Abstract
The prevalence of asthma and allergic diseases is disproportionately distributed among different populations, with an increasing trend observed in Western countries. Here we investigated how the environment affected genotype-phenotype association in a genetically homogeneous, but geographically separated population. We evaluated 18 single nucleotide polymorphisms (SNPs) corresponding to 8 genes (ADAM33, ALOX5, LT-α, LTC4S, NOS1, ORMDL3, TBXA2R and TNF-α), the lung function and five respiratory/allergic conditions (ever asthma, bronchitis, rhinitis, dermatitis and atopy) in two populations of Inuit residing either in the westernized environment of Denmark or in the rural area of Greenland. Our results showed that lung function was associated with genetic variants in ORMDL3, with polymorphisms having a significant interaction with place of residence. LT-α SNP rs909253 and rs1041981 were significantly associated with bronchitis risk. LT-α SNP rs2844484 was related to dermatitis susceptibility and was significantly influenced by the place of residence. The observed gene-phenotype relationships were exclusively present in one population and absent in the other population. We conclude that the genotype-phenotype associations relating to bronchitis and allergy susceptibility are dependent on the environment and that environmental factors/lifestyles modify genetic predisposition and change the genetic effects on diseases.
Introduction
The global prevalence of asthma and other allergic conditions such as rhinitis, dermatitis and atopy, have been increasing for the past 30 to 40 years1–3. This rising trend is attributed to regions that are becoming more urbanised and ‘Westernised’4. The risk factors contributing to allergen hypersensitivity originate from an individual’s genetics, its accompanying lifestyles and the surrounding environment. To date, genetic association studies suffer from inconsistencies in identifying the susceptibility genes for asthma and allergy5. The genes/loci that are identified to be associated with the disease vary in different ethnic populations and in people from different geographic regions5. Thus, gene-environment interactions are likely to be critical determinants of asthma and allergy susceptibility.
The Inuit present a unique population to investigate the complex gene and environment interaction as they are homogenous and genetically distinct from other ethnicities6. This population descends from generations living in the harsh Artic environment. Their acculturation to the modern world, including migrating to Western countries, has brought a change in allergic condition prevalence in Inuit7. For the present study, we recruited two Inuit populations both born in Greenland but the first resided in their native country while the second migrated to the Western European country of Denmark. The comparison of the immigrants with Greenlandic Inuit is an ideal way to investigate how environmental factors affect genetic effects on disease susceptibility. The two Inuit populations were unselected for respiratory/allergic conditions, adjusted for several confounders particularly genetic admixture, and representative of the general Inuit population in each residing environment so that the findings resulting from this study can be generalised.
We selected 8 candidate genes which are related to asthma/allergic conditions or pulmonary function based on literature reports8–14. From these 8 genes, the genetic effects of 19 prospective single nucleotide polymorphisms (SNPs) on the lung function and five respiratory/allergic conditions (ever asthma, bronchitis, rhinitis, dermatitis and atopy) were compared in the two populations of Inuit. The interaction with place of residence was further investigated in the association studies. We hypothesised that the selected genetic variants were significantly associated with respiratory conditions or allergic prevalence in Inuit, and that the genetic effects were influenced by the residing environment or life styles. As differences of smoking condition and ancestral ethnicity were identified between the two populations in the current study, we also investigated the genotype-phenotype association profile, taking into account smoking effects and using the Inuit population with full ethnicity.
Results
Demographics
The demographic data for the two study populations are shown in Table 1. A lower proportion of Danish male Inuit (27.4%) compared to Greenlandic male Inuit (46.7%) was recruited in this study (P < 0.001). The proportion of smokers in the Greenlandic Inuit (88.1%) was higher than that in the Danish Inuit (78.8%, P < 0.001). In regards to the lung function, Greenlandic Inuit had lower values of FEV1 and FVC than Danish Inuit in both male and female populations (P < 0.05). After adjustment of gender, age, height, ethnicity and smoking status, this difference remained significant in the female population.
Table 1.
Demographic and phenotypic data for Inuit residing in Greenland and Denmark.
| Greenland (n = 615) | Denmark (n = 643) | P value | ||
|---|---|---|---|---|
| Gender (male) | 46.7 (287) | 27.4 (176) | <0.001 | |
| Age (yr) | 42.4 ± 14.78 | 43.4 ± 12.40 | 0.209 | |
| Height (cm) | 163.1 ± 9.48 | 163.1 ± 10.10 | 0.995 | |
| Ethnicity# | ||||
| Full Inuit | 91.5 (558) | 53.2 (337) | ||
| Non-full Inuit | 8.5 (52) | 46.8 (297) | <0.001 | |
| Smoker | 88.1 (542) | 78.8 (507) | <0.001 | |
| Lung Function | ||||
| FEV1 (L) | Male | 3.7 ± 0.95 | 3.9 ± 0.89 | 0.017 |
| Female | 2.7 ± 0.71 | 2.8 ± 0.64 | 0.016 | |
| FVC (L) | Male | 4.7 ± 1.13 | 4.9 ± 1.04 | 0.041 |
| Female | 3.3 ± 0.79 | 3.4 ± 0.72 | 0.014 | |
| Allergic/respiratory conditions | ||||
| Ever asthma | 8.5 (52) | 9.2 (59) | 0.646 | |
| Bronchitis | 25.6 (157) | 13.9 (89) | <0.001 | |
| Rhinitis | 42.7 (173) | 60.0 (386) | <0.001 | |
| Dermatitis | 57.7 (355) | 36.3 (233) | <0.001 | |
| Atopy | 24.0 (30) | 23.4 (121) | 0.896 | |
Data are presented as Mean ± SD or % (n); #Full Inuit means that all 4 grandparents are Inuit; Non-full Inuit means at least 1 non-Inuit grandparent.
