Abstract
Summary
Our aim was to evaluate the gap in osteoporosis treatment and the impact of osteoporosis treatment on subsequent fragility fractures. We found osteoporosis medication use lowered risk of subsequent fractures by 21% and that black race, higher CCI scores, dementia, and kidney diseases reduced the likelihood of osteoporosis medication use.
Introduction
The goal of this study was to evaluate the predictors of osteoporosis medication use and compare the risk of fragility fractures within 1 year of a fragility fracture between osteoporosis treated and untreated women.
Methods
We conducted a retrospective, observational cohort study using the national Medicare database. Elderly women (≥65 years) who were hospitalized or had an outpatient/ER service for fragility fracture between January 1, 2011 and December 31, 2011 were included. The outcomes of interest were the correlates of and time-to-osteoporosis medication use and risk of a subsequent fracture within 12 months for treated and untreated women. Cox regression was used to evaluate the predictors of treatment use and the risk of fracture based on treatment status.
Results
Women (28,722) (27.7%) were treated with osteoporosis medication within 12 months of index fracture, and 74,979 (72.2%) were untreated. A number of patient characteristics were associated with a reduced likelihood of osteoporosis medication use, including black race, higher Charlson comorbidity index scores, presence of dementia, and kidney diseases in the baseline. The predictor most strongly and positively associated with osteoporosis medication use after fracture was osteoporosis medication use before fragility fracture (HR = 7.87; 95% CI 7.67–8.07). After adjusting for baseline characteristics, osteoporosis medication use lowered the risk of subsequent fractures by 21% (HR = 0.79, 95% CI 0.75–0.83) over 12 months compared to women without treatment.
Conclusions
Demographics and clinical characteristics were strong predictors of osteoporosis medication use. In the US Medicare population, osteoporosis treatment significantly reduced the risk of fragility fractures.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-4072-6) contains supplementary material, which is available to authorized users.
Keywords: Fracture risk assessment, Osteoporosis, Therapeutics
Introduction
Osteoporosis affects an estimated 200 million women worldwide and leads to almost 9 million fractures per year, of which 16.9% occur among persons aged 80–89 years while 4.3% occur among women aged 90+ years [1, 2]. In addition, it has been reported that hip fractures constituted a major proportion (23%) of the 1.7 million hospitalizations for fragility fractures [3].
Over the years, antiresorptive and anabolic medication treatments have improved the physical health of patients by reducing the risk of fracture [4]. Overall, randomized control trial (RCT) data have demonstrated that osteoporosis medications have reduced vertebral fractures by 40–70%, non-vertebral fractures by 20–36%, and hip fractures by up to 40% [5–8]. Recent real world evidence (RWE) studies have confirmed treatment efficacy reported from RCTs and prior head-to-head trials and have shown that osteoporosis treatments reduced the risk of fractures by 20–60% [9, 10]. Despite the availability of these efficacious treatments, a gap in osteoporosis treatment still exists due to several factors, including the underdiagnosis of osteoporosis in women, differences in the interpretation of bone mineral density (BMD) testing measurements (using dual-energy x-ray absorptiometry [DEXA]) across physicians [11], lower Medicare DEXA reimbursement, apparent concerns of adverse events, and lack of fracture liaison services in the US [12]. Therefore, a large proportion of women at risk for fractures remain undertreated [13–15].
To better understand this treatment gap, and the characteristics of women affected by this gap, several studies have examined predictors of osteoporosis medication use. Key patient characteristics associated with low rates of osteoporosis medication use include younger age, lower education level, high BMD T-score, high BMI, lack of glucocorticoid use, and absence of osteoporosis treatment prior to fragility fracture [11, 16, 17].
Due to the increased risk of osteoporosis diagnoses among women compared to men, post-menopausal women were the population of interest in our study [18, 19]. In light of these previous studies and the growing aging population in the US, there remains a dearth of RWE among osteoporotic women in the Medicare system. To better understand the wide extent of these treatment gaps among women, we used the 100% Medicare database, which is a primary strength in this analysis. To garner additional real-world evidence among Medicare enrolled osteoporotic women with a fragility fracture, this study aims to examine correlates of time to osteoporosis medication prescription within 12 months of index fragility fracture and the relative impact of treatment on risk for subsequent fractures over 12 months of follow-up.
Material and methods
Data source and study population
This was a retrospective observational cohort study based on US administrative claims data from fee-for-service Medicare beneficiaries (Study period: January 1, 2010 - December 31, 2012). This observational study was conducted under the provisions of Privacy Rule 45 CFR 164.514(e) and was exempt from Investigational Review Board review and approval since there was no collection or use of personally-identifiable information in the conduct of this study [20]. Women who had an index fragility fracture between January 1, 2011 and December 31, 2011 were followed for 12 months to identify whether they had been prescribed osteoporosis medication and examined for their risk of subsequent fractures within 12 months from index date.
Data of patients were obtained from the 100% fee-for-service Centers for Medicare & Medicaid Services (CMS) database. The Medicare database captured a comprehensive collection of demographic and clinical information. Study variables were measured from the database using enrollment records, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, Healthcare Common Procedure Coding System codes, and National Drug Codes, as appropriate.
Patient selection criteria
Women were included in the study if they had (1) an inpatient hospital stay with a primary discharge diagnosis of fragility fracture, defined as a fragility fracture (closed fractures without trauma codes) of the hip, pelvis, femur, clavicle, humerus, forearm and wrist, tibia/fibula, or spine (Supplemental Table 1) or (2) at least 2 medical claims for clavicle, humerus, forearm, wrist, tibia/fibula, or spine fractures in an outpatient setting not more than 90 days from the same fracture site between January 1, 2011 and December 31, 2011. Fractures with a trauma code identified within 7 days before or after any fracture claim date were excluded. The index date was defined as the date of the first observed fragility fracture claim in an outpatient setting or the date of discharge in an inpatient setting. Further, women were included if they were aged at least 65 years as of the index date and had continuous Medicare enrollment in Parts A, B, and D for at least 12 months before and after the index date. Women were excluded from the study if they had claim-based evidence of cancer, Paget’s disease, or treatments for Paget’s disease (Supplemental Table 1) during the study period.
