Abstract
Advances in cancer research in the past have led to an evolving understanding of cancer pathogenesis and the development of novel drugs that significantly improve patient outcomes. However, many patients still encounter treatment resistance, recurrence, or metastasis and eventually die from progressing disease. Experimental evidence indicates that a subpopulation of cancer cells, called cancer stem cells (CSCs), possess “stemness” properties similar to normal stem cells, including self-renewal, differentiation, and proliferative potential. These stemness properties are lost during differentiation and are governed by pathways such as STAT3, NANOG, NOTCH, WNT, and HEDGEHOG, which are highly dysregulated in CSCs due to genetic and epigenetic changes. Promising results have been observed in preclinical models targeting these CSCs through the disruption of stemness pathways in combination with current treatment modalities. This has led to anti-CSC–based clinical trials in multiple stages of development. In this review, we discuss the role of CSCs and stemness pathways in cancer treatment and how they relate to clinical observations. Because CSCs and the stemness pathways governing them may explain the negative clinical outcomes observed during treatment, it is important for oncologists to understand how they contribute to cancer progression and how they may be targeted to improve patient outcomes.
History of Cancer Stem Cells in Oncology
The concept in oncology that the development and growth of cancer occurs through cancer stem cells (CSCs) has been popularized over the past decade. The evidence for the CSC concept has been evolving since pathologists began examining cancer cells under the microscope more than 150 years ago [1, 2]. In the latter half of the twentieth century, evidence from functional studies for CSCs began to emerge from experiments involving colony formation from cancer cells in vitro and in vivo [3–9]. There is now substantial evidence that CSCs play a role in the development and growth of most human malignancies [10]. Therefore, it is important for oncologists to understand the biology of CSCs, how CSCs contribute to cancer progression, and how they may be targeted using new, potentially more-effective therapies being developed.
To understand CSCs, it is important to realize that many normal stem cells can be found in the body throughout a lifetime. The concept of a stem cell was first articulated in 1877 by Ernst Haeckel to explain the idea that the fertilized egg is the cell that gives rise to all the other cells in the body [11]. The concept of stem cells was then rapidly incorporated as a way to explain the existence of other cell types in the embryo and adult organism. Stem cells have been recognized as clonogenic cells in radiation oncology for many years [12, 13]. Although CSCs and normal stem cells share many of the same properties, CSCs are unique in that they can initiate and maintain cancer [10, 14–16].
Definitions of Stem Cell and Stemness
A stem cell is defined primarily by its function, but a number of markers have been found that can be used to enrich cell isolates for stem cells in functional assays that compare stem cells with their progeny. Recent evidence indicates that CSCs can arise from normal stem cells or from progenitor cells. There are two main types of normal stem cells [16–18]: embryonic stem cells and somatic stem cells (also called adult stem cells) [19]. Embryonic stem cells are derived from the early divisions of the fertilized egg and give rise to all cells during human development [17]. Somatic stem cells are usually present in developed tissues and organs. The primary function of somatic stem cells is to maintain and repair tissues and organs [19]. Scientists are now able to reprogram adult/somatic cells into a state similar to embryonic stem cells, resulting in induced pluripotent stem cells [20]. These cells have the potential to help repair damaged organs, improve and revolutionize organ transplant, and to test drugs in development [19]. This overview of the stem cell/progenitor origin of cancer will mainly discuss properties of somatic stem cells, because the majority of cancers arise from somatic tissue.
The two basic properties of stem cells are self-renewal and differentiation into multiple lineages. Some stem cells may also exhibit high proliferative potential [21]. The capability to self-renew gives these cells the ability to maintain themselves and the capacity for tissue regeneration. The ability to differentiate gives them the capacity to produce cells with specialized properties that are necessary for organ function. As stem cells differentiate, they begin to lose their “stemness,” i.e. their ability to act as a stem cell as described above. These changes establish a hierarchy of cell populations that underlie organogenesis. Tumors, similar to normal organs, despite very often aberrant and limited terminal differentiation, are possibly composed of a relatively flattened hierarchical collection of cancer cells and stromal cells interacting with structures (e.g. extra-cellular matrix). However, tumors are structurally and functionally abnormal compared with normal organs [22].
Within this hierarchical cellular organization of tissues, the stem cells usually reside in specialized microenvironments or niches [13, 21, 23]. In any given tissue, homeostasis is maintained through a balance between stem cell self-renewal and differentiation that maintains a constant number of cells in the tissue [21]. This balance requires the ability of normal stem cell populations to precisely maintain their numbers through regulation of symmetric and asymmetric stem cell division [21]. Stem cell populations can be quite heterogeneous. In some tissues, stem cells have also been found to be relatively quiescent, i.e. they have tremendous proliferative capacity, but are often not actively cycling. They have longer cell-cycle times than proliferating non-stem cells, presumably due to their arrest in the G0-phase of the cell cycle [21]. This property of quiescence may be a mechanism that protects the stem cell from acquiring mutations that could occur in DNA replication during cell division [24]. In other tissues, particularly skin and gut, stem cell populations are highly proliferative. Organs with high turnover would be expected to have a high proliferative capacity in the stem-cell compartment [24].
The term “stem cell” defines a specific cell type that possesses the main properties of self-renewal, multilineage differentiation potential, and, in some cases, proliferation. The term “stemness” refers to the degree to which a cell possesses these functional properties. Thus, stemness is a more elusive term, and this raises the question as to whether it is specific enough to help distinguish between a stem cell and a non-stem cell [25]. Because of the elusiveness of the term stemness, there have been efforts to provide a mathematical expression for stemness [26] and to identify a gene expression profile for stemness [27].
How Do Cancer Stem Cells Differ from Normal Stem Cells?
Although CSCs in malignancies have the capacity for self-renewal, the processes involved in self-renewal are dysregulated, which leads to CSC overpopulation, driving tumor growth. This dysregulation is thought to be due to an increase in symmetric cell division (producing two stem cell daughters) compared with asymmetric division (producing one stem cell daughter and one non-stem cell daughter) [16, 28]. Most CSCs, but not normal stem cells, carry genetic mutations and epigenetic changes. Such changes can lead to dysregulation of signaling pathways, particularly those pathways that are known to be involved in embryonic development (e.g. NANOG, WNT family member [WNT]/β-CATENIN, HEDGEHOG, NOTCH). Further details on the importance of these pathways are discussed below. CSCs also have the ability for multilineage differentiation, but this ability is impaired [10, 16, 29]. Indeed in the clinic, pathologists have recognized that cancers are composed of a heterogeneous population of partially differentiated cell types that resemble the cell types found in the tissue of the tumor’s origin. This pathologic evidence points to a stem cell or progenitor origin for cancers, because only multipotent CSCs could produce so many different cell types [2, 10, 29]. Moreover, many malignant tumors have been pathologically classified as poorly differentiated, and these tumors often contain many malignant cells that phenotypically resemble normal stem cells or progenitors [6].
Tumor biologists investigating CSCs have found that CSCs usually comprise a subpopulation of cells within a tumor. This is often based on research showing that a small proportion of cells in a cancer possess the ability to form tumors (i.e. xenografts) when transplanted into animals [14]. In this way, CSCs functionally differ from normal stem cells, i.e. normal stem cells do not have tumor-initiating ability. The degree to which tumor cells can initiate xenograft tumors in mice (based on cell number, latency time, and serial passaging) is also a measure of a cancer cell’s stemness.