Inuit residing in Denmark had a higher prevalence of rhinitis (60.0% vs 42.7%, P < 0.001), but a lower prevalence of bronchitis (13.9% vs 25.6%, P < 0.001) and dermatitis (36.3% vs 57.7%, P < 0.001) compared with Greenlandic Inuit. These differences remained significant after adjustment for gender, age, height, ethnicity and smoking status. There was no significant difference between the two Inuit groups of ever asthma and atopy prevalence. Additionally these diseases were related to one another, as demonstrated by the interrelationship diagram in Fig. 1. We studied the relevance of different phenotypes by pooling the two Inuit populations together and adjusting for gender, age, height, ethnicity and place of residence and observed a positive correlation between smoking status and bronchitis prevalence and a negative correlation between lung function parameters (FEV1 and FVC) and bronchitis prevalence (P < 0.01).
Figure 1.
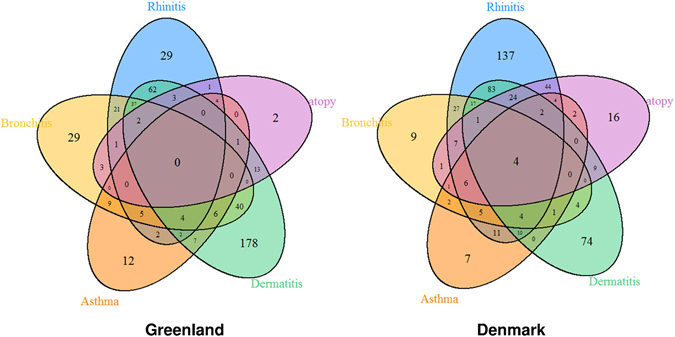
Interrelations of the respiratory/allergic diseases: Venn diagram shows the interrelations of the respiratory/allergic diseases in the two populations.
Genotype frequencies
We did not observe any deviation from the Hardy-Weinberg Equilibrium for all SNP genotype frequencies in both populations (P > 0.05; Supplementary Table S1). Apparent differences in genotype frequencies between the two Inuit populations (P < 0.05) were observed in ADAM33 rs528557, the three ALOX5 SNPs, LT-α rs2844484, TBXA2R rs4523, TNF-α rs1799964 and rs1800629. In the pure Inuit population (both grandparents and parents are Inuit), only significant differences were found for ALOX5 SNPs (rs892690 and rs2115819), TBXA2R rs4523 and TNF-α rs1799964.
Lung function parameters and SNPs
The lung function data for the 18 SNP genotypes are shown in the Supplementary Table S2. After adjusting for gender, age, height, ethnicity and smoking status, ORMDL3 SNPs (rs12603332 and rs4065275) were significantly associated with lung function (FEV1 and FVC) in Greenlandic Inuit whereas ADAM33 rs2787094 was found to associate with FEV1 in Danish Inuit (P = 0.045). When we further tested these associations using the sensitivity analysis, the association of polymorphisms with lung function remained significant for ORMDL3 SNPs (P < 0.05, Supplementary Table S8A), but not for ADAM33 rs2787094 (P = 0.473, Supplementary Table S8B). The homozygous CC genotype of rs12603332 and the GG genotype of rs4065275 in ORMDL3 were associated with higher values of FEV1 and FVC than the homozygous CT and GA genotypes in Greenlandic Inuit respectively (Fig. 2). Moreover, the interaction of the ORMDL3 SNPs (rs12603332 and rs4065275) with place of residence were statistically significant for the lung function (FEV1 and FVC) (P < 0.05, Supplementary Table S9). Apart from these, we did not observe significant associations of any other SNPs with lung function in either the whole population or the full Inuit population.
Figure 2.
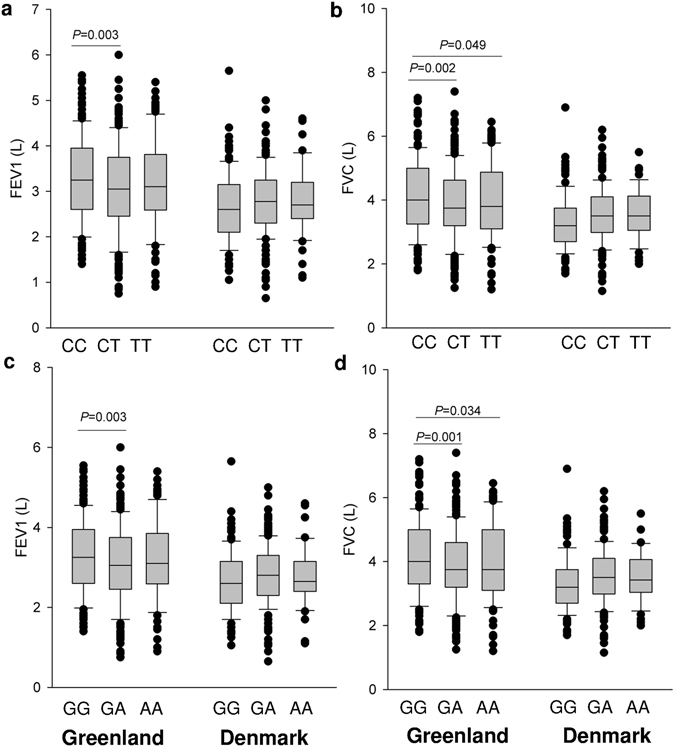
Associations of lung function and genotypes in the sensitivity analysis: Lung function (FEV1 and FVC) values in Greenlandic and Danish Inuit with full ethnicity were stratified by the genotypes of ORMDL3 SNPs rs12603332 (a and b) and rs4065275 (c and d). P values were adjusted for age, gender, height, and smoking. Box and whisker plots represent median with 10th and 90th centiles.