Baseline patient characteristics
Demographics and clinical characteristics were measured for the 12 months prior to the index fragility fracture to determine potential predictors of treatment. Baseline characteristics included demographics, comorbidities (identified via ICD-9-CM diagnosis codes), Charlson Comorbidity Index (CCI) score [21], medication use (osteoporosis medications, glucocorticoids, and anticonvulsant drugs), and healthcare utilization.
Outcome variables
The primary outcome was time to osteoporosis medication within 12 months after the index fragility fracture. Osteoporosis medication use was defined as the occurrence of a claim for a bisphosphonate (alendronate, risedronate, pamidronate, etidronate, zoledronate, or tiludronate), calcitonin, denosumab, raloxifene, or teriparatide, as evidenced from Part B and/or Part D Medicare files. Time to osteoporosis medication, defined as the period from the index fragility fracture to the first osteoporosis medication claim date, was calculated in days. Women were evaluated overall and stratified into three subgroups by their index fragility fracture site: fractures in the hip or pelvis or femur regions denoted as “hip/pelvis/femur fractures,” fractures in the vertebral regions denoted as “clinical vertebral fractures,” fractures in the non-vertebral regions of clavicle, humerus, forearm, wrist, or tibia/fibula denoted as “other non-vertebral fractures.”
Subsequent fracture events were captured during the 12-month follow-up period, identified using the same criteria as the index fragility fracture, and reported by overall as well as clinical vertebral, hip/pelvis/femur, and other non-vertebral fractures for treated and untreated women. If the subsequent fracture during the follow-up period was the same fracture site as the index fragility fracture, a gap of ≥180 days was required for it to be considered a subsequent fracture. However, if the subsequent fracture was at a different site than the index fracture, it was considered an incident fracture and no gap was required. Women treated for osteoporosis were defined as those prescribed an osteoporosis treatment during the 12-month follow-up period after index fragility fracture. Untreated women diagnosed with osteoporosis were defined as those who had no treatment within 12 months after the initial fragility fracture. For treated women, subsequent fractures were identified after treatment initiation during the follow-up period and censored at treatment discontinuation or 12 months after the index fragility fracture. Treatment discontinuation was defined as no evidence of an osteoporosis prescription at any time after the run-out date of the previous prescription fill.
Statistical analysis
Counts, percentages, means, and standard deviations were provided for appropriate variables. T-tests and chi-square tests were used to detect statistical differences among continuous and categorical variables, respectively. Baseline variables including age, US geographical region, race, clinical risk scores, comorbidities, osteoporosis-related medications and procedures, physician specialty, and healthcare resource utilization were measured during the 12-month baseline period. Cumulative incidence of treatment use and subsequent fractures were evaluated descriptively using a cumulative density plot. In order to evaluate predictors of treatments, certain variables were also measured post-index as time-dependent variables. Time-dependent variables included subsequent fracture, BMD test (yes/no), and rheumatology and endocrinology visits. First, a Cox proportional hazards model was used to evaluate the association between time to osteoporosis medication prescription and predictors of prescription use. The dependent variable was time to treatment and the independent variables (i.e., predictors) were baseline characteristics and time-dependent variables, including subsequent fractures, BMD tests, and rheumatology and endocrinology visits. A variance inflation factor of ≥ 10 was used to assess multicollinearity of the dependent variables.
To evaluate the risk of fracture between treated and untreated women, a Cox proportional hazards model was used to evaluate time to subsequent fracture within 12 months of the index fragility fracture. Women in the untreated cohort were followed until the subsequent fracture or 12 months after the index date. Women in the treated cohort were followed until the discontinuation of osteoporosis treatment, subsequent fracture, or 12 months after index date.
Sensitivity analysis
Two sensitivity analyses were conducted. First, baseline osteoporosis medication use was examined. Patients prescribed baseline osteoporosis medication were considered prevalent users, and patients without baseline osteoporosis medication use were considered new users. The results were stratified (predictors of treatment and fracture risk) to determine if there was a difference between prevalent and new osteoporosis medication users. Second, index fragility was stratified by fracture place of service (inpatient or outpatient) to determine if there was a difference in treatment utilization or subsequent fracture risk.
All analyses were carried out using SAS version 9.3 (SAS Institute, Inc., Cary, NC, US). Values of p < 0.05 were considered, a priori, to be statistically significant.
Results
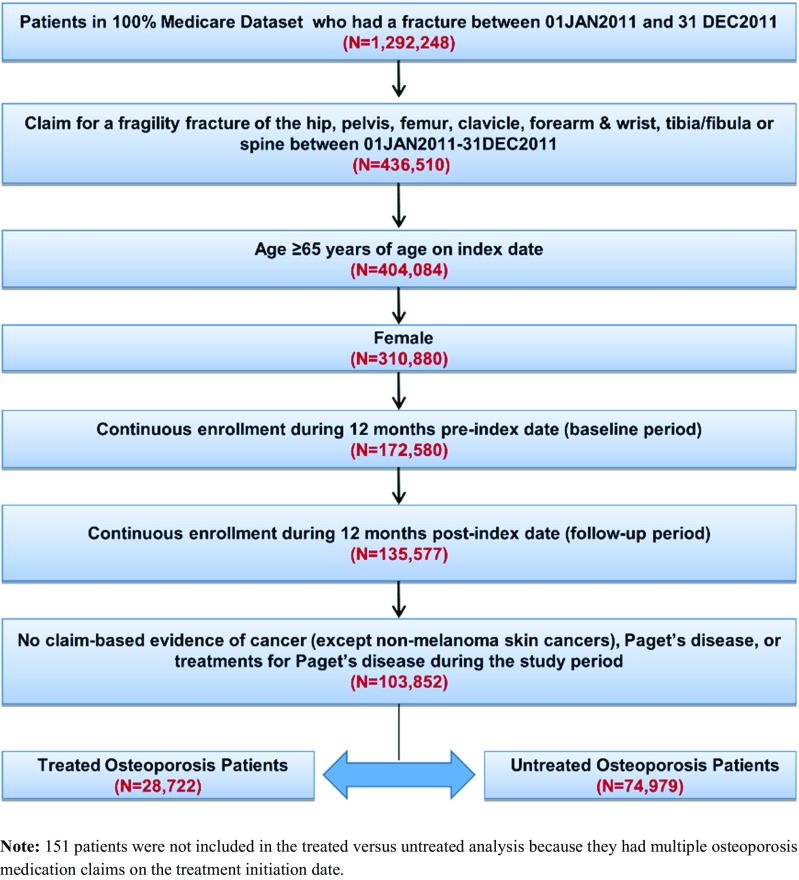
Of the 1,292,248 total Medicare enrollees who had a fracture in 2011, a total of 103,852 (8%) women ≥65 years of age with a fragility fracture were identified based on the selection criteria, of which a majority had hip/pelvis/femur fractures (n = 55,158; 53%), followed by other non-vertebral fractures (n = 29,509; 28%) and clinical vertebral fractures (n = 19,252; 19%). Overall, 28,722 (27.7%) of women were treated with osteoporosis medication within 12 months of index fracture and 74,979 (72.2%) were untreated (Fig. 1).