CSCs and normal stem cells may share stem cell markers, particularly cell surface markers, and use common stemness-related signaling pathways [29]. However, in normal stem cells, signaling pathways that maintain stemness are tightly regulated and not mutated. In contrast, in CSCs, stemness signaling pathways are often dysregulated due to acquired genetic mutations and epigenetic changes, giving these cells uncontrolled self-renewal and impaired differentiation to develop into cancer cells. Moreover, CSCs may 1) possess innate resistance to multiple chemotherapeutic agents, 2) be responsible for tumor recurrence, and 3) be the origin of distant metastases [2, 10, 29, 30]. Resistance to chemotherapeutic agents and radiation occurs because CSCs, like normal stem cells, are relatively quiescent and only slowly proliferating. They may also possess mechanisms of multidrug resistance (e.g. ATP-binding cassette [ABC] transporters), antiapoptotic proteins (e.g. B-cell lymphoma 2 [BCL2]), and enhanced DNA repair mechanisms [31, 32].
How CSCs Are Identified and Isolated
The identification and isolation of CSCs from the large bulk of cells in a cancer or from cancer cell lines has been essential to the ongoing research into the role of CSCs in tumor initiation, development, diagnostics, and therapeutics [10, 33]. Initially CSCs could only be identified functionally using their ability to undergo self-renewal and multilineage differentiation. However, it has now become possible to identify normal stem cells and CSCs through the use of specific surface markers and other modalities (Table 1) [10, 34, 35]. Few of these markers for stem cells and CSCs are shared across all tissue/tumor types [10]. The markers for cell surface proteins (like CD44 and CD133) and enzymatic activity (e.g. aldehyde dehydrogenases [ALDH]) can be used to sort and isolate stem cells and CSCs [36, 37].
Table 1.
| ALDH+, ALDH1high, α2β1 high, A2B5 +, ABCG2high |
| BCRP1+, BMI1+ |
| CD15, CD20+, CD24+, CD24−/low, CD29, CD34, CD38−, CD44+, CD49f+, CD71−, CD90−, CD117−, CD123+, CD133+, CD138−, CD166+, CEA+, CK20+, CXCR4+ |
| ESA+, EpCAM+ |
| HLA-DR− |
| Lineage−, LGR5+ |
| NANOG |
| OCT4 |
| SOX2, SSEA+ |
| YAP1+ |
− negative; + positive; ALDH aldehyde dehydrogenases; BCRP1 breakpoint cluster region pseudogene 1; BMI1 B-cell-specific Moloney murine leukemia virus integration site 1; CEA carcinoembryonic antigen; CK20 cytokeratin 20; CXCR4 C-X-C chemokine receptor type 4; ESA epithelial-specific antigen; EpCAM epithelial cell adhesion molecule; HLA-DR human leukocyte antigen–antigen D related; LGR5 leucine-rich repeat-containing G-protein coupled receptor 5; OCT4 organic cation/carnitine transporter 4; SOX2 sex determining region Y-box 2; SSEA stage-specific embryonic antigen; YAP1; yes-associated protein 1
Once identified, CSCs can then be isolated with fluorescence activated cell sorting (FACS). The cell surface markers CD133, CD24, and CD44 are often employed to isolate CSCs from several different tumor types [10, 14, 33, 38]. For example, Al-Hajj et al. [14] demonstrated that CSCs from breast cancers display the ESA+CD44+CD24−/(low) surface marker phenotype. Leukemic stem cells display the CD34+CD38− surface marker phenotype that permits isolation of them from normal hematopoietic stem cells [15]. Further investigation into leukemic stem cells has identified other markers including CD123, TIM3, CD47, and CD96 [39]. Another common method to isolate CSCs is based on the enzymatic activity of ALDH, which is a marker for CSCs from many cancers. This method, called the ALDEFLUOR assay, has been used to purify CSCs from primary surgical tissues including breast cancers [36] and colon cancers [37]. However, ALDH as a CSC marker may be limited to certain subtypes of cancers, such as luminal subtype of breast cancer [40].
In addition to cell surface markers (like CD133) and enzymatic markers (like ALDH), CSCs can be purified (usually by FACS) via their ability to pump out substances (like DNA dyes), which results in a dye-low or dye-negative “side population” (SP) that is distinct from most of the sorted cells [41]. Analysis of multiple ovarian cancer cell lines and tumors identified ovarian CSCs in the SP. These SP cells exhibited multiple stemness properties including resistance to drugs [41].
After they are isolated, candidate CSCs are typically analyzed to see if they display functional properties of CSCs. This step can involve sphere-forming assays (ability to form spheres or colonies in a serum-free or soft agar medium) or xenograft assays in immunocompromised mice. Tumor-initiating ability and serial transplantation in vivo are regarded as gold standard assays, which are complemented by the in vitro clonogenic assays [36]. Clonogenic assay are adaptable for performing high throughput screens that identify drugs that target CSCs [42]. Afterwards, in vitro findings can be validated utilizing the more labor-intensive xenograft models [43]. Experimental results from studies on CSCs may be translated into clinically useful approaches, particularly in development of agents that eliminate or control CSC populations, which may lead to more effective cancer therapies. For example, a gene-silencing method was used to identify STAT3 as critical for maintaining cancer stemness, while remaining expendable in hematopoietic stem cells [43]. In silico screening and computational modeling were used to identify a drug capable of targeting CSCs by inhibiting the STAT3 pathway. This drug is now in clinical development [43].
The Use of Reporter Systems Based on Stem Cell Transcription Factor Activity
Although cell surface markers are typically used to enrich for CSCs, this approach has limitations owing to the plasticity of CSCs, which can interconvert between non-CSCs and CSC-like states. Moreover, the ability of surface markers to track CSCs in real time is limited. These drawbacks, and others, have led to the development of reporter systems that have several unique advantages over cell surface markers for enrichment and analysis of CSCs [33].
The design of reporter systems is typically based on the fact that transcription factors, such as NANOG, sex-determining region Y-box (SOX)2, organic cation/carnitine transporter (OCT) 4, and self-renewal pathways, such as NOTCH and WNT, are upregulated in CSCs [44]. Reporter systems can mark stem cells based on this upregulated transcription factor expression using a model system that contains a genetic construct that leads to expression of a tagged (typically fluorescent) transcription factor protein when the gene of interest is transcriptionally activated in cells.
Several transcription factor-type reporter systems, such as NOTCH, SOX2, enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), STAT3, and NANOG, have been developed for enrichment and analysis of CSCs [33, 45–49]. The development of these reporters has enabled investigators to rapidly enrich for stem cells, monitor their functionality, track them in vivo, and lineage trace their progeny.