Ever asthma and SNPs
No significant difference in the prevalence of ever asthma was found for genotypes of the examined SNPs (Supplementary Table S3). However, in the sensitivity analysis, Greenlander with homozygous AA genotype of ALOX5 SNP rs892690 had a significantly increased risk of ever asthma compared to GG genotype (P < 0.05, Supplementary Table S8A).
Bronchitis and SNPs
Significant differences in the prevalence and risk of bronchitis were found in genotypes of LT-α and TNF-α, but not in ALOX5, LTC4S, TBXA2R, ADAM33, NOS1 and ORMDL3 in the Inuit populations residing both in Greenland and in Denmark (Supplementary Table S4). LT-α SNP rs2844484 was significantly associated with bronchitis, but only within the Danish population. On the other hand, two TNF-α SNPs (rs1800630 and rs1800629) were exclusively associated with bronchitis within the Greenlandic population. Using the sensitivity analysis, we further revealed the significant associations of LT-α rs909253 and rs1041981 with bronchitis in Danish Inuit, while the association of TNF-α rs1800630 with bronchitis did not remain significant (Supplementary Table S8).
The sensitivity analysis showed that in Danish Inuit, the prevalence of bronchitis was significantly lower among heterozygous CT for LT-α rs2844484 (8.8%) than among homozygous CC (19.4%) and TT (22.2%). Danish Inuit with genotype CC for LT-α rs909253 (Fig. 3a) and AA for and rs1041981 (Fig. 3b) had a significantly increased prevalence of bronchitis, compared to other genotypes. In Greenland, the Inuit with genotype GA of TNF-α rs1800629 had a significantly higher prevalence of bronchitis (38.9%) than that in GG (25.4%). However, interactions of the SNPs with place of residence were not statistically significant in the prevalence of bronchitis (Supplementary Table S9).
Figure 3.
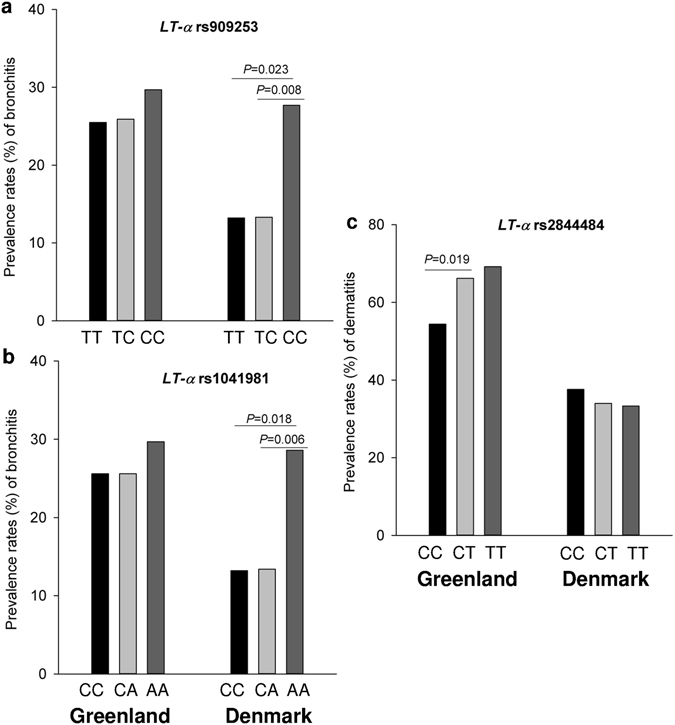
Prevalence of bronchitis/dermatitis and genotypes in the sensitivity analysis: Prevalence rates (%) of bronchitis in Greenlandic and Danish Inuit with full ethnicity were stratified by the genotypes of LT-α SNP rs909253 (a) and rs1041981 (b), while dermatitis prevalence was stratified by the genotypes of LT-α SNP rs2844484 (c). P values were adjusted for age, gender, height, and smoking.
Rhinitis and SNPs
No significant associations were found between rhinitis and any SNPs for Inuit residing in either Greenland or Denmark (Supplementary Table S5). Furthermore, the sensitivity analysis did not reveal any significant associations in the two populations (Supplementary Table S8).
Dermatitis and SNPs
As shown in Supplemental Table 6, within the Greenlandic population, Inuit with LT-α rs2844484 homozygous CC (54.5%) had a significantly lower prevalence of dermatitis compared with Inuit with CT genotype (P = 0.035). The same significant relationship (P = 0.025, Fig. 3c and Supplementary Table S8A) was detected within the Greenlandic group using the sensitivity analysis. The place of residence variable was significantly interacted with LT-α rs2844484 on dermatitis (P = 0.009, Supplementary Table S9). No significant differences in the prevalence and risk of dermatitis and interaction with place of residence were found in other SNPs examined within the two Inuit populations.