Fig. 1.
Patient selection flow diagram
Study Sample Characteristics and Unadjusted Outcome Patterns
Women had an average age of 82.0 ± 8.1 years. Women with a hip/pelvis/femur fracture were older and had higher mean CCI scores compared to those with clinical vertebral or other non-vertebral fractures. Overall, 12.3% of women had a BMD test prior to the index fragility fracture, and those with clinical vertebral fracture had the highest proportion of glucocorticoid use (54.4%) and anticonvulsant therapy use (11.4%) during the baseline (Table 1). Additionally, 26% of women were prescribed osteoporosis medication in the baseline period.
Table 1.
Descriptive baseline characteristics for fragility fracture patients among Medicare population in the US
| Baseline characteristics | All fractures | Hip/pelvis/femur | Clinical vertebral | Other non-vertebral | ||||
|---|---|---|---|---|---|---|---|---|
| Sample size (N) | 103,852 | 55,158 | 19,252 | 29,509 | ||||
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Age (mean) | 82.0 | 8.1 | 83.6 | 7.7 | 81.7 | 7.7 | 79.1 | 8.1 |
| Age group | ||||||||
| 65–74 | 21,968 | 21.2% | 8117 | 14.7% | 3870 | 20.1% | 9992 | 33.9% |
| 75–84 | 38,857 | 37.4% | 19,797 | 35.9% | 7827 | 40.7% | 11,261 | 38.2% |
| ≥85 | 43,027 | 41.4% | 27,244 | 49.4% | 7555 | 39.2% | 8256 | 28.0% |
| Geographic location | ||||||||
| Northeast | 18,827 | 18.1% | 10,142 | 18.4% | 3164 | 16.4% | 5527 | 18.7% |
| North Central | 28,196 | 27.2% | 14,128 | 25.6% | 5684 | 29.5% | 8414 | 28.5% |
| South | 41,049 | 39.5% | 22,498 | 40.8% | 7475 | 38.8% | 11,098 | 37.6% |
| West | 15,637 | 15.1% | 8296 | 15.0% | 2921 | 15.2% | 4429 | 15.0% |
| Unknown | 143 | 0.1% | 94 | 0.2% | 8 | 0.0% | 41 | 0.1% |
| Race | ||||||||
| White | 94,363 | 90.9% | 49,972 | 90.6% | 17,667 | 91.8% | 26,785 | 90.8% |
| Black | 3549 | 3.4% | 2155 | 3.9% | 358 | 1.9% | 1036 | 3.5% |
| Hispanic | 2440 | 2.3% | 1263 | 2.3% | 422 | 2.2% | 757 | 2.6% |
| Other/missing | 3500 | 3.4% | 1768 | 3.2% | 805 | 4.2% | 931 | 3.2% |
| Baseline comorbid indices | ||||||||
| Charlson Comorbidity Index | 2.7 | 2.2 | 2.9 | 2.2 | 2.6 | 2.2 | 2.3 | 2.2 |
| Baseline comorbid conditions | ||||||||
| Congestive heart failure | 18,679 | 18.0% | 11,255 | 20.4% | 3490 | 18.1% | 3948 | 13.4% |
| COPD | 25,975 | 25.0% | 14,227 | 25.8% | 5402 | 28.1% | 6359 | 21.5% |
| Cerebrovascular disease | 4801 | 4.6% | 2907 | 5.3% | 829 | 4.3% | 1071 | 3.6% |
| Depression/bipolar disorders | 24,939 | 24.0% | 14,673 | 26.6% | 4500 | 23.4% | 5779 | 19.6% |
| Diabetes mellitus | 27,479 | 26.5% | 14,609 | 26.5% | 4654 | 24.2% | 8230 | 27.9% |
| Kidney diseases | 13,892 | 13.4% | 8727 | 15.8% | 2178 | 11.3% | 2991 | 10.1% |
| Liver diseases | 1082 | 1.0% | 486 | 0.9% | 273 | 1.4% | 324 | 1.1% |
| Peripheral vascular diseases | 7494 | 7.2% | 4461 | 8.1% | 1399 | 7.3% | 1640 | 5.6% |
