Abstract
The present study aimed to investigate the mechanisms underlying the antidepressant-like effects of puerarin via the chronic unpredictable stress (CUS) procedure in rats. Similar to Sertraline (Ser), Chronic treatment of puerarin (60 and 120 mg/kg, i.g) elicited the antidepressant-like effects by reversing the decreased sucrose preference in sucrose preference test (SPT), by blocking the increased latency to feed in novelty-suppressed feeding test (NSFT) and the increased immobility time in forced swimming test (FST) without affecting locomotor activity. However, acute puerarin treatment did not ameliorate the antidepressant- and anxiolytic- like effects in FST and NSFT, respectively. In addition, enzyme linked immunosorbent assay (ELISA) and high performance liquid chromatography-electrochemical detection (HPLC-ECD) showed that chronic treatment of puerarin (60 and 120 mg/kg, i.g) reversed the decreased levels of progesterone, allopregnanolone, serotonin (5-HT) and 5-Hydroxyindoleacetic acid (5-HIAA) in prefrontal cortex and hippocampus of post-CUS rats. Furthermore, puerarin (60 and 120 mg/kg, i.g) blocked the increased corticotropin releasing hormone (CRH), corticosterone (Cort) and adrenocorticotropic hormone (ACTH). Collectively, repeated administration of puerarin alleviated the behavioral deficits induced by chronic stress which was associated with the biosynthesis of neurosteroids, normalization of serotonergic system and preventing HPA axis dysfunction.
Introduction
Major depressive disorder (MDD) is a debilitating and chronic disorder with high probability of medical and psychiatric co-morbidity, functional impairment, as well as significant societal and personal costs1, 2. Although several novel drug targets have been identified, there are no major breakthroughs in the treatment of the disorder3. In view of the pathways, a number of drugs are considered beneficial to combat depressive-like behavior. For instance, selective serotonin reuptake inhibitors (SSRIs), such as sertraline (Ser) and paroxetine, are the usual treatment options4. Nonetheless, long-term use of these drugs induces multiple inevitable side effects, including cognitive dysfunction, sexual dysfunction, dependence, weight gain, sedation and withdrawal5, 6. Based on these drawbacks, considerable effort has been invested in search for better drugs for more effective treatments of depressive-like behavior.
There has been considerable popular interest in using natural extracts and plant preparations to treat depressive-like disorder7. Puerarin is a flavonoid glycoside that is extracted from the root of the leguminous plants pueraria lobata and Thomson Kudzuvine Root, and its chemical name is 8-β-D-glucopyranosyl-4′,7-dihydroxyisoflavone8, 9. Puerarin displays a series of beneficial activities on cardiovascular disease, hangover, osteoporosis, fever, and liver injury10–14. In neurological study, puerarin exerts the potential effects on attenuating memory and learning disorders15. Moreover, depressive-like behaviors and chronic pain with spared nerve injury (SNI) in mice are ameliorated by puerarin16. However, the molecular and cellular mechanisms underlying the antidepressant-like effects of puerarin are remain unclear.
A greater understanding of the mechanisms underlying depressive-like illness is required to provide new perspectives on the cause and the potential identification of novel therapeutic targets to treat depressive-like behavior. Chronic stress results in the dysregulation of hypothalamic-pituitary-adrenal (HPA) axis, which maybe one of the factors to MDD17, 18. The HPA axis, includes a feedback loop composed of the hypothalamus, pituitary and adrenal glands. Hyperactivity of the HPA axis in stress/depressive-like behavior is thought to be particularly related to reduced feedback inhibition by endogenous hormones of corticotropin releasing hormone (CRH), corticosterone (Cort) and adrenocorticotropic hormone (ACTH), which are commonly detected hormones in clinical patients and animal models19–21.
In addition, evidences also suggested that abnormal monoaminergic neurotransmission is one of most important mechanisms underlying depressive-like behavior. Numerous studies demonstrated that monoamines were important neurotransmitters involved in the etiology of depressive-like behavior3, 4. Actually, most of the antidepressants act on more than one mechanisms, such as inhibition of the reuptake of serotonin (5-HT) and its metabolites. Evidences from various studies indicated that the levels of monoamine neurotransmitters in the brain were increased after the treatments of antidepressants22.
Moreover, the down-regulation of neurosteroid biosynthesis has been implicated as one of the possible contributors to the development of depressive disorders23, 24. Neuroactive steroids (e.g progesterone and allopregnanolone) have been shown to elicit antidepressant-like properties. For instance, normalization of cerebrocortical allopregnanolone levels may contribute to the pharmacological profile of the antidepressants (i.e SSRIs) in rats25, 26.
Based on the above findings, we used a chronic unpredictable stress (CUS) procedure of rats, a well validated stress-related animal model of depressive-like behavior to further evaluate whether puerarin attenuates the CUS behavioral deficits in various behavioral tests. To investigate the molecular and cellular mechanisms underlying the effects of puerarin, we then assessed biosynthesis of neurosteroids production, HPA axis activation and the levels of monoamines in post-CUS rats after chronic puerarin treatment.