Atopy and SNPs
Significant differences in the prevalence and risk of atopy and association with atopy were only found in genotypes of ALOX5 and LTC4S (Supplementary Table S7). However, these differences did not persist after the sensitivity analysis (Supplementary Table S8). There were no significant interactions for place of residence association with the SNPs on atopy (Supplementary Table S9).
Correction for multiple tests
As shown in Table 2, the identified associations were further adjusted for multiple tests. The effects of ALOX5 rs892690 on ever asthma, LT-α rs2844484 and TNF-α rs1800629 on bronchitis were not considered significant with the adjusted thresholds, while the others remained significant after the correction.
Table 2.
Correction for multiple comparisons in the significant associations identified.
| Whole population (P value) | Full Inuit (P value) | M eff | Significance Threshold | |
|---|---|---|---|---|
| ORMDL3 rs12603332 & FEV1 in Greenland | 0.058 | 0.014 | 1.0018 | 0.0253 |
| ORMDL3 rs12603332 & FVC in Greenland | 0.027 | 0.009 | 1.0018 | 0.0253 |
| ORMDL3 rs4065275 & FEV1 in Greenland | 0.033 | 0.011 | 1.0018 | 0.0253 |
| ORMDL3 rs4065275 & FVC in Greenland | 0.023 | 0.005 | 1.0018 | 0.0253 |
| ALOX5 rs892690 & Ever asthma in Greenland | 0.025 | 0.029 | 2.686 | 0.0170 |
| LT-α rs2844484 & Bronchitis in Denmark | 0.002 | 0.044 | 1.8635 | 0.0253 |
| LT-α rs909253 & Bronchitis in Denmark | 0.090 | 0.019 | 1.8635 | 0.0253 |
| LT-α rs1041981 & Bronchitis in Denmark | 0.070 | 0.014 | 1.8635 | 0.0253 |
| TNF-α rs1800629 & Bronchitis in Greenland | 0.035 | 0.023 | 2.7127 | 0.0170 |
| LT-α rs2844484 & Dermatitis in Greenland | 0.035 | 0.025 | 1.8635 | 0.0253 |
M eff : the effective number of SNPs tested within each gene.
Genotype and gene expression associations
Associations between genotype of the selected SNPs (ORMDL3 rs12603332 and rs4065275, LT-α rs909253, rs1041981 and rs2844484) and blood-specific gene expression levels were analysed using GTEx portal. We revealed that ORMDL3 gene expression was up-regulated in blood cells of people with allele T of rs12603332 (P = 1.1e-25) or allele A of rs4065275 (P = 1.6e-25). The polymorphisms of LT-α rs909253 and rs1041981 were not significantly related to mRNA expression of LT-α (P > 0.05). Compared to CC, genotype CT of rs2844484 was related to a higher LT-α gene expression (P = 0.0065).
Discussion
In this study, we compared the genetic effects of 18 single nucleotide polymorphisms in 8 asthma and allergic candidate genes on the lung function and respiratory/allergic conditions in two populations of Inuit: one born and residing in Greenland and the other first generation Inuit immigrants of Denmark. We discovered several significant associations with respiratory/allergic conditions in the eight candidate genes after adjusting for the confounding factors (e.g. gender, age, height, ethnicity and smoking), using the sensitivity analysis and correcting for multiple testing. These include 1) Lung function parameters are associated with genetic variants in ORMDL3, with polymorphisms having a significant interaction with place of residence; 2) Frequency of bronchitis is associated with variants in the LT-α gene; 3) Dermatitis risk is related to the genotype distribution of LT-α SNP rs2844484, with a significant interaction with place of residence. The gene-phenotype relationship was exclusively observed in one population and not the other, indicating that environment plays a vital role of regulating the genetic effects.
Comparison of the pulmonary function in the two populations indicated that Denmark Inuit had higher lung function FEV1 and FVC values than Greenlandic Inuit. However, after correcting for the confounding variables (e.g. gender, age, height, ethnicity and smoking status) in the analysis, we found that only female Inuit in Denmark had a better lung function performance than the corresponding population in Greenland. In analysing the respiratory conditions and allergic phenotypes, we did not observe a significant difference of prevalence in asthma, but a higher prevalence of rhinitis and a lower prevalence of bronchitis (13.9%) and dermatitis (36.3%) in Danish Inuit compared with Greenlandic Inuit. The high prevalence of bronchitis and dermatitis in Greenlandic Inuit observed in this study is consistent with the reported frequency of these diseases in Greenlander7. Indigenous Inuit were previously reported to have reduced respiratory health due to high tobacco consumption7. In the present study, we also noted that the proportion of smokers was higher in Greenlandic Inuit compared to Danish Inuit. A positive association between bronchitis and smoking status and a negative association between bronchitis frequency and lung function were revealed when investigating two populations as a whole. Thus, we conclude that smoking tobacco is a risk factor for bronchitis, and the development of bronchitis is likely to contribute to the compromised lung function of Inuit.