| Rheumatoid arthritis | 4868 | 4.7% | 2653 | 4.8% | 1087 | 5.6% | 1130 | 3.8% |
| Dementia | 19,375 | 18.7% | 13,397 | 24.3% | 2582 | 13.4% | 3412 | 11.6% |
| Parkinson’s | 3296 | 3.2% | 1957 | 3.6% | 627 | 3.3% | 715 | 2.4% |
| Thyroid-related disease | 5092 | 4.9% | 2645 | 4.8% | 1032 | 5.4% | 1418 | 4.8% |
| Physician specialty | ||||||||
| Rheumatology | 6799 | 6.5% | 3225 | 5.8% | 1739 | 9.0% | 1841 | 6.2% |
| Endocrinology | 4840 | 4.7% | 2342 | 4.2% | 975 | 5.1% | 1526 | 5.2% |
| Baseline medication and procedures | ||||||||
| Bisphosphonate | 23,091 | 22.2% | 11,422 | 20.7% | 5738 | 29.8% | 5948 | 20.2% |
| Denosumab | 114 | 0.1% | 47 | 0.1% | 41 | 0.2% | 26 | 0.1% |
| Calcitonin | 2559 | 2.5% | 1182 | 2.1% | 947 | 4.9% | 433 | 1.5% |
| Teriparatide | 470 | 0.5% | 222 | 0.4% | 139 | 0.7% | 110 | 0.4% |
| Raloxifene | 2337 | 2.3% | 1153 | 2.1% | 519 | 2.7% | 669 | 2.3% |
| Tamoxifen | 29 | 0.0% | 18 | 0.0% | 4 | 0.0% | 7 | 0.0% |
| Glucocorticoids | 45,252 | 43.6% | 22,290 | 40.4% | 10,479 | 54.4% | 12,517 | 42.4% |
| Bone mineral density (BMD) test | 12,787 | 12.3% | 5609 | 10.2% | 3111 | 16.2% | 4073 | 13.8% |
| Anticonvulsants | 11,441 | 11.0% | 6148 | 11.1% | 2200 | 11.4% | 3099 | 10.5% |
| Baseline all-cause healthcare utilization | ||||||||
| Inpatient stay | 79,296 | 76.4% | 51,922 | 94.1% | 12,563 | 65.3% | 14,835 | 50.3% |
| ER visit | 45,297 | 43.6% | 23,460 | 42.5% | 9917 | 51.5% | 11,954 | 40.5% |
| Outpatient visit | 88,701 | 85.4% | 45,964 | 83.3% | 17,468 | 90.7% | 25,335 | 85.9% |
| Pharmacy claim | 101,781 | 98.0% | 53,997 | 97.9% | 19,059 | 99.0% | 28,791 | 97.6% |
| DME claim | 45,733 | 44.0% | 23,233 | 42.1% | 9368 | 48.7% | 13,154 | 44.6% |
| SNF visit | 15,522 | 14.9% | 9311 | 16.9% | 2945 | 15.3% | 3281 | 11.1% |
| HHA visit | 25,794 | 24.8% | 14,398 | 26.1% | 5692 | 29.6% | 5722 | 19.4% |
| Hospice visit | 1133 | 1.1% | 883 | 1.6% | 90 | 0.5% | 160 | 0.5% |
DME durable medical equipment, ER emergency room, HHA home health aide, SNF skilled nursing facility
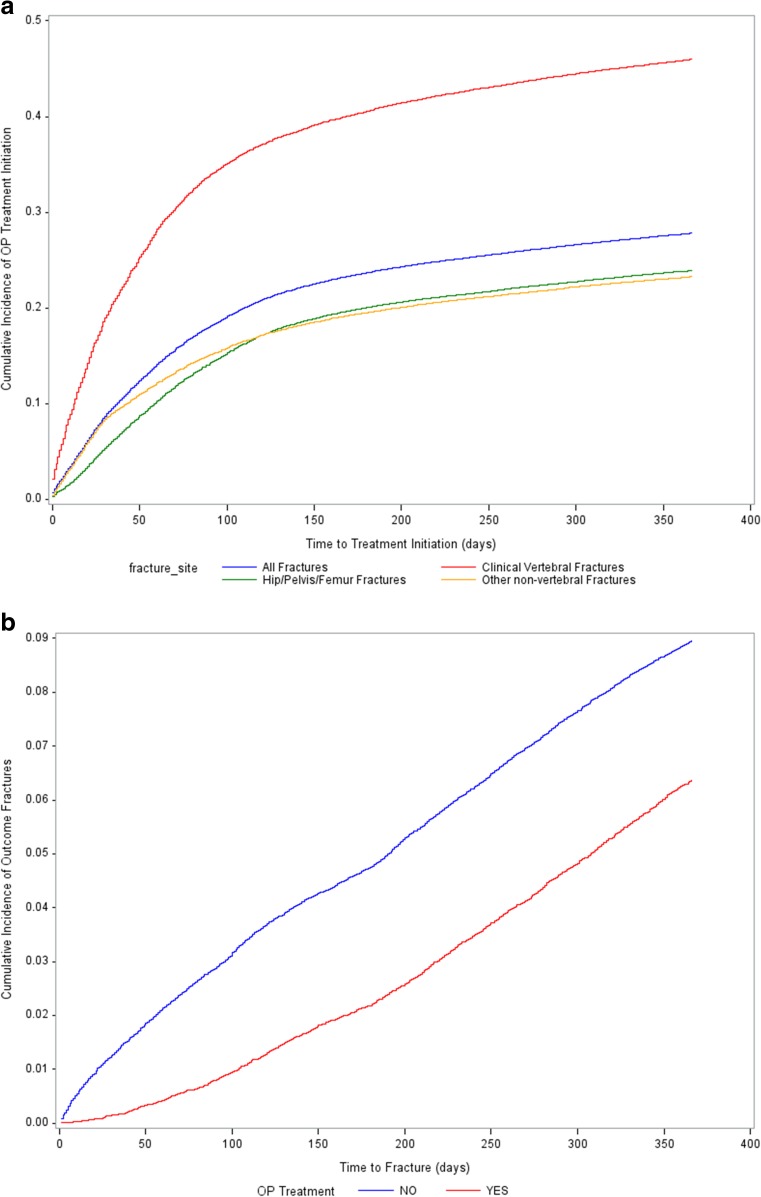
Figure 2a shows the cumulative incidence of osteoporosis treatment during the 12-month follow-up period by index fragility fracture site. The highest proportion of female patients who were prescribed osteoporosis medication within 12 months of index fracture was found to be in clinical vertebral fracture patients (46.0%) followed by hip/pelvis/femur (23.9%) and other non-vertebral fracture patients (23.3%). The mean time to treatment use was 72.7 ± 82.5, 88.7 ± 90.7, and 98.4 ± 86.6 days for clinical vertebral, hip/pelvis/femur, and other non-vertebral fractures, respectively (Supplemental Table 2). Among female patients, 24.0% discontinued treatment, and the mean time to discontinuation was 118.9 days.