Materials and Methods
Drugs and reagents
Both puerarin and sertraline (Ser) were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.), dissolved in dimethyl sulphoxide (DMSO, <0.1%) and diluted to indicate concentration with 0.9% normal saline. All compounds were administered by intragastric gavage (i.g.) in a volume of 2 mL/kg between 08:00 and 09:00 h. Ser was administrated as a positive control in all behavioral tests at a dose (15 mg/kg i.g) based on previous PTSD studies27, 28. Doses of puerarin (30, 60 and 120 mg/kg i.g) were based on a previous study showing that puerarin ameliorated the depressive-like behavior in rodents16.
Animals
The Sprague-Dawley rats (male, 200 ± 20 g) were maintained in the conditions of controlled humidity (45–50%), temperature (23 ± 1 °C), and lighting (12 h/d). The rats were housed in a 12-h light/dark cycle starting at least 5 days before the experiments with access to food and water freely available. The experiments were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996) and approved by the institution of Academy of Military Medical Sciences. All efforts were made to minimize animal suffering and reduce the number of animals used in the experiments.
Preparation of the chronically stressed rats
The CUS model was performed as described previously with some modifications24. Except for control, the animals were subjected to the following stressors (from Mondays to Saturadays, except Sundays): (1) low-intensity stroboscopic illumination (80 flashes/min); (2) overnight illumination; (3) soiled cage (180 mL water in 90 g sawdust bedding); (4) food or water deprivation (24 h); (5) forced swimming (5 min at 10 °C); (6) 45° cage tilt; (7) white noise (approx. 110 dB); (8) restraint (2 h); and (9) tail pinch (2 min). All of the stressors were applied randomly and continuously. The rats in the control group were left undisturbed in the home cages, except for the 14 h period of water deprivation prior to each sucrose test. The outline of design for CUS was shown in Table (Table 1).
Table 1.
Chronic unpredictable stress schedule.
| Groups | Condition | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| Monday | Soiled cage: 24 h | Restraint: 2 h | Restraint: 2 h | Force swimming: 5 min |
| Tuesday | Overnight stroboscopic: 12 h | Tail pinch: 1 min | Overnight stroboscopic: 12 h | Food derivation: 24 h |
| Wednesday | Force swimming: 5 min | Overnight stroboscopic: 12 h | Food derivation: 24 h | Overnight illumination: 12 h |
| Thursday | Tail pinch: 1 min | Overnight illumination: 12 h | Water deprivation: 24 h | Soiled cage: 24 h |
| Friday | White noise: 1 h | Cage tilt: 24 h | Overnight illumination: 12 h | White noise: 1 h |
| Saturday | Water deprivation: 24 h | Water deprivation: 24 h | Force swimming: 5 min | Overnight stroboscopic: 12 h |
Behavioral paradigms
After the acclimatization, rats were singly placed in cages and subjected to sucrose training and sucrose baseline test. The animals were divided into six statistically equivalent groups according to their sucrose intake in the final baseline test. After the 4-week CUS procedure, behavioral assessments including sucrose preference test (SPT) (from day 36 to 40), novelty-suppressed feeding test (NSFT) (from day 42 to 43), forced swimming test (FST) (from day 45 to 46), and open field test (OFT) (day 48) were performed on different days. The drugs were given to the rats from day 29 to the end of the behavioral tests. All the behavioral tests were performed 1 h after drugs administration. Control animals received 0.9% normal saline. The schedule of behavioral tests and drug treatment was outlined in Fig. 1.
Figure 1.
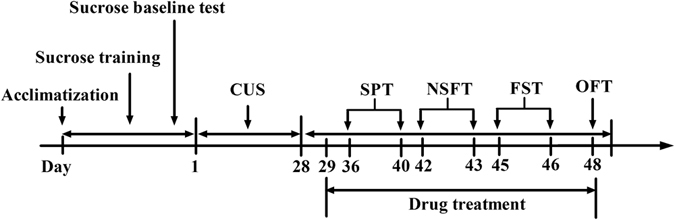
The outline of design for behavioral tests and the schedule of drug treatment.
SPT
SPT is widely applied to measure the depressive-like behavior and performed as described previously24, 29, 30. Rats were placed in individual cages to train to consume 1% (w/v) sucrose solution for 48 h without food and water supply. Following 16 h water deprivation, one bottle of 1% sucrose solution was replaced by water. The position of the sucrose and water bottles for the SPT was counterbalanced. The SPT was performed for 1 h. During the test, rats could select between two preweighed bottles, one with 1% sucrose solution and the other with tap water. The sucrose preference was calculated as sucrose intake/(sucrose intake + water intake) ×100%.
NSFT
The NSFT provides a sensitive and reliable measure of depressive-like behavior and motivation level in animals which mimics the situation in human31, 32. The test was performed according to the literature with minor modifications24. Briefly, after fasting for 24 h, each rat was placed in the corner of the plastic box (76 × 76 × 46 cm) with several pallets placed in the center. The latency to begin eating within 5 min was recorded (defined as chewing or biting the pallet, instead of merely sniffing or toying with it). Moreover, home-cage food consumption in 5 min was immediately determined to assess effects of drugs on feeding drive.