Out of the 18 SNPs that we examined, ORMDL3 variants (rs12603332 and rs4065275) were significantly associated with lung function in Greenlandic Inuit after controlling the confounding variables and showed an interaction with place of residence. ORMDL3 is involved in endoplasmic reticulum–mediated Ca2+ homeostasis and unfolded protein response which potentially activate inflammatory processes and T-lymphocyte induction15. We found that the T allele of rs12603332 and the A allele of rs4065275 had a reduced lung function compared to the C or G allele, respectively. A change from C to T allele in rs12603332 was reported to result in an increase in ORMDL3 transcription16. Indeed, according to the GTEx database17, ORMDL3 rs12603332 and rs4065275 are expression quantitative trait loci (eQTL). Allele T of rs12603332 or allele A of rs4065275 was correlated to overexpression of ORMDL3 gene. Thus, increased ORMDL3 may provoke an inflammatory response in lung tissue and affect pulmonary performance. It should be noted that ORMDL3 is well known as an asthma susceptibility gene as confirmed in genetic association studies in several ethnic populations16 and also associated with smoking exposure18. Further testing on how smoking and other environmental factors regulate the effect of ORMDL3 SNPs on lung function is of interest, particularly in light of the uncertainty surrounding ORMDL3’s direct relation to asthma pathogenesis.
We also found that Danish Inuit with the minor allele homozygous genotypes CC and AA for rs909253 and rs1041981 in LT-α, respectively, had 2-fold increased risk of developing bronchitis. LT-α rs909253 is located in the first intron of the gene while LT-α rs1041981 is located in the chromosomal region that transcribes for codon 60 and translates into an amino acid change, from threonine to asparagine. The C allele of rs909253 was reportedly associated with increased synthesis of LT-α19. However, GTEx analysis did not support that the polymorphisms of both variants were significantly related to functional expression of LT-α. Interestingly, these SNPs were confirmed to be in high linkage disequilibrium by a genome wide association study on lung function20 and the current study (Supplementary Fig. 1). Individuals with a haplotype consisting of CC genotype of rs909253 and AA genotype of rs1041981 produced substantially higher amounts of LT-α in their peripheral blood mononuclear cells21. Thus, the individual SNPs may have minimal functional impact and it is likely that LT-α rs909253 and rs1041981 affect the functional expression in linkage disequilibrium forming a functional haplotype. Functionally, LT-α is a major player of the lymphotoxin signalling pathway in regulating the development and maintenance of secondary lymphoid organs and controlling organ design22. Previously it was reported that rs909253 was associated with pulmonary function in a cohort of Caucasian smokers23. Since the inflammation response to external environmental factors involves this cytokine, the bronchitis associations found in Inuit further support that LT-α play a biological or pathological role in the development of bronchitis in the Inuit population. In addition, the two genetic variants in LT-α were only associated with bronchitis in Danish Inuit, but not in Greenlandic Inuit. These findings indicate that the environmental factors interact with different genetic variants and alter the genetic effects on bronchitis. However, living place was not significantly interacted with the genotype–phenotype associations. Other important factors were proposed in the development of respiratory illness in Greenlandic Inuit, including indoor chimney heating, dry frost at low temperatures, passive smoke exposure during infancy, respiratory infections during childhood etc7. Studies are needed to explore whether these environmental factors are responsible for mediating genetic predisposition of LT-α to bronchitis.
A genetic variant (rs2844484) in the LT-α gene was also revealed to associate with dermatitis. LT-α rs2844484 is positioned upstream from the start of LT-α and this SNP is part of a region identified as a hypersensitive site shown to contain upstream stimulatory factors24. This family of transcription factors has the capability to vary LT-α expression when under stress and immune-related conditions24. It is also suggested that LT-α plays a crucial role in the inflammation mechanism associated with immune responses to infectious agents, by stimulating chemokines and adhesion molecules25. When infectious agents including foreign antigens and pathogens invade the skin, ectopic lymphoid tissue develops under the regulation of LT-α22. We found that Greenlandic Inuit with minor allele (CT) in the LT-α SNP rs2844484 had an increased risk of dermatitis, and the place of residence is significantly interacted with the association of LT-α SNP rs2844484 with dermatitis prevalence. In addition, the GTEx data also supported that individual with genotype CT of rs2844484 had a higher LT-α gene expression, relative to CC. Considering the regulatory role of LT-α cytokine in the immune defensive system, our data suggest that the LT-α gene variant affects the dermatitis prevalence, and this association is regulated by environmental factors.
One limitation of our study is that the sample size is small after stratifying by genotype, particularly for SNP genotypes associated with low disease prevalence. Nevertheless, we identified several significant genetic effects as well as interactions with place of residence after testing in the sensitivity analysis and correcting for multiple tests. The relatively small sample size for the population study also reduces the statistical power and has potentially type 2 errors (false negative). A larger sample size population with a similar genetic background is needed to validate or replicate our results. Consequently, the results should be interpreted with caution and we encourage further studies to confirm these findings.
Taken together, our data show that polymorphisms of ORMDL3 are related to lung function and affected by place of residence. Moreover, the candidate gene LT-α variants are significantly associated with either respiratory inflammation or allergic conditions. The effects of heterozygote on respiratory function and allergic traits are different in these associations. We argue that the protective effect of heterozygote is partly attributed to the natural consequence of adaptation, in which the fitness of the heterozygote is superior to either homozygote26. The associations of the heterozygous genotypes with lung function and disease prevalence may also result from potential linkage of the SNP with other functional genetic variants or by a chance. The results should be interpreted with caution. Interestingly, these associations are environment dependent, indicating that environmental factors or life styles could modify genetic predisposition, and change the genetic effects on diseases.