STAT Reporter Systems
The STAT3 protein is a member of the family of transcriptional factors that plays a role in embryogenesis and has been identified as an oncogene. For example, Nanog was found to maintain the pluripotency of mouse ESC by interacting with Stat3 [50]. Additionally, STAT3 has been found to be constitutively activated in many human malignancies and to play a pivotal role in tumor growth and metastasis [50, 51]. For example, in the normal human mammary glands, STAT3 is typically not expressed, but tumor samples from breast cancer patients often show STAT3 expression. Recent evidence has also identified a role for STAT3 signaling in breast tumor-initiating cells (TICs), an alternative name of CSCs. To study whether STAT3 is preferentially activated in TICs, Wei et al. [52] constructed a series of lentiviral fluorescent reporters, which enable FACS, in vivo/in situ localization, and molecular characterization of STAT3 responsive cells. Using an in vivo xenograft model of human breast cancer, the study showed that STAT3 signaling reporter activity is associated with a subpopulation of cancer cells enriched for mammosphere-forming efficiency, as well as TIC function in limiting dilution transplantation assays. These findings indicate that STAT3 is a functional marker for human breast CSCs and a potential target for CSC-directed therapy.
NANOG Reporter Systems
NANOG is a homeodomain-containing transcription factor involved in the self-renewal of embryonic stem cells and in maintenance of pluripotency [53, 54]. Increased NANOG expression is found in several cancer types, such as breast, kidney, lung, gastric, brain, ovarian, pancreatic, and head and neck cancers, and its elevated expression can have prognostic significance for patients with these cancers [55–63]. NANOG levels have been found to be increased in CSCs, which contributes to their self-renewal capacity and ability to initiate and promote tumor development and growth.
Based on the importance of NANOG in CSCs, several CSC reporter systems have been created using a NANOG promoter-fluorescent reporter gene strategy. Such a NANOG promoter-driven green fluorescent protein (GFP) reporter was used to study CSC populations in different tumor types. For example, using this reporter system in studies of prostate cancer, NANOG–GFP-positive cells exhibited CSC characteristics, such as enhanced clonal growth [64]. NANOG overexpressing cancer cells, including both prostate and breast, also displayed drug-resistance and capacity for tumor regeneration as well as hormone-resistant tumor growth. Another recent reporter-based study by Wiechert et al. [65] allows for determination of the CSC state in real time and showed that cisplatin induces a CSC state in treatment of ovarian cancer. Another study [66] on triple-negative breast cancer (TNBC) that employed a GFP NANOG reporter system to monitor differences between CSCs and non-CSCs in TNBC was used to identify new CSC surface markers that are elevated in TNBC. Taken together, these reporter studies reveal that NANOG serves as a reliable marker for CSCs in a variety of cancers and represents a strong potential CSC-specific target for the development of future treatment approaches.
Stochastic Model versus Cancer Stem Cell Model of Tumor Growth
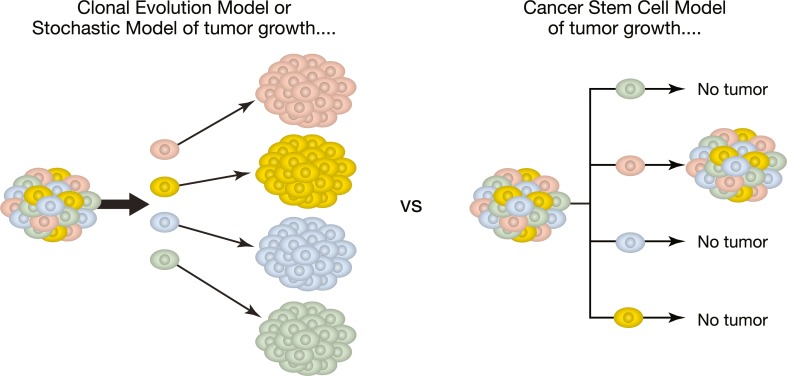
Two different, but not necessarily mutually exclusive, models have been established to explain the development of cancers (Fig. 1) [67]. One is the stochastic, also called the clonal evolution, model that postulates that every cell in a cancer has the same potential to produce a new tumor. In this model, the acquisition of mutations and epigenetic changes over time results in an increasing ability of clones of cells to become malignant. There can also be some clones that develop to drive the cellular heterogeneity of tumors. Moreover, through this mechanism, cancer cells within a tumor can become invasive, develop metastases at distant sites, overcome immune surveillance, and gain resistance to treatment leading to tumor recurrence [68].
Fig. 1.
Theories of tumor development: clonal versus cancer stem cell model. [67]. This figure has been reproduced unchanged under a Creative Commons License
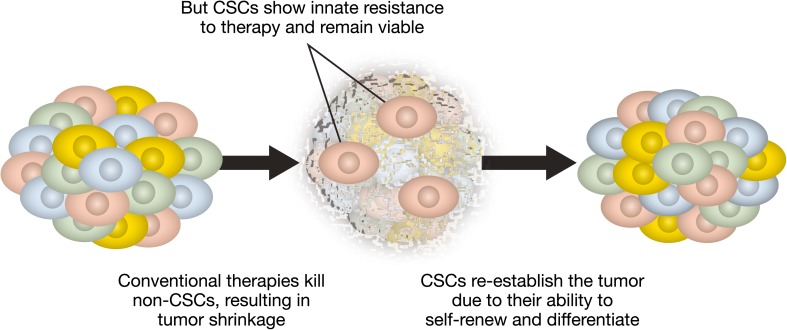
The other model is the cancer stem cell model (Figs. 1 and 2) that postulates that only a subpopulation of CSCs in a cancer are responsible for tumor development and for a tumor cell’s malignant behavior, including invasion and metastasis. That CSCs carry properties for self-renewal and multilineage differentiation gives them the unique ability to generate the bulk of other cells in a cancer [16, 67]. Based on this model, a cancer’s resistance to chemotherapy is ultimately due to the subset of CSCs that survive treatment and lead to recurrent disease even after apparent complete remission [67].
Fig. 2.
Using cancer stem cell model to explain treatment resistance [67] CSCs cancer stem cells. This figure has been reproduced unchanged under a Creative Commons License
These two models have different implications for therapeutic response. In the clonal evolution model, to achieve a durable response, it is necessary to therapeutically eliminate all clones of tumor cells that have the ability to invade and metastasize. Based on the CSC model, a durable therapeutic response requires killing all the CSCs, because they have the ability to propagate the tumor by producing non-CSCs that make up the tumor bulk (Fig. 2) [67].
Investigation into both these models has provided important information on processes that contribute to tumorigenesis. Tumor cells within a cancer are heterogeneous. It has been shown that cells in the same tumor can differ in their morphology, genetics, cell surface markers, proliferation kinetics, tumorigenicity, and response to therapy. On the one hand, this observation supports the clonal evolution model. On the other hand, it can be reasoned that it is CSC properties of self-renewal and multilineage differentiation that contribute to this heterogeneity [69, 70]. This reasoning supports the CSC model of tumor growth. The other evidence in support of the CSC model comes from tumor-initiation assays that show that only a small subset of cells in a cancer have tumorigenic ability in animal models. Studies that provided early evidence for this result involved transplantation of leukemic cells and breast cancer cells into immune-deficient mice [7, 14, 15, 71].
How “Stemness” Conveys High Tumorigenicity
The dysregulation of specific cellular signaling pathways can result in the formation of CSCs, lead to dysregulation of stem cell self-renewal, and result in increased stemness of tumor cells during neoplastic transformation [16, 34]. Most of these pathways are known to be essential for stemness properties of normal adult stem cells, including self-renewal, differentiation, and proliferation, as well as for development of various organs during embryogenesis. The most studied and characterized stemness pathways are NANOG, WNT/β-CATENIN, NOTCH, Janus kinase (JAK)/STAT, and phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase (AKT), all of which have been shown to contribute to the formation of CSCs, when dysregulated [33]. However, the role of any given pathway can differ among cancers. Moreover, the influence of the tumor microenvironment in the setting of dysregulation of these pathways may drive tumor growth in complex, poorly understood ways. Furthermore, stemness pathways, when overactivated, can confer innate resistance to chemo- and radiotherapies. Therefore, targeting these aberrant signaling pathways, which are important for the formation of CSCs, offers a new strategy for cancer therapy.