Fig. 2.
a Unadjusted time-to-osteoporosis treatment initiation comparing different fracture sites. b Unadjusted time-to-fracture comparing treated and untreated osteoporosis patients
Cumulative incidence of subsequent fractures was measured over 12 months post-index date. Overall, 8.3% of women had a subsequent fracture, and the mean time-to-fracture was 175.8 ± 108.6 days (Supplemental Table 3). Hip/pelvis/femur fractures were the most common (57.5%) followed by other non-vertebral fractures (25.0%) and clinical vertebral fractures (18.1%). Women exposed to an osteoporosis treatment during the follow-up period had a significantly lower risk of fractures compared to those without treatment exposure (6.4 vs 9.0%, p < 0.001; Fig. 2b, Supplemental Table 3). There was a small proportion of women (3.3%) with a subsequent fracture prior to osteoporosis medication use during the follow-up period, and the mean time from subsequent fracture-to-medication prescription use was 54.4 ± 57.9 days (Supplemental Table 2).
Untreated women had a subsequent fracture much sooner compared to treated women (165.2 ± 109.4 vs 216.2 ± 95.3 days, p < 0.001). Importantly, the difference in cumulative incidence of fractures among treated and untreated women was evident from the beginning of the follow-up period (Fig. 2b). Among women with a fracture, there was a higher proportion of women with hip/pelvis/femur fractures (59.1 vs 51.5%, p < 0.001) and other non-vertebral fractures (25.4 vs 23.2%, p < 0.001) among untreated compared to treated women. However, there was a lower proportion of women with clinical vertebral fractures among untreated compared to those who were treated (16.0 vs 25.8%).
Multivariable analysis
The multivariable Cox proportional hazards model for predictors of osteoporosis treatment are shown in Table 2. Women diagnosed with dementia (HR 0.92, 95% CI: 0.89-0.96), and those who were black (HR 0.92, 95% CI 0.85-1.00), were significantly less likely to be prescribed osteoporosis treatment. For all fractures, women who had a BMD test after their first fragility fracture were significantly more likely to be prescribed an osteoporosis medication (HR 4.66, 95% CI 4.49–4.84). In addition, having a subsequent fracture during the follow-up increased the likelihood of being prescribed osteoporosis treatment by 48.7%, compared to no subsequent fracture (HR 1.49, 95% CI 1.39–1.59). The strongest predictor of treatment after a fragility fracture was use of osteoporosis treatment in the baseline period (HR 7.87, 95% CI 7.67–8.07). For specific factors of treatment initiation for each facture site, refer to Supplemental Table 4. There was no evidence of multicollinearity among the covariates in the Cox proportional hazards model.
Table 2.
Cox proportional hazards model for time-to-initiation of osteoporosis medication within 12 months of the index fracture date
| Time-to-initiation | |||
|---|---|---|---|
| All fractures | |||
| Covariates | Hazard Ratio | 95% CI | P value |
| Age group | |||
| 65–74 (reference) | |||
| 75–84 | 1.05 | 1.02–1.09 | 0.001 |
| ≥85 | 0.99 | 0.96–1.02 | 0.484 |
| Geographic location | |||
| Northeast (reference) | |||
| North Central | 1.16 | 1.12–1.20 | <0.001 |
| South | 1.02 | 0.99–1.06 | 0.210 |
| West | 1.14 | 1.10–1.19 | <0.001 |
| Unknown | 0.78 | 0.53–1.16 | 0.216 |
| Race | |||
| White (reference) | |||
| Black | 0.92 | 0.85–1.00 | 0.046 |
| Hispanic | 1.19 | 1.10–1.27 | <0.001 |
| Other/missing | 1.32 | 1.25–1.39 | <0.001 |
| Baseline comorbid conditions | |||
| Charlson Comorbidity Index | 0.98 | 0.97–0.98 | <0.001 |
| Congestive heart failure | 0.97 | 0.94–1.01 | 0.150 |
| Congestive obstructive pulmonary disease | 1.09 | 1.06–1.13 | <0.001 |
| Kidney diseases | 0.94 | 0.90–0.98 | 0.003 |
| Liver diseases | 1.08 | 0.97–1.21 | 0.160 |
| Rheumatoid arthritis | 1.03 | 0.97–1.08 | 0.340 |
| Dementia | 0.92 | 0.89–0.96 | <0.001 |
| Baseline medication and procedures | |||
| Any osteoporosis medication | 7.87 | 7.7–8.07 | <0.001 |
| BMD test | 1.13 | 1.1–1.17 | <0.001 |
| Glucocorticoids | 1.02 | 1.0–1.05 | 0.059 |
| Time-varying covariates during follow-up | |||
| Subsequent fracture | 1.49 | 1.39–1.59 | <0.001 |
| BMD Test | 4.66 | 4.49–4.84 | <0.001 |
| Rheumatology visit | 1.43 | 1.36–1.51 | <0.001 |
| Endocrinology visit | 1.28 | 1.20–1.37 | <0.001 |
When predictors of treatment were stratified by prior medication use, the results were generally consistent (Supplemental Table 5). Older age, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, glucocorticoid use, and time-varying subsequent fractures, BMD test, rheumatology visits, and endocrinology visits were significant predictors of treatment initiation. When predictors of treatment were stratified by place of service, the results were consistent, except that dementia was only a significant predictor among patients who had their fragility fracture in the inpatient setting (Supplemental Table 6).
After adjusting for covariates, including age, geographic region, comorbidities, and medication, the treated group was associated with a 20.9% lower risk of a subsequent fracture while on osteoporosis therapy, compared to the untreated group during 12 months after the index fragility fracture (HR 0.79, 95% CI 0.75–0.83; Table 3). Older age (≥85 years: HR 1.50, 95% CI 1.40–1.59; 75–84 years: HR 1.29, 95% CI 1.21–1.38), diagnosis for rheumatoid arthritis (HR 1.15, 95% CI 1.05–1.27) or depression (HR 1.20, 95% CI 1.14–1.26), and baseline glucocorticoid (HR 1.08, 95% CI 1.03–1.13) and anticonvulsant use (HR 1.19, 95% CI 1.11–1.26) were other factors associated with increased risk of subsequent fracture.