Materials and Methods
Study populations
Two populations of Inuit were studied, the Inuit population residing in Qasigiannguit (North/West Greenland) and the first generation Inuit immigrants residing in Denmark. The mean length of residence in Denmark of the migrants was 23 years and they had lived 52% of their life in Denmark. The two Inuit populations were originally recruited as part of a larger study conducted by The Greenlandic Study Population Group27. All Inuit from both populations were born in Greenland. Inuit ethnicity was self-reported and ethnicity was documented by number of Inuit grandparents for each subject27.
Qasigiannguit is a small town, near the Arctic Circle, which is only accessible by boat during the short summer season. A high proportion of this population works outside as fishermen or hunters (60.4%) and consumes mainly traditional Greenlandic food (84.9%), such as marine mammals7.
The second Inuit population examined in this study are first generation immigrants residing in Copenhagen, the capital of Denmark. Copenhagen residents are considered to live in a predominantly ‘Western’ European culture and lifestyle. A low proportion of first generation immigrant Inuit in Denmark works as fishermen or hunters (0.4%), and rarely consumes traditional Greenlandic food (4.5%)7.
DNA was available of 625 Inuit from Qasigiannguit and 680 Inuit from Denmark of which 98.4% (n = 615) and 94.6% (n = 643) provided phenotypic data, respectively. The genotype and phenotype study was approved by the ethics committees of Bispebjerg University hospital, Denmark, and School of Paediatrics and Child Health, the University of Western Australia. All subjects gave informed consent prior to participation. All experiments were performed in accordance with relevant guidelines and regulations of Bispebjerg University hospital and School of Paediatrics and Child Health, the University of Western Australia.
Study design
This study investigated the environmental interaction between the individual genetic effects of the eight candidate genes (ADAM33, ALOX5, LT-α, LTC4S, NOS1, ORMDL3, TBXA2R, and TNF-α) on lung function and allergic conditions in Greenlandic and Danish Inuit. The cohorts are an unselected sample of adult participants, representative of their respective Inuit populations. The original data collection occurred during the years 1997–200127. This comprised of an interview, a questionnaire, clinical examinations, lung function studies and blood sample collection, and was performed by a team of seven investigators, consisting of trained staff from Denmark and staff from the local hospital with constant supervision by a senior investigator from the Greenlandic Population Study Group27.
Phenotypic characterization
Respiratory and allergic conditions (ever asthma, bronchitis, rhinitis, dermatitis and atopy) were initially defined by the questionnaire27. Atopy was further determined using skin prick tests (SPTs) according to the European Academy of Allergy and Clinical Immunology (EAACI) guidelines. The allergens used for SPTs are commonly present in both Greenlandic and Danish environments, including birch, timothy grass, mugwort, animal dander (horse, dog, and cat), house dust mites (Dermatophagoides pteronyssinus and D. farina) and moulds (Alternaria alternate and Cladosporium herbarum). Atopy was recorded if a wheal size >3 mm to one of the allergens27.
Lung function parameter values were calculated from the completed capacities using a 7L wedge spirometer (Vitalograph Spiropharma, Copenhagen, Denmark), including reduced expiratory volume in one second (FEV1) and forced vital capacity (FVC) values28.
Selection of single nucleotide polymorphism (SNP)
The SNP selection criteria are summarized in the flowchart of Fig. 4. Specifically, the search started with the Online Mendelian Inheritance in Man website (https://omim.org) to obtain historical molecular genetics information and landmark papers about each candidate gene. Subsequently the Web of Knowledge database was employed to retrieve possible candidate gene SNPs from literatures published from 2004 to 2012. The Genetic Association Database (https://geneticassociationdb.nih.gov) was aligned to obtain other possible references using the “broad phenotype (disease)” category. NCBI dbSNP databases (http://www.ncbi.nlm.nih.gov/SNP/) and fastsnp (https://fastsnp.ibms.sinica.edu.tw/) were used as additional resources for analysing SNP details. The SNP information was collated into a database, e.g. the rs number, minor allele frequency (MAF), location within gene, associations with asthma and allergic diseases, ethnicity. Finally, the recorded SNPs from each candidate gene were ranked by P value, irrespective of positive or negative associations. SNPs were selected based on significant association and with MAF > 0.15. We selected 19 SNPs for the study: ADAM33 (rs612709, rs528557, rs44707 and rs2787094); ALOX5 (rs4986832, rs892690 and rs2115819); LT-α (rs2844484, rs909253 and rs1041981); LTC4S (rs3776944 and rs730012); NOS1 (rs7977109); ORMDL3 (rs12603332 and rs4065275/4378650); TBXA2R (rs4523); TNF-α (rs1799964, rs1800630 and rs1800629);
Figure 4.