For example, the HEDGEHOG pathway is reported to contribute to chronic myeloid leukemia (CML) pathogenesis, and treatment of CML-bearing mice with the smoothened antagonist cyclopamine, which inhibits this pathway, leads to depletion of leukemic stem cells and to increased survival [72]. Other key pathways, including the NOTCH and NANOG signaling pathways, are activated in several human cancer types, including breast cancer and malignant gliomas [45, 62]. Treatment with antibodies directed against delta-like ligand (DLL) 4 or NOTCH1 has been reported to decrease breast CSC numbers and to improve response of patient-derived xenografts to taxanes [68, 73, 74]. Similar responses have been shown for gamma-secretase inhibitors that block the NOTCH signaling pathway [75]. Agents are also being developed to target the WNT signaling pathway, which, when dysregulated, drives the growth of a number of malignancies, including CML and colon cancer [76]. In mouse models of CML, therapy designed to target WNT signaling results in depletion of residual CSCs in bone marrow following imatinib therapy and, when combined with indomethacin, in prolonged overall survival [77, 78].
EMT, Stemness, and Metastasis
Epithelial-mesenchymal transition (EMT) is one mechanism that can lead to increased stemness during tumor development [79, 80]. This mechanism is important because the majority of human cancers arise from epithelial tissues. Epithelial cells possess apical-basolateral polarity [69, 81], are attached to a basement membrane, and are connected together via specialized junctions that allow them to organize into regular layers. In contrast, mesenchymal cells lack apical-basolateral polarity and do not form regular layers because their adhesions with adjacent cells are only formed focally [81]. EMT involves a series of steps occurring in epithelial cells that lead to their transformation into fibroblast-like, mesenchymal, motile cells [82]. This transformation causes reduced cell–cell adhesion, increased stemness, and generation of CSCs with the ability to migrate and disseminate [69, 81, 82]. The acquisition of EMT by cancer cells leads to local tumor invasion and the migration into vasculature eventually resulting in metastases [81, 82]. The activation or repression of various stemness pathways can modulate the EMT during the various stages of metastasis [82]. For example, stemness pathways such as WNT/B-CATENIN and HEDGEHOG are associated with an increased EMT phenotype including motility and invasiveness, resistance to apoptosis, and increased ability to self-renew and differentiate [81]. On the other hand, mesenchymal epithelial transition (MET) and increased cell–cell adhesion can enable colonization at distant organ sites and increase tumor-initiating ability [79]. Thus, both EMT and MET are related to stemness and CSC capacity for metastasis, and it will be challenging to finely tune EMT and MET for optimal outcomes. Agents designed to target stemness signaling pathways that affect EMT have been candidates for development of novel cancer therapies. Such agents include drugs that target cytokine signaling via C-X-C chemokine receptor 1 (CXCR1) and CXCR2 [83]. Interleukin 8, which targets CXCR1/2 signaling, has been reported to induce a state of stemness by affecting EMT [84]. Moreover, reparixin, an inhibitor of CXCR1/2 signaling, reduced the proportion and activity of CSCs in vitro and increased efficacy of docetaxel in vivo by decreasing mouse xenograft tumor growth [85].
CSCs May Differ As Well
As previously discussed, stemness is an elusive term. Stem cells are defined by the degree to which they possess stemness characteristics (especially self-renewal and multilineage differentiation). From this definition, one could argue that stem cells may possess varying levels of stemness and may also exhibit different properties. Multiple studies have shown heterogeneity in the CSC population. Similar to normal counterparts, CSCs display cellular plasticity, meaning they can alternate between epithelial and mesenchymal-like stem-cell states [86]. Mesenchymal-like CSCs are associated with metastasis, may bear a CD24−/CD44+ phenotype, are quiescent, and more present at the invasive tumor front [86]. Epithelial-like CSCs express ALDH, are more proliferative, and are located in the inner part of the tumor [86]. Alternation between these states has been postulated to be involved in the ability to invade into tissue stroma, disseminate throughout the patient, and grow at metastatic sites [86]. Other groups have shown differences in CSC makers depending on area investigated (within the tumor bulk or at the invading front) [87]. In breast cancer, a subset of CSCs expressing a certain variant of the CSC marker CD44 called CD44v, exhibited significantly higher metastatic potential to the lungs. In patient samples from the same study, expression of CD44v, but not the standard isoform of CD44, correlated with poor prognosis [88]. As in ovarian cancer, CSCs expressing both CXCR4 and CD133 exhibited the greatest tumor-initiating potential, resistance to cisplatin, and overexpression of the ABCG2 drug efflux pump compared with CD133−/CXCR4+ and CD133+/CXCR4− CSCs, as well as CD133−/CXCR4− cells [89]. Similar phenomenon was reported in other cancers as well [90]. The transformation of CSCs into mesenchymal invasive phenotypes has been shown to be mediated by stemness pathways. In renal cells cancer, overexpression of the Notch signaling pathway upregulates the expression of CXCR4 in CSCs [91]. Stemness is maintained in drug-resistant lung cancer cells through CXCR4-mediated STAT3 signaling pathway [92]. This suggests that multiple CSCs may exist in the same tumor.
Implications of Stemness for Cancer Therapy
Conventional Therapies Are Effective in Removing the Bulk of the Tumor, but May Increase the Proportion of CSCs
CSCs have been reported to be innately resistant to conventional therapies (i.e. chemotherapy, surgery, and radiation) in the treatment of many different tumor types, including brain [34, 93], head and neck [94], lung [95], breast [96, 97], liver [98], gastric [99, 100], pancreatic [101], ovarian [32], lymphoma [102], colorectal [103], prostate [104], and cervical [105].
These studies show that stemness can contribute to the innate resistance to conventional therapies by targeting the tumor bulk. Chemotherapy is designed to kill rapidly proliferating cells, which, in many but not all cancers, are typically non-CSCs. As some CSCs are relatively slowly cycling quiescent cells [31, 38], they are innately resistant to chemotherapy (which usually targets proliferating cells) [38]. Moreover, like normal stem cells, CSCs have a higher repair mechanism [38]. Thus, conventional chemotherapy can increase the fraction of CSCs within a cancer. Once the cancer develops resistance to treatment, the CSCs will often re-establish tumor growth, leading to an increased fraction of non-CSCs within the malignancy [38].
Conventional Therapies Can Increase the Subpopulation of CSCs by Inducing the Expression of Stemness Pathways
Multiple mechanisms have been identified that mediate this innate resistance in CSCs. It has also been shown that, within any given tumor, different mechanisms of resistance may be activated in different CSC populations. Therapeutic strategies designed to overcome CSC resistance to chemotherapy are discussed below. Radiation and chemotherapy can induce expression of stemness pathways in non-CSCs. Consequently, these therapies may activate cellular stress response programs and can enhance stemness characteristics in non-CSCs and therefore increase their ability to selectively survive. Thus, radiation and chemotherapy can lead to enrichment of a CSC subpopulation with higher innate resistance to these therapies [106–108].