Table 3.
Cox proportional hazards model for time-to-fracture within 12 months following the initial fracture claim
| Covariates | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Cohort | |||
| Untreated | |||
| Treated | 0.79 | 0.75–0.83 | <0.001 |
| Age group | |||
| 65–74 (reference) | |||
| 75–84 | 1.29 | 1.21–1.38 | <0.001 |
| ≥85 | 1.50 | 1.40–1.59 | <0.001 |
| Geographic location | |||
| Northeast (reference) | |||
| North Central | 1.08 | 1.01–1.15 | 0.018 |
| South | 0.96 | 0.90–1.02 | 0.161 |
| West | 0.94 | 0.87–1.01 | 0.086 |
| Unknown | 0.09 | 0.01–0.63 | 0.015 |
| Race | |||
| White (reference) | |||
| Black | 0.59 | 0.51–0.68 | <0.001 |
| Hispanic | 0.81 | 0.69–0.95 | 0.009 |
| Other/missing | 1.02 | 0.90–1.15 | 0.778 |
| Baseline comorbid conditions | |||
| Charlson Comorbidity Index | 1.06 | 1.04–1.07 | <0.001 |
| Chronic obstructive pulmonary disease | 1.04 | 0.99–1.10 | 0.122 |
| Depression/bipolar disorders | 1.20 | 1.14–1.26 | <0.001 |
| Diabetes mellitus | 0.91 | 0.86–0.96 | 0.001 |
| Kidney diseases | 0.90 | 0.83–0.96 | 0.003 |
| Liver diseases | 1.25 | 1.04–1.51 | 0.019 |
| Rheumatoid arthritis | 1.15 | 1.05–1.27 | 0.003 |
| Dementia | 1.09 | 1.03–1.15 | 0.002 |
| Baseline medication and procedures | |||
| Glucocorticoids | 1.08 | 1.03–1.13 | 0.001 |
| Anticonvulsants | 1.19 | 1.11–1.26 | <0.001 |
The results were consistent when stratified by prior medication prescription use and place of service (Supplemental Tables 7, 8). Prevalent osteoporosis medication users had a greater reduction in fracture risk (HR 0.51; 95% CI 0.47–0.55) compared to new osteoporosis medication users (HR 0.80; 95% CI 0.73–0.87).
Discussion
The current study was the first real-world retrospective observational analysis to examine the correlates of osteoporosis treatment and the relative impact of treatment on fracture risk in a large Medicare patient population. In the past, many studies have suggested the significant clinical and economic burden this disease has on the patient as well as society [22–24]. To date, few studies have explored and addressed the real-world factors that impact the treatment gap between index fragility fracture and risk of subsequent fractures post-treatment among female Medicare enrollees [25, 26].
Our analysis showed that only 28% of patients initiated treatment after the index fracture and that the treatment gap was almost 3 months. Almost half of all clinical vertebral fracture patients were prescribed an osteoporosis medication within 12 months of the index fracture and initiated treatment earlier than hip/pelvis/femur and other non-vertebral fracture patients. Clinical vertebral fractures are often associated with chronic pain and subsequent fractures; therefore, this may explain the greater likelihood of osteoporosis treatment use among clinical vertebral fracture patients [27, 28]. Also, among treated patients, the time from treatment to subsequent fracture was much longer for clinical vertebral fractures. This may be attributed to the fact that clinical vertebral fracture patients were more likely to be on treatment and the positive impact of the treatment reflected on the longer time-to-subsequent fracture for clinical vertebral fracture patients.
We conducted multivariate analysis for time to treatment use to identify potential determinants for the treatment gap among osteoporotic women. Numerous other studies have also shown that black patients are less likely to be on osteoporosis medication, which is consistent with our study, where black patients were 8% less likely to use osteoporosis medication [29–31]. The presence of kidney diseases and dementia at baseline were found to be predictors of a lower likelihood of treatment. Moreover, several osteoporosis medications are not approved to be used in patients with severe renal disease. At age 80 and above, the presence of renal and mental health complications raises the challenges to improve physical health by any margin.
In an elderly population, dementia may have a differential effect on osteoporosis treatment use and fracture risk. Patients with osteoporosis have been shown to have a 46% greater risk for dementia compared to non-osteoporosis control patients (HR 1.46, 95% CI 1.37-1.56) [32], while another study showed that dementia was an independent risk factor for developing hip fractures compared to non-dementia patients (HR 1.92, 95% CI 1.48–2.49) [33]. In addition, another article suggested that dementia patients are found to be 66% less likely to use osteoporosis medications (OR 0.34, 95% CI 0.19–0.59) compared to those without dementia [34]. Similarly, our results showed a dementia diagnosis was associated with a 7.6% lower likelihood of initiating osteoporosis treatment (p < 0.001) and 8.8% increased risk of fracture (p = 0.002).
Conversely, baseline osteoporosis medication and BMD testing showed an increased likelihood for further osteoporosis treatments being prescribed. A prior study suggested that more than half of patients (58%) resumed osteoporosis treatment during the 1 year after a BMD test [35]. Therefore, BMD testing has a temporal relationship with increased osteoporosis medication use over follow-up. In our current study, BMD test as a time-varying covariate was significantly associated with higher likelihood of osteoporosis medication use over time (HR 4.66, 95% CI 4.49–4.84). Furthermore, a recent research study suggested that among high-risk osteoporosis patients, a rheumatologist is 21% more likely to prescribe osteoporosis medications compared to a non-rheumatologist [36], which is consistent with our study that showed a rheumatology visit was associated with a 43% increased likelihood of osteoporosis medication use.