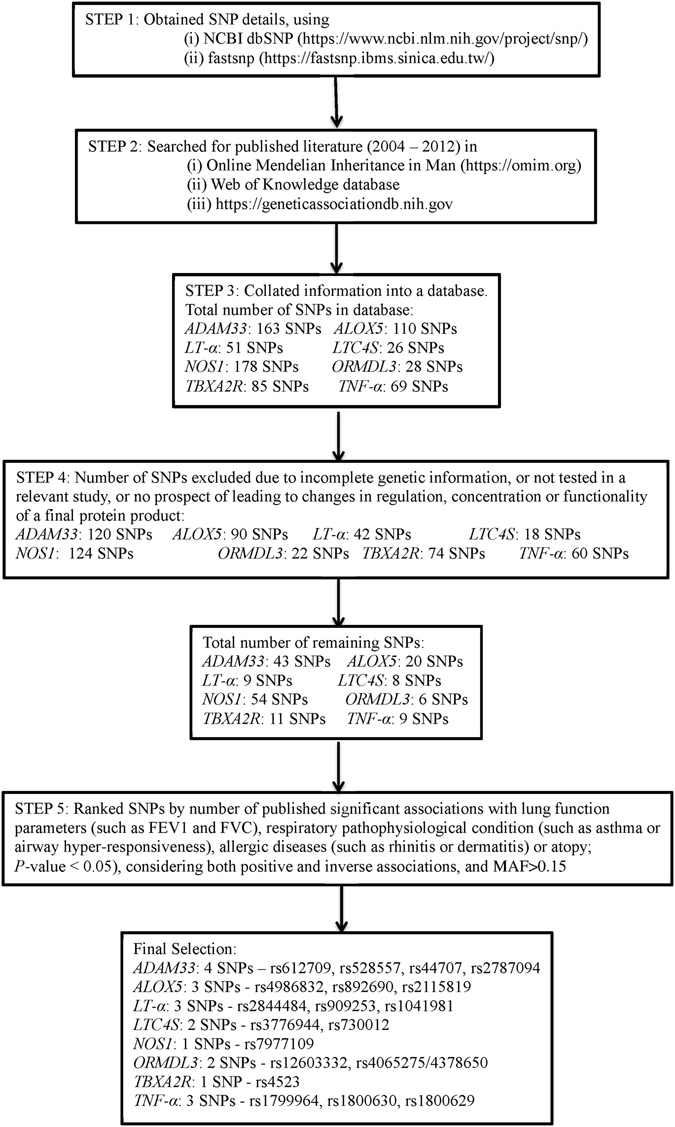
Selection of single nucleotide polymorphisms (SNPs): Flow chart summarises the procedures for the selection of SNPs in the study. MAF: minor allele frequency.
Genotyping
The genomic DNA was prepared from peripheral blood using standard methods27. The DNA was then genotyped for the selected SNPs using the Sequenom MassARRAY® system, with the iPLEX Gold primer extension process and MALDI-TOF mass spectrometry analysis. All SNPs were successfully genotyped except LTC4S SNP rs3776944 that was thereafter not included in the analysis.
Genotype-tissue expression (GTEx) analysis
The five SNPs (ORMDL3 rs12603332 and rs4065275, LT-α rs909253, rs1041981 and rs2844484) were finally selected for further analysis. Utilizing the GTEx database (http://gtexportal.org/home, version V6)17, we investigated the association between genotypes and gene expression level in whole blood.
Statistical analysis
Assessments of Hardy Weinberg Equilibriums (HWE) were obtained via the Innate Immunity Programs for Genomic Applications collaboration website (https://regepi.bwh.harvard.edu/IIPGA2/Bioinformatics/). The genotype frequencies were compared using Pearson χ2 test, further stratified by ethnicity. All genotype-phenotype association analyses were performed using a co-dominant model in which genotypes of SNPs were treated as categorical variables. Specifically, the associations were investigated using linear regression between SNPs and lung function and using logistic regression analyses between SNPs and disease prevalence. The regression analyses were adjusted for potential confounders e.g. gender, age, height, ethnicity, and smoking. The outcome variable was either the value of FEV1 and FVC or the frequency of respiratory/allergic phenotypes (Yes/No), while the independent variables were SNP genotype and the potential confounders. A sensitivity analysis was further performed by analysing the genotype-phenotype associations in Inuit with full ethnicity (all four grandparents are Inuit).
The interactive effect of place of residence on genotype-phenotype associations was investigated by using the likelihood-ratio test after estimation in the regression models by pooling the two populations together. Both the unrestricted (with interaction) and the restricted (without interaction) models were fitted using the maximum-likelihood method, and subsequently these were compared to identify significant interactions. Differences with P < 0.05 were considered statistically significant.
All analyses mentioned above were conducted using the statistical package SPSS (version 20.0: SPSS Inc., Chicago, IL, USA) except for the likelihood-ratio tests for which STATA (StataCorp LP, College Station, TX, USA) was used.
In addition, a standard Bonferroni correction for multiple testing is considered too conservative without taking into account linkage disequilibrium between SNPs in the same gene. This can result in a significant overcorrection and subsequent loss of power. Inconsistencies that arise from population differences may be resolved by using a gene based approach rather than either a SNP-based or a haplotype-based approach29, 30. As the strong linkage disequilibrium was identified in the present study (Supplementary Fig. 1), we employed a gene-based approach and applied multiple comparison correction with respect to the number of SNPs in that gene. We used the method proposed by Li and Ji31 to calculate the number of independent components underlying the linkage disequilibrium structure of the set of SNPs, as implemented in Single Nucleotide Polymorphism Spectral Decomposition (SNPSpD)32.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This study is supported by fellowships from the Thoracic Society of Australia and New Zealand and Curtin University (awarded to GZ). We also thank Asthma Allergic Fonden, Nationalforeningen til bekaempelse af Lungesygdomme and Helsefonden, who donated their financial to support this study.