Several stemness pathways are known to be dysregulated in cancers, and when they are activated in CSCs, they can contribute to their innate resistance. For example, STAT3 is a transcription factor that is constitutively activated in many malignancies, and it plays a pivotal role in tumor growth and metastasis [51, 52]. When constitutively activated in CSCs, STAT3 contributes to the innate resistance of CSCs to conventional cancer therapies. Gastric CSCs that have overactivation of STAT3 cells exhibit increased resistance to docetaxel [109]. Moreover, small molecule inhibitors that decrease STAT3 signaling tend to prevent cancer relapse and block metastasis [51, 110].
Conventional Therapies May Lead to Treatment Resistance by Temporarily Halting CSC Division, Rendering Drugs Designed to Target Proliferating Cells Ineffective
CSC quiescence is another mechanism for resistance. CSCs can be relatively quiescent and typically in a nondividing state, which contributes to innate resistance to chemotherapeutic agents, which are designed to kill proliferating cells. To overcome this resistance, some therapeutic approaches have been designed to induce CSCs to become actively cycling cells. For example, it has been shown that induction of CML and acute myeloid leukemia (AML) cells with granulocyte colony-stimulating factor enhances the efficacy of chemotherapy [111–113].
Conventional Therapies May Create a Tumor Microenvironment that Supports CSCs
Stimuli from the CSC microenvironment is still another mechanism for resistance. Because CSCs reside in a stem cell niche that contains other supportive cells, such as vascular, mesenchymal, and inflammatory cells, the microenvironment provides factors that sustain CSCs. This microenvironmental influence on CSCs includes signaling that contributes to innate or adaptive resistance to chemotherapy [107, 108]. For example, pharmacologically targeting the microenvironment of CML cells that secrete factors required for tumorigenesis sensitize tumors to systemic agents [114, 115]. With growing evidence supporting the role of the tumor microenvironment in supporting CSC-mediated tumor propagation, investigators have postulated that indirect targeting of CSCs by targeting stromal cells in the tumor microenvironment. One prominent target is carcinoma-associated fibroblasts [41].
CSCs Overexpress Prosurvival Proteins that May Prevent Therapy-Mediated Cell Death
Overexpression of antiapoptotic proteins has also been shown to be an important factor in promoting innate resistance in CSCs and in reducing the efficacy of chemotherapeutic agents [116]. It is well known that the BCL2 antiapoptotic family of proteins protects CSCs from apoptosis and enhances their ability to repopulate [117]. BCL2 overexpression in CSCs from several cancers, including prostate and breast cancers, has been found to play a role in chemotherapy resistance [118, 119]. For example, treating the blast crisis of CML with a pan-BCL2 inhibitor sensitized leukemic stem cells to tyrosine kinase inhibition [120].
CSCs Express High Levels of Transporters that Can Pump Drugs out of Cells
ABC transporters are proteins expressed in membranes cells that can pump substances out of cells (including drugs, lipids, and other substances) [121]. CSCs overexpress ABC drug transporters including ABCB1 and ABCG2 [41]. In ovarian cancer, CSCs overexpressing high levels of these drug transporters are more resistant to paclitaxel and ionophore antibiotics [41]. These CSCs were sensitive to drugs that blocked ABC transporters (including fumitremorgin C and verapamil) [41] Combination of ABC transporter inhibitors with chemotherapy is currently under preclinical investigation in other cancers including pancreatic cancer [122].
Other factors that can contribute to CSC resistance to therapeutic agents include microRNAs, activation of DNA damage repair systems, epigenetic mechanisms (e.g. DNA methylation and histone alterations), and maintenance of low levels of reactive oxygen species (ROS). All these factors represent potential targets to overcome the innate resistance of CSCs [31, 38, 121, 123].
New Approaches and Challenges to Target CSCs
Because the role of CSCs in driving cancer development and growth involves a complex multifactorial process involving intrinsic factors, such as the genetic and epigenetic makeup of tumors, and extrinsic factors, such as the tumor microenvironment and immune response, many different approaches developed to target CSC pathways (e.g. STAT3, NOTCH, WNT, and HEDGEHOG) are currently under clinical evaluation [31, 34, 38, 121, 124, 125].
The ultimate goal is to develop drugs that target CSCs effectively without affecting normal stem cells or non-stem cells. The FDA has already approved treatment of basal cell squamous carcinoma with two drugs designed to inhibit HEDGEHOG pathways by targeting SMO (LDE225/Sonidegib and GDC-0449/Vismodegib) [126]. Early phase 2 clinical data suggested that there is no clinical benefit in combining vismodegib with first-line standard of care for mCRC [127]. A simple explanation for this observation may be that the HEDGEHOG pathway is not the primary driver of CRC pathogenies [127]. A more complex explanation is the phenomenon of crosstalk between CSC pathways like HEDGEHOG, NOTCH, and WNT [128]. WNT pathway hyperactivation has been shown to be more prominent in CRC [76]. Recent preclinical data on approaches developed to target cancer-specific fusion receptors in the WNT pathway have shown promising results in CRC [129]. CD47 is overexpressed in AML CSCs compared to their normal counterparts [130]. CD47 also serves as a prognostic marker in AML and other cancers. Blocking CD47 in AML CSCs has resulted in outcome benefit in experimental models [130] and is being tested in clinical trials. Together this suggests a need for targeting identifying the specific prominent stemness pathways in difference cancers. Multiple trials targeting CSCs are under clinical investigation.
Targeting Epigenetic Mechanisms: The Next Step in Eradicating CSCs
Epigenetic programming, involving DNA methylation, chromatin remodeling, and microRNA expression, is known to be key to normal stem cell differentiation. A similar process is thought to cause tumor cells to regain stem cell-specific features [131]. Dysregulation of epigenetic mechanisms, such as DNA methylation, leads to abnormal epigenetic memory that can contribute to progression of CSCs. Consequently, agents that target epigenetic programming are being investigated as anti-CSC therapies [124, 125]. One class of agents, DNA methyltransferases (DNMTs) inhibitors, has already been integrated into therapy for hematologic malignancies [132, 133]. The DNMT inhibitors azacitidine and aza-deoxycytidine (aza-dC) have been integrated in treatment for myelodysplastic syndromes and AML. Although these epigenetic-type treatment approaches are proving successful in therapy for hematologic malignancies, the recent demonstration of resistance of leukemic stem cells to azacitidine, has prompted rethinking of how to develop new drugs and drug combinations that have increased effectiveness against CSCs [133]. One such new approach for AML involves a combination of the differentiation-inducing agent retinoic acid with an epigenetic-type agent (tranylcypromine) that inhibits histone H3 lysine 4 demethylase 1. Aza-dC–based treatment is also being explored in other ways, including low-dose regimens for myelodysplastic syndromes and examining activity in treatment of other tumor types (e.g. medulloblastoma) [133].