These findings may suggest that women value mobility, physical health, and the prevention of future fractures as important healthcare priorities, resulting in increased baseline screening and baseline osteoporosis medication as well as the consequent greater uptake in future osteoporosis medication. Additionally, prior osteoporosis medication may also suggest that the patient’s physician was more likely to prescribe medications for osteoporosis in the future. Importantly, we found that a second fracture increased the likelihood of initiating osteoporosis treatment in the sample by 48.7%, suggesting that an additional fracture after the index fragility fracture can necessitate prescription of osteoporosis medication. With timely osteoporosis treatment during follow-up, risk of subsequent fractures can be managed and the effectiveness of osteoporosis treatment can be improved.
Our analysis resulted in treated-osteoporotic women being associated with a 21% lower likelihood of a subsequent fracture during the 12-month follow-up period. Among prevalent osteoporosis medication users, we found a 41% lower risk of fractures, and new users had a 20% lower risk of fractures during the study follow-up period. The results imply that continued exposure to osteoporosis treatment has a greater impact on fracture risk reduction compared to new exposure. Our study has a shorter follow-up period compared to RCT and other RWE studies; therefore, the impact may be greater with a longer follow-up. Additionally, a systematic review of RCTs demonstrated the impact of osteoporosis medication on time to fracture reduction, ranging from 6-36 months of treatment [37]. Our RWE study results among Medicare women can be tied to the fact that osteoporosis treatments show an increased effectiveness against subsequent fractures and support the efficacy demonstrated through RCTs in the past.
Limitations
There are several important limitations to this study. CMS Medicare data does not include information for beneficiaries enrolled in a Medicare-managed care plan. The study sample was limited to Medicare fee-for-service enrollees; therefore, it is not generalizable to the entire elderly population in the US. While claims data are valuable for the efficient and effective examination of healthcare outcomes, treatment patterns, resource utilization, and costs, claims data are collected for the purpose of payment and not research. Therefore, certain limitations were associated with claims data use. First, the presence of a Part D claim for a filled prescription did not indicate whether the medication was actually consumed or taken as prescribed. Second, medications filled over-the-counter or provided as samples by the physician would not be observed in the claims data. Third, the presence of a diagnosis code on a medical claim was not a positive presence of disease, as the diagnosis code may be incorrectly coded or included as rule-out criteria rather than actual disease. For example, vertebral fractures are usually underdiagnosed in clinical practice and could potentially be misclassified, especially among vertebral compression fractures [38]. Finally, certain information was not readily available in claims data (or could be randomly missing) that may have influenced study outcomes, such as clinical and disease-specific parameters, including BMD T-scores, tobacco use, low calcium intake, and eating disorders. These unmeasured variables could lead to residual confounding. In addition, there is evidence of continued fracture reduction after 12 months and it has been shown that each additional month up to 24 months reduced non-vertebral fractures by another 7% [39]. Therefore, a 12-month follow-up may have underestimated the full benefit of longer treatment.
Our analysis was conducted from a large, comprehensive, administrative Medicare claims database that offers an adequate sample size to infer meaningful relationships between index fragility fracture, osteoporosis medication use, and subsequent fractures during the 12-month follow-up period for osteoporotic women. We examined a comprehensive list of osteoporosis medications among Medicare enrollees, which gave us a robust estimate of the predictors of osteoporosis medication use. In addition, we were able to adjust for all the observable confounders that could mediate and/or confound the relationship in our models. Furthermore, we conducted two sensitivity analyses, which confirmed our results.
In conclusion, clinical vertebral fracture patients were the most likely to be treated with osteoporosis medications compared to hip/pelvis/femur and other non-vertebral fracture patients in our Medicare sample. After adjusting for baseline demographic and clinical characteristics, treated osteoporotic women had a significantly lower risk for subsequent fractures compared to untreated osteoporotic women; this adds sufficient value to the need of timely and effective osteoporosis treatment among elderly osteoporotic women with fragility fractures. Considering that osteoporosis treatment use post-fracture is low (below 50% for all sites) and mean times to treatment ranged from 2 to 3 months, our findings reflect that osteoporosis treatment management can be improved. There is a need for coordination of care to close this treatment gap to improve treatment rates as well as timing of osteoporosis treatment post-fracture to reduce future fractures effectively. Collaborations across orthopedic providers and payers and implementing an integrated model of care [40] may improve access and use of osteoporosis treatments in the US Medicare population.
Electronic supplementary material
(DOCX 14.4 kb).
(DOCX 14.4 kb).
(DOCX 15.6 kb).
(DOCX 20.6 kb).
(DOCX 16.1 kb).
(DOCX 17.9 kb).
(DOCX 17 kb).
(DOCX 16.8 kb).
Acknowledgements
Neel Vaidya, of STATinMED Research, provided editorial assistance.
Compliance with ethical standards
Conflict of interest
L. Xie, A. Keshishian and O. Baser are employees of STATinMED Research, a paid consultant to Eli Lilly Inc. in connection with the study design, data analysis, and development of this manuscript.
N. Boytsov, R. Burge, K. Krohn, L. Lombard, and X. Zhang are employees and stock owners of Eli Lilly and Co.
Funding for this research was provided by Eli Lilly and Co.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-4072-6) contains supplementary material, which is available to authorized users.