Author Contributions
Y.S., M.J.S., J.G., V.B., P.L., and G.Z. conceived the study and performed statistical analysis. Y.S., and G.Z. prepared the manuscripts. V.B., and C.P. involved the recruitment and sample collection. M.J.S., S.K.K., and G.Z. performed the genotyping. I.A.L., and E.K.M. provided statistical input and interpretation of the results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yong Song and Michelle J Schwager contributed equally to the work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06791-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Marco R, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 2.von Mutius, E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 28 Suppl 5, 45–49; discussion 50–41 (1998). [DOI] [PubMed]
- 3.Strachan D, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatr Allergy Immunol. 1997;8:161–176. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G, Goldblatt J, LeSouef P. The era of genome-wide association studies: opportunities and challenges for asthma genetics. J Hum Genet. 2009;54:624–628. doi: 10.1038/jhg.2009.97. [DOI] [PubMed] [Google Scholar]
- 6.Helgason A, et al. mtDNA variation in Inuit populations of Greenland and Canada: migration history and population structure. Am J Phys Anthropol. 2006;130:123–134. doi: 10.1002/ajpa.20313. [DOI] [PubMed] [Google Scholar]
- 7.Backer V, et al. Respiratory symptoms in greenlanders living in Greenland and Denmark: a population-based study. Ann Allergy Asthma Immunol. 2004;93:76–82. doi: 10.1016/S1081-1206(10)61450-0. [DOI] [PubMed] [Google Scholar]
- 8.Holloway JW, et al. The discovery and role of ADAM33, a new candidate gene for asthma. Expert Rev Mol Med. 2004;6:1–12. doi: 10.1017/S1462399404007963. [DOI] [PubMed] [Google Scholar]
- 9.Undarmaa S, et al. Replication of genetic association studies in asthma and related phenotypes. J Hum Genet. 2010;55:342–349. doi: 10.1038/jhg.2010.32. [DOI] [PubMed] [Google Scholar]
- 10.Kalayci O, et al. ALOX5 promoter genotype, asthma severity and LTC production by eosinophils. Allergy. 2006;61:97–103. doi: 10.1111/j.1398-9995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 11.Grasemann H, Yandava CN, Drazen JM. Neuronal NO synthase (NOS1) is a major candidate gene for asthma. Clin Exp Allergy. 1999;29(Suppl 4):39–41. [PubMed] [Google Scholar]
- 12.Casale TB, Costa JJ, Galli SJ. TNF alpha is important in human lung allergic reactions. Am J Respir Cell Mol Biol. 1996;15:35–44. doi: 10.1165/ajrcmb.15.1.8679220. [DOI] [PubMed] [Google Scholar]
- 13.Huang SC, et al. Association of a lymphotoxin-alpha gene polymorphism and atopic asthma in Taiwanese children. Pediatr Neonatol. 2008;49:30–34. doi: 10.1016/S1875-9572(08)60008-X. [DOI] [PubMed] [Google Scholar]
- 14.Wang TN, et al. Gene-gene synergistic effect on atopic asthma: tumour necrosis factor-alpha-308 and lymphotoxin-alpha-NcoI in Taiwan’s children. Clin Exp Allergy. 2004;34:184–188. doi: 10.1111/j.1365-2222.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu KJ, Turvey SE. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin Immunol. 2013;9:4. doi: 10.1186/1710-1492-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galanter J, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med. 2008;177:1194–1200. doi: 10.1164/rccm.200711-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouzigon E, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–1994. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- 19.Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet. 2003;33:469–475. doi: 10.1038/ng1124. [DOI] [PubMed] [Google Scholar]
- 20.Soler Artigas M, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–1090. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messer G, et al. Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med. 1991;173:209–219. doi: 10.1084/jem.173.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruse CE, et al. Tumour necrosis factor gene complex polymorphisms in chronic obstructive pulmonary disease. Respir Med. 2007;101:340–344. doi: 10.1016/j.rmed.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Taylor JM, Wicks K, Vandiedonck C, Knight JC. Chromatin profiling across the human tumour necrosis factor gene locus reveals a complex, cell type-specific landscape with novel regulatory elements. Nucleic Acids Res. 2008;36:4845–4862. doi: 10.1093/nar/gkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjelmstrom P, et al. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedrick PW. What is the evidence for heterozygote advantage selection? Trends Ecol Evol. 2012;27:698–704. doi: 10.1016/j.tree.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Bjerregaard P, et al. Inuit health in Greenland: a population survey of life style and disease in Greenland and among Inuit living in Denmark. Int J Circumpolar Health. 2003;62(Suppl 1):3–79. doi: 10.3402/ijch.v62i0.18212. [DOI] [PubMed] [Google Scholar]
- 28.Backer V, Nepper-Christensen S, Ulrik CS, von Linstow ML, Porsbjerg C. Factors associated with asthma in young Danish adults. Ann Allergy Asthma Immunol. 2002;89:148–154. doi: 10.1016/S1081-1206(10)61930-8. [DOI] [PubMed] [Google Scholar]
- 29.Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 32.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