Another epigenetic mechanism that is being targeted because it is known to be important in the regulation of CSCs is histone acetylation. Histone deacetylases (HDACs) are chromatin-remodeling enzymes, and it has been shown that HDACs can modulate chemotherapeutic resistance in hematologic neoplasms. For example, treatment with a HDAC inhibitor suppressed CML stem cells following imatinib therapy [134]. In a subsequent study, it was demonstrated that the selective HDAC inhibitor sirtuin suppressed CML stem cell growth and engraftment in vivo [135]. MicroRNAs are also thought to be another epigenetic mechanism that regulates CSCs. For example, in prostate cancer, microRNA-34a has been shown to repress the stem cell protein CD44 and to inhibit CSCs and tumor metastasis [83]. Other microRNAs, Let-7 and miR200, have been shown to inhibit breast CSC self-renewal and differentiation [136–138]. Several other epigenetic mechanisms that are known to regulate CSCs, including inhibition of DNA methylation and chromatin remodeling, are also being investigated as potential therapeutic targets [31, 38]. Because epigenetic mechanisms are key regulators of CSCs, new epigenetic-type agents and drug combinations that target and kill CSCs without adversely affecting normal stem cells to avoid adverse toxicity in cancer patients hold great promise to advance the effectiveness of therapy in oncology.
Managing Adverse Events Associated with Targeted Agents
Boussios et al. [139] discussed the incidence, presentation, and management of cancer treatment-related toxicity after reviewing more than 350,000 publications related to cancer treatment. Drugs that block specific molecular pathways have been shown to highly effective in many types of cancer. These drugs target molecular pathways that are also present in normal cells, resulting in adverse events. GI-related toxicities, especially diarrhea, nausea, and vomiting are common when treating with targeted agents. Although the specific mechanisms of induction are still not well understood, the onset of these adverse events are well documented and can be controlled. For example, diarrhea is commonly treated with loperamide [139], which is recommended as first-line treatment of cancer treatment–induced diarrhea by the American Society of Clinical Oncology (ASCO) [140]. Nausea and vomiting is managed with antiemetics including serotonin (5-HT3) receptor agonists and dexamethasone. ASCO recommends a combination of a neurokinin 1 [NK1] receptor antagonist, a 5-hydroxytryptamine-3 [5-HT3] receptor antagonist and dexamethasone for cancer treatment-induced diarrhea [141]. Managing adverse events associated with targeted agents including anti-CSC pathway drugs will be integral in optimal disease management.
Concluding Remarks
Despite advances in cancer research, systemic chemotherapy is unable to cure many patients who have advanced cancers. In this overview, evidence is presented indicating that CSCs drive tumor development and growth. Unfortunately, most current cytotoxic or cytostatic therapies have limited ability to eliminate CSCs, and thus cancers still acquire resistance to treatment, recur, and metastasize. Theoretically, if the subpopulation of CSCs can be eliminated or stemness can be reduced, it would be an avenue to tumor control or even cancer cure [16].
Although much effort is being spent to decrease cancer stemness by targeting self-renewal signaling pathways, molecular CSC markers, and microenvironmental influences on stemness, there are many future challenges, because the biologic nature of CSCs is complex [31]. For example, CSCs exhibit plasticity in their phenotype and functional properties—a mechanism that can contribute to development of systemic therapies [124]. Moreover, CSCs and normal stem cells have many properties in common, and targeting CSCs may adversely affect normal stem cells leading to untoward toxicity [124]. Perhaps the immediate focus needs to be on discovering ways to distinguish and inhibit residual CSCs that are phenotypically different from normal stem cells in advanced cancer patients who have experienced partial or complete remission from systemic treatment to prevent disease progression or relapse. If therapeutic approaches can be designed to control CSC overpopulation in patients, perhaps cancer can be clinically controlled as a chronic disease, much like diabetes or heart disease.
Acknowledgements
Medical writing and editorial support were provided by CATX, Inc., and Alfred Adomako, PhD, of Adelphi Communications, New York, and were funded by Boston Biomedical, Cambridge, MA. Boston Biomedical was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy.
Compliance with Ethical Standards
Funding
Medical writing and editorial support were funded by Boston Biomedical, Cambridge, MA.
None of the named authors received any compensation for their contributions to this work.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Julius Cohnheim: (1839-1884) experimental pathologist. JAMA. 1968;206:1561–2. [PubMed]
- 2.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 3.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 4.Courtenay VD, Selby PJ, Smith IE, Mills J, Peckham MJ. Growth of human tumour cell colonies from biopsies using two soft-agar techniques. Br J Cancer. 1978;38:77–81. doi: 10.1038/bjc.1978.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackillop WJ, Ciampi A, Till JE, Buick RN. A stem cell model of human tumor growth: implications for tumor cell clonogenic assays. J Natl Cancer Inst. 1983;70:9–16. [PubMed] [Google Scholar]
- 6.Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Ailles LE, Gerhard B, Kawagoe H, Hogge DE. Growth characteristics of acute myelogenous leukemia progenitors that initiate malignant hematopoiesis in non-obese diabetic/severe combined immunodeficient mice. Blood. 1999;94:1761–1772. [PubMed] [Google Scholar]
- 9.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017. [DOI] [PubMed]
- 11.Machle AH. Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes Rec R Soc Lond. 2011;65:359–378. doi: 10.1098/rsnr.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kummermehr J, Trott K-R. Stem Cells. In: Potten CS, editor. Tumor stem cells. London: Academic Press; 1997. pp. 363–400. [Google Scholar]
- 13.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond Ser B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falzacappa MV, Ronchini C, Reavie LB, Pelicci PG. Regulation of self-renewal in normal and cancer stem cells. FEBS J. 2012;279:3559–3572. doi: 10.1111/j.1742-4658.2012.08727.x. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Stem cell basics. https://stemcells.nih.gov/sites/default/files/SCprimer2009.pdf. Accessed 15 March 2017.