References
- 1.Kanis JA (2007) WHO technical report. Report. University of Sheffield. https://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. Accessed 28 March 2017
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality (2011) Healthcare cost and utilization project. HCUP Central Distributor. https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.pdf. Updated November 2015; Accessed 28 March 2017
- 4.Eichner SF, Lloyd KB, Timpe EM. Comparing therapies for postmenopausal osteoporosis prevention and treatment. Ann Pharmacother. 2003;37(5):711–724. doi: 10.1345/aph.1C246. [DOI] [PubMed] [Google Scholar]
- 5.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282(14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 7.Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 8.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 9.Boytsov N, Zhang X, Sugihara T, Taylor K, Swindle R. Osteoporotic fractures and associated hospitalizations among patients treated with teriparatide compared to a matched cohort of patients not treated with teriparatide. Curr Med Res Opin. 2015;31(9):1665–1675. doi: 10.1185/03007995.2015.1066765. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson J. Clinical effectiveness of bisphosphonates for prevention of fragility fractures: a systematic review and network meta-analysis. Value Health. 2015;18(7):A634. doi: 10.1016/j.jval.2015.09.2250. [DOI] [PubMed] [Google Scholar]
- 11.Lems WF. Fracture risk estimation may facilitate the treatment gap in osteoporosis. Ann Rheum Dis. 2015;74(11):1943–1945. doi: 10.1136/annrheumdis-2015-208245. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, Cauley J, Compston J, Kiel DP, Rosen C, Saag KG, Shane E. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32(3):424–430. doi: 10.1002/jbmr.3074. [DOI] [PubMed] [Google Scholar]
- 13.Guggina P, Flahive J, Hooven FH, et al. Characteristics associated with anti-osteoporosis medication use: data from the global longitudinal study of osteoporosis in women (GLOW) USA cohort. Bone. 2012;51(6):975–980. doi: 10.1016/j.bone.2012.08.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asche C, Nelson R, McAdam-Marx C, Jhaveri M, Ye X. Predictors of oral bisphosphonate prescriptions in post-menopausal women with osteoporosis in a real-world setting in the USA. Osteoporos Int. 2010;21(8):1427–1436. doi: 10.1007/s00198-009-1079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siris ES, Modi A, Tang J, Gandhi S, Sen S. Substantial under-treatment among women diagnosed with osteoporosis in a US managed-care population: a retrospective analysis. Curr Med Res Opin. 2014;30(1):123–130. doi: 10.1185/03007995.2013.851074. [DOI] [PubMed] [Google Scholar]
- 16.Brennan RM, Wactawski-Wende J, Crespo CJ, Dmochowski J. Factors associated with treatment initiation after osteoporosis screening. Am J Epidemiol. 2004;160(5):475–483. doi: 10.1093/aje/kwh245. [DOI] [PubMed] [Google Scholar]
- 17.Cole RP, Palushock S, Haboubi A. Osteoporosis management: physicians’ recommendations and womens’ compliance following osteoporosis testing. Women Health. 1999;29(1):101–115. doi: 10.1300/J013v29n01_08. [DOI] [PubMed] [Google Scholar]
- 18.Bonnick SL. Osteoporosis in men and women. Clin Cornerstone. 2006;8(1):28–39. doi: 10.1016/S1098-3597(06)80063-3. [DOI] [PubMed] [Google Scholar]
- 19.Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: a review. J Womens Health (Marchmt) 2014;23(7):563–572. doi: 10.1089/jwh.2013.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services (1996) Health Insurance Portability and Accountability Act of 1996. Public law 104–191, 104th Congress. https://aspe.hhs.gov/report/health-insurance-portability-and-accountability-act-1996. Accessed August 1, 2016
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 23.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(Suppl 6):S164–S169. [PubMed] [Google Scholar]
- 25.Liu SK, Munson JC, Bell JE, et al. Quality of osteoporosis care of older Medicare recipients with fragility fractures: 2006 to 2010. J Am Geriatr Soc. 2013;61(11):1855–1862. doi: 10.1111/jgs.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun H, Curtis JR, Guo L, Kilgore M, et al. Patterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiaries. BMC Musculoskelet Disord. 2014;15:112. doi: 10.1186/1471-2474-15-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Geel TA, Huntjens KM, van den Bergh JP, Dinant GJ, Geusens PP. Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep. 2010;8(3):118–122. doi: 10.1007/s11914-010-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vedantam R. Management of osteoporotic vertebral compression fractures: a review. Am J Clin Med. 2009;6(4):14–18. [Google Scholar]
- 29.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68(10):1236–1242. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, McClure LA, Delzell E, Howard VJ, Orwoll E, Saag KG, Safford M, Howard G. Population-based fracture risk assessment and osteoporosis treatment disparities by race and gender. J Gen Intern Med. 2009;24(8):956–962. doi: 10.1007/s11606-009-1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CW, Karaca-Mandic P, McCullough JS, Weaver L. Access to oral osteoporosis drugs among female Medicare part D beneficiaries. Womens Health Issues. 2014;24(4):e435–e445. doi: 10.1016/j.whi.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang KH, Chung CJ, Lin CL, Sung FC, Wu TN, Kao CH. Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age (Dordr) 2014;36(2):967–975. doi: 10.1007/s11357-013-9608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Hung C, Lin S et al. (2014) Increased risk of hip fractures in patients with dementia: a nationwide population-based study. BMC Neurology 14(1):175 [DOI] [PMC free article] [PubMed]
- 34.Haasum Y, Fastbom J, Fratiglioni L, Johnell K. Undertreatment of osteoporosis in persons with dementia? A population-based study. Osteoporos Int. 2012;23(3):1061–1068. doi: 10.1007/s00198-011-1636-8. [DOI] [PubMed] [Google Scholar]
- 35.Yood RA, Andrade SE, Mazor KM, Fouayzi H, Chan W, Kahler K. Bone density consequences of initiation and compliance with therapy for osteoporosis. Arthritis Care Res. 2010;62(10):1440–1445. doi: 10.1002/acr.20261. [DOI] [PubMed] [Google Scholar]
- 36.Overman R (2013) Evaluation of osteoporosis medication initiation in patients based on t-score and FRAX® absolute fracture risk in a large health care system. American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting. University of North Carolina and Cleveland Clinic, San Diego
- 37.Inderjeeth CA, Chan K, Kwan K, Lai M. Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab. 2012;30(5):493–503. doi: 10.1007/s00774-012-0349-1. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47(1):69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsay R, Miller P, Pohl G, Glass EV, Chen P, Krege JH. Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporosis Int. 2009;20(6):943–948. doi: 10.1007/s00198-008-0766-0. [DOI] [PubMed] [Google Scholar]
- 40.Strassberger C, Unger L, Weber AT, Defer A, Bonnaire FA. Management of osteoporosis-related bone fractures: an integrated concept of care. Arch Orthop Trauma Surg. 2010;130(1):103–109. doi: 10.1007/s00402-009-0989-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14.4 kb).
(DOCX 14.4 kb).
(DOCX 15.6 kb).
(DOCX 20.6 kb).
(DOCX 16.1 kb).
(DOCX 17.9 kb).
(DOCX 17 kb).
(DOCX 16.8 kb).