van der Cammen TJ, Verschoor CJ, van Loon CP, van Harskamp F, de Koning I, Schudel WJ et al. (1998). Risk of left ventricular dysfunction in patients with probable Alzheimer’s disease with APOE*4 allele. J Am Geriatr Soc, 46(8): 962-7. [DOI] [PubMed] [Google Scholar]
- [40].Beltrami M, Palazzuoli A, Ruocco G, Aspromonte N (2016). The predictive value of plasma biomarkers in discharged heart failure patients: the role of plasma BNP. Minerva Cardioangiol, 64(2): 147-56. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl Table A.
Genotypes, allele frequencies and Hardy-Weinberg Equilibrium (HWE) of the studied polymorphisms
| SNP | Genotype | N | Allele | N | % | HWE (p) |
|---|---|---|---|---|---|---|
|
P2X7R rs208294 |
T-T C-T C-C |
38 100 52 |
C T |
152 138 |
0.90 0.10 |
0.42 |
|
P2X7R rs3751143 |
A-A C-A C-C |
111 55 8 |
A C |
166 63 |
0.72 0.28 |
0.72 |
|
APOE rs7412 |
C-C C-T |
181 17 |
C T |
198 17 |
0.92 0.08 |
0.53 |
|
APOE rs429358 |
T-T C-T |
157 32 |
C T |
32 189 |
0.14 0.86 |
0.20 |
Suppl Table B.
Proportional hazards regression analysis showing BNP impact on reduced overall survival
| Β coefficient | p | |
|---|---|---|
| BNP | 0.0003 | 0.032 |
| Age | 0.046 | 0.084 |
| Sex (f) | -0.162 | 0.340 |
| Charlson comorbidity index | 0.079 | 0.257 |