- 20.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–666. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 24.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laplane L. Cancer stem cells: philosophy and therapies. Cambridge: Harvard University Press; 2016. [Google Scholar]
- 26.Boman BM, Fields JZ, Cavanaugh KL, Gujetter A, Runquist OA. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;6:3304–3313. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 27.Vathipadieka V, Saxena D, Mok SC, Hauschka PV, Ozbun L, Birrer MJ. Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boman BM, Wicha M, Fields JZ, Runquist O. Symmetric division of cancer stem cells: a key mechanism in tumor growth that should be targeted in future therapeutic approaches. J Clin Pharmacol Ther. 2007;81:893–898. doi: 10.1038/sj.clpt.6100202. [DOI] [PubMed] [Google Scholar]
- 29.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 30.Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, et al. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75(6):924–929. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal SJ, Rodriguez-Bravo V, Galsky M, Cordon-Cardo C, Domingo-Domenech J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2014;33:4451–4463. doi: 10.1038/onc.2013.411. [DOI] [PubMed] [Google Scholar]
- 32.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2011;18:869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarrar A, Chumakova A, Hitomi M, Lathia J. Enrichment and interrogation of cancer stem cells. In: Liu H, Lathia J, editors. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. 1st. London: Academic Press; 2016. pp. 59–100. [Google Scholar]
- 34.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;(Suppl 1):S3–17. [DOI] [PubMed]
- 35.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Dhfyan A, Alhoshani A, Korashy HM. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-catenin and Akt activation. Mol Cancer. 2017;16(1):14. doi: 10.1186/s12943-016-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Fisher RC, Signs S, Molina LA, Shenoy AK, Lopez MC, et al. Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells reduces tumor growth due to apoptosis. Oncotarget. 2016. [DOI] [PMC free article] [PubMed]
- 38.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology. 2014;28:1101–1107. [PubMed] [Google Scholar]
- 39.Wang X, Huang S, Chen JL. Understanding of leukemic stem cells and their clinical implications. Mol Cancer. 2017;16(1):2. doi: 10.1186/s12943-016-0574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64(11):937–946. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 41.Boesch M, Zeimet AG, Reimer D, Schmidt S, Gastl G, Parson W, et al. The side population of ovarian cancer cells defines a heterogeneous compartment exhibiting stem cell characteristics. Oncotarget. 2014;5(16):7027–7039. doi: 10.18632/oncotarget.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathews LA, Keller JM, Goodwin BL, Guha R, Shinn P, Mull R, et al. A 1536-well quantitative high-throughput screen to identify compounds targeting cancer stem cells. J Biomol Screen. 2012;17(9):1231–1242. doi: 10.1177/1087057112458152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112(6):1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saygin C, Samour M, Chumakova A, Jarrar A, Lathia JD, Reizes O. Reporter systems to study cancer stem cells. Methods Mol Biol. 2016;1516:319–333. doi: 10.1007/7651_2016_360. [DOI] [PubMed] [Google Scholar]
- 45.D'Angelo RC, Ouzounova M, Davis A, Choi D, Tchuenkam SM, Kim G, et al. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Mol Cancer Ther. 2015;14:779–787. doi: 10.1158/1535-7163.MCT-14-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoltz K, Sinyuk M, Hale JS, Wu Q, Otvos B, Walker K, et al. Development of a Sox2 reporter system modeling cellular heterogeneity in glioma. Neuro-Oncology. 2015;17:361–371. doi: 10.1093/neuonc/nou320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511(7508):246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 48.van Vlerken LE, Kiefer CM, Morehouse C, Li Y, Groves C, Wilson SD, et al. EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter. Stem Cells Transl Med. 2013;2:43–52. doi: 10.5966/sctm.2012-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres J, Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat Cell Biol. 2008;10:194–201. doi: 10.1038/ncb1680. [DOI] [PubMed] [Google Scholar]
- 50.Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep. 2015;5:17663. doi: 10.1038/srep17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei W, Tweardy DJ, Zhang M, Zhang X, Landua J, Petrovic I, et al. STAT3 signaling is activated preferentially in tumor-initiating cells in claudin-low models of human breast cancer. Stem Cells. 2014;32:2571–2582. doi: 10.1002/stem.1752. [DOI] [PubMed] [Google Scholar]
- 53.Hastreiter S, Schroeder T. Nanog dynamics in single embryonic stem cells. Cell Cycle. 2016;15(6):770–771. doi: 10.1080/15384101.2015.1137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 55.Jeter CR, Liu B, Lu Y, Chao HP, Zhang D, Liu X, et al. NANOG reprograms prostate cancer cells to castration resistance via dynamically repressing and engaging the AR/FOXA1 signaling axis. Cell Discov. 2016;2:16041. doi: 10.1038/celldisc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasmim M, Bruno S, Azzi S, Gallerne C, Michel JG, Chiabotto G, et al. Isolation and characterization of renal cancer stem cells from patient-derived xenografts. Oncotarget. 2016;7(13):15507–15524. doi: 10.18632/oncotarget.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo T, Kong J, Liu Y, Li Z, Xia J, Zhang Y, et al. Transcriptional activation of NANOG by YBX1 promotes lung cancer stem-like properties and metastasis. Biochem Biophys Res Commun. 2017;487(1):153–159. doi: 10.1016/j.bbrc.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Amsterdam A, Raanan C, Schreiber L, Freyhan O, Schechtman L, Givol D. Localization of the stem cell markers LGR5 and Nanog in the normal and the cancerous human ovary and their inter-relationship. Acta Histochem. 2013;115:330–338. doi: 10.1016/j.acthis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Hu C, Xu L, Liang S, Zhang Z, Zhang Y, Zhang F. Lentivirus-mediated shRNA targeting Nanog inhibits cell proliferation and attenuates cancer stem cell activities in breast cancer. J Drug Target. 2016;24(5):422–432. doi: 10.3109/1061186X.2015.1082567. [DOI] [PubMed] [Google Scholar]
- 60.Hart LS, Dolloff NG, Dicker DT, Koumenis C, Christensen JG, Grimberg A, et al. Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell Cycle. 2011;10:2331–2338. doi: 10.4161/cc.10.14.16418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habu N, Imanishi Y, Kameyama K, Shimoda M, Tokumaru Y, Sakamoto K, et al. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer. 2015;15:730. doi: 10.1186/s12885-015-1732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, et al. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–775. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 63.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, et al. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–626. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 64.Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, et al. Nanog promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiechert A, Saygin C, Thiagarajan PS, Rao VS, Hale JS, Gupta N, et al. Cisplatin induces stemness in ovarian cancer. Oncotarget. 2016;7:30511–30522. doi: 10.18632/oncotarget.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiagarajan PS, Hitomi M, Hale JS, Alvarado AG, Otvos B, Sinyuk M, et al. Development of a fluorescent reporter system to delineate cancer stem cells in triple-negative breast cancer. Stem Cells. 2015;33:2114–2125. doi: 10.1002/stem.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fanali C, Lucchetti D, Farina M, Corbi M, Cufino V, Cittadini A, et al. Cancer stem cells in colorectal cancer from pathogenesis to therapy: controversies and perspectives. World J Gastroenterol. 2014;20:923–942. doi: 10.3748/wjg.v20.i4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marjanovic ND, Weinberg RA, Chaffer CL. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013;59:168–179. doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 72.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–77. [DOI] [PubMed]
- 74.Qiu M, Peng Q, Jiang I, Carroll C, Han G, Rymer I, et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013;328:261–270. doi: 10.1016/j.canlet.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 75.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu Y, Chen Y, Douglas L, Li S. Beta-catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 78.Heidel FH, Bullinger L, Feng Z, Wang Z, Neff TA, Stein L, et al. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10:412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao D, Dai C, Peng S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 80.Fabregat I, Malfettone A, Soukupova J. New insights into the crossroads between EMT and stemness in the context of cancer. J Clin Med. 2016;5:1–12. doi: 10.3390/jcm5030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2013;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ram R, Brasch HD, Dunne JC, Davis PF, Tan ST, Itinteang T. The identification of three cancer stem cell subpopulations within moderately differentiated lip squamous cell carcinoma. Front Surg. 2017;4:12. doi: 10.3389/fsurg.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu J, Li G, Zhang P, Zhuang X, Hu G. A CD44v(+) subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017;8(3) doi: 10.1038/cddis.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cioffi M, D'Alterio C, Camerlingo R, Tirino V, Consales C, Riccio A, et al. Identification of a distinct population of CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci Rep. 2015;5:10357. doi: 10.1038/srep10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo KM, et al. CXCR4 is involved in CD133-induced EMT in non-small cell lung cancer. Int J Oncol. 2017;50(2):505–514.91. doi: 10.3892/ijo.2016.3812. [DOI] [PubMed] [Google Scholar]
- 91.Xiao W, Gao Z, Duan Y, Yuan W, Ke Y. Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):41. doi: 10.1186/s13046-017-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209–221. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- 93.Oliva CR, Zhang W, Langford C, Suto MJ, Griguer CE. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4–1 regulatory subunit. Oncotarget. 2017;8(23):37568–83. [DOI] [PMC free article] [PubMed]
- 94.Mitra D, Malkoski SP, Wang XJ. Cancer stem cells in head and neck cancer. Cancers. 2011;3:415–427. doi: 10.3390/cancers3010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan R, Sethi P, Jyoti A, McGarry R, Upreti M. Investigating the radioresistant properties of lung cancer stem cells in the context of the tumor microenvironment. Radiat Res. 2016;185:169–181. doi: 10.1667/RR14285.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 97.Korkaya H, Kim G-I, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warmann S, Hunger M, Teichmann B, Flemming P, Gratz KF, Fuchs J. The role of the MDR1 gene in the development of multidrug resistance in human hepatoblastoma: clinical course and in vivo model. Cancer. 2002:951795–801. [DOI] [PubMed]
- 99.Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol. 2014;20:5420–5426. doi: 10.3748/wjg.v20.i18.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishikawa S, Konno M, Hamabe A, Hasegawa S, Kano Y, Ohta K, et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int J Oncol. 2013;42:1437–1442. doi: 10.3892/ijo.2013.1837. [DOI] [PubMed] [Google Scholar]
- 101.Rao CV, Mohammed A. New insights into pancreatic cancer stem cells. World J Gastroenterol. 2015;7:547–555. doi: 10.4252/wjsc.v7.i3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee CG, Das B, Lin TL, Grimes C, Zhang X, Lavezzi T, et al. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol. 2012;158:79–90. doi: 10.1111/j.1365-2141.2012.09123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yun EJ, Zhou J, Lin CJ, Hernandez E, Fazli L, Gleave M, et al. Targeting cancer stem cells in castration-resistant prostate cancer. Clin Cancer Res. 2016;22(3):670–679. doi: 10.1158/1078-0432.CCR-15-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–7. [DOI] [PMC free article] [PubMed]
- 105.Xie Q, Liang J, Rao Q, Xie X, Li R, Liu Y, et al. Aldehyde dehydrogenase 1 expression predicts chemoresistance and poor clinical outcomes in patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy prior to radical hysterectomy. Ann Surg Oncol. 2016;23:163–170. doi: 10.1245/s10434-015-4555-7. [DOI] [PubMed] [Google Scholar]
- 106.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Borovski T, Beke P, van Tellingen O, Rodermond HM, Verhoeff JJ, Lascano V, et al. Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene. 2012;32:1539–1548. doi: 10.1038/onc.2012.172. [DOI] [PubMed] [Google Scholar]
- 108.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 109.Hajimoradi M, Mohammad Hassan Z, Ebrahimi M, Soleimani M, Bakhshi M, Firouzi J, et al. STAT3 is overactivated in gastric cancer stem-like cells. Cell J. 2016;17:617–628. doi: 10.22074/cellj.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang FX, Zhang WG, He AL, Cao XM, Chen YX, Zhao WH, et al. Effect of granulocyte colony-stimulating factor priming combined with low-dose cytarabine and homoharringtonine in higher risk myelodysplastic syndrome patients. Leuk Res. 2016;48:57–61. doi: 10.1016/j.leukres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Qu Q, Liu L, Zhang Y, Li X, Wu D. Increasing aclarubicin dosage of the conventional CAG (low-dose cytarabine and aclarubicin in combination with granulocyte colony-stimulating factor) regimen is more efficacious as a salvage therapy than CAG for relapsed/refractory acute myeloid leukemia. Leuk Res. 2015;39(12):1353–1359. doi: 10.1016/j.leukres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 113.Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Vekemans MC, et al. Dutch-Belgian Hemato-oncology cooperative Group (HOVON); German AML study Group (AMLSG); Swiss collaborative Group for Clinical Cancer Research (SAKK) favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood. 2012;119:5367–5373. doi: 10.1182/blood-2011-11-389841. [DOI] [PubMed] [Google Scholar]
- 114.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 115.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26:985–990. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeuner A, Francescangeli F, Contavalli P, Zapparelli G, Apuzzo T, Eramo A, et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-XL inhibition in non-small cell lung cancer. Cell Death Differ. 2014;21(12):1877–1888. doi: 10.1038/cdd.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia Y, Chen J, Zhu H, Jia ZH, Cui MH. Aberrantly elevated redox sensing factor Nrf2 promotes cancer stem cell survival via enhanced transcriptional regulation of ABCG2 and Bcl-2/Bmi-1 genes. Oncol Rep. 2015;34(5):2296–2304. doi: 10.3892/or.2015.4214. [DOI] [PubMed] [Google Scholar]
- 118.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lang JY, Hsu JL, Meric-Bernstam F, Chang CJ, Wang Q, Bao Y, et al. BikDD eliminates breast cancer initiating cells and synergizes with lapatinib for breast cancer treatment. Cancer Cell. 2011;20:341–356. doi: 10.1016/j.ccr.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goff DJ, Court Recart A, Sadarangani A, Chun HJ, Barrett CL, Krajewska M, et al. A pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12:316–328. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim JK, Jeon HY, Kim H. The molecular mechanisms underlying the therapeutic resistance of cancer stem cells. Arch Pharm Res. 2015;38:389–401. doi: 10.1007/s12272-014-0531-1. [DOI] [PubMed] [Google Scholar]
- 122.Zhao L, Zhao Y, Schwarz B, Mysliwietz J, Hartig R, Camaj P, et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int J Oncol. 2016;49(1):99–110. doi: 10.3892/ijo.2016.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B. 2013;3:65–75. [Google Scholar]
- 124.Talukdar S, Emdad L, Das SK, Sarkar D, Fisher PB. Evolving strategies for therapeutically targeting cancer stem cells. Adv Cancer Res. 2016;131:159–191. doi: 10.1016/bs.acr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50(3):117–125. [DOI] [PMC free article] [PubMed]
- 126.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8(2). [DOI] [PMC free article] [PubMed]
- 127.Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19(1):258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 128.Takebe N, Miele L, Harris PJ, et al. Targeting notch, hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Storm EE, Durinck S, de Sousa e Melo F, Tremayne J, Kljavin N, Tan C, et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- 130.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shukla S, Meeran SM. Epigenetics of cancer stem cells: pathways and therapeutics. Biochim Biophys Acta. 2014;1840(12):3494–3502. doi: 10.1016/j.bbagen.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 132.Saunthararajah Y, Sekeres M, Advani A, Mahfouz R, Durkin L, Radivoyevitch T, et al. Evaluation of noncytotoxic DNMT1-depleting therapy in patients with myelodysplastic syndromes. J Clin Invest. 2015;125(3):1043–1055. doi: 10.1172/JCI78789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wongtrakoongate P. Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries. World J Stem Cells. 2015;7(1):137–148. doi: 10.4252/wjsc.v7.i1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. Let-7 regulates self-renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 137.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K, et al. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–72. [DOI] [PMC free article] [PubMed]
- 139.Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25(2):106–118. [PMC free article] [PubMed] [Google Scholar]
- 140.Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22(14):2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 141.Hesketh PJ, Bohlke K, Lyman GH, Basch E, Chesney M, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol. 2016;34(4):381–386. doi: 10.1200/JCO.2015.64.3635. [DOI] [PubMed] [Google Scholar]