FST
FST is a well-established measurement for evaluating the effects of antidepressants and the assessment is highly reliable to predict the validity of antidepressants33, 34. The procedure comprised two sections (the pretest and the test) with identical apparatus and conditions (height 40 cm, diameter 20 cm, containing 25 cm of water maintained at 25 °C). During the pretest section, rats were forced to swim for 5 min. After 24 h, rats were placed in the same apparatus for 5 min and the section was designated as a test section. The duration of immobility during 5 min was measured.
OFT
To evaluate whether the reversion of depressive-like behavior by puerarin is dependent on an affect on locomotor activity, we assessed the number of crossings, rears, and fecal pallets in rats. The test was performed based on the literature with minor adjustments35. Each rat was placed in the corner of a plastic box (dimensions: 76 × 76 × 46 cm) that the base was divided into 16 equal squares for the 5-min acclimation period. Following that, the number of crossings (with all four paws placed into a new square), rears (with both front paws raised from the floor), and fecal pallets was rated by the observers who were blind to the grouping for 5 min by camera. The OFT was cleaned with a 5% ethanol/water solution after each test to remove any confounding olfactory cues and dried thoroughly between sections.
Neurosteroids measurement
Altered levels of progesterone and allopregnanolone have been implicated as one of the possible contributors to the development of depressive-like behavior23, 36. The levels of neurosteroids in the antidepressant-like activity of puerarin were evaluated by enzyme linked immunosorbent assay (ELISA). The brain tissues preparation was based on a literature24. The brains were removed and carefully dissected to remove the prefrontal cortex and hippocampus at the end of OFT in 24 h after the last drug treatment. The brain regions were extracted by 1 mL extraction buffer per 100 mg tissue and then homogenized in the ice-cold lysis buffer containing 137 mM NaCl, 1% NP40, 10% glycerol, 20 mM Tris-HCl (pH 8.0), 1 μg/mL leupeptin, 1 mM PMSF 10 μg/mL aprotinin, and 0.5 mM sodium vanadate. The tissue homogenate solutions were centrifuged at 10,000 g for 25 min at 4 °C, and then the supernatants were collected. Progesterone and allopregnanolone were quantified by Enzyme Immunoassay kit (Progesterone: No. ADI-900–011, 15.62–500 pg/mL, Enzo Life Sciences, USA; Allopregnanolone: No. E1963Ge, 31.2–2000 pg/mL, EIAab, China). Six samples in each group were used to determine optical density (OD) values at 450 nm in ELISA plate reader (Beckman, USA) and used for statistical analyzes.
Levels of Cort, CRH and ACTH measurement
The blood was sampled and collected at the end of behavioral tests in 24 h after the last drug treatment. The samples were centrifuged (2000 g, 30 min) at 4 °C and stored at −80 °C until further analyses. The test was performed based on the previous literature37. Levels of Cort, CRH and ACTH in serum were determined by ELISA kits (Cort: No. KGE009, 0.1–25 ng/mL, R&D Systems, USA; CRH: No. DL-CRH-Ra, 12.35–1000 pg/mL, Dldevelop, China; ACTH: No. DL-ACTH-Ra, 12.35–1000 pg/mL, Dldevelop, China) according to the manufacturer’s instructions. A sample (or standard) and conjugate were added to each well, and the plate was incubated for 1 h at room temperature. After several washes and proper color development, the OD value was determined at 450 nm by an ELISA plate reader (Beckman, USA).
The determination of monoamine neurotransmitter levels
To further explore neurochemical mechanisms involved in antidepressant-like effect of puerarin, the levels of prefrontal cortex, hippocampus monoamine neurotransmitters and their metabolites were detected by high performance liquid chromatography-electrochemical detection (HPLC-ECD) after behavioral assessments. The detection was performed as described previously7, 28. Also, the brain tissues were dissected at the end of behavioral tests in 24 h after the last drug treatment on ice by a binocular dissection microscope and homogenized in an ice-cold tissue lysis buffer containing 0.1 g/L of L-cysteine, 0.5 mM Na2EDTA and 0.4 M HClO4 (5.0 μL/mg). The samples were centrifuged at 12,000 × g for 30 min at 4 °C and then filtered through a 0.45 μm pore membrane. The sample or standard solution was injected into the reversed-phase SunFire™ C18 column (250 mm × 4.6 mm, 5 μm) (Model C-18, DIKMA Technologies Ltd., China). Separation was performed in an isocratic elution mode at a column temperature of 20 °C using a mobile phase containing 0.1 M sodium acetate buffer (pH3.7) with 85 mM citric acid, 15% methanol, 0.9 mM sodium octanesulfonate, and 0.2 mM Na2EDTA at a flow rate of 1.0 mL/min. The monoamine neurotransmitters and the metabolites (5-HT, 5-HIAA, DOPAC, DA, AD, HVA and NE) were presented as ng/g wet weight of tissue.
The antidepressant- and anxiolytic-like effects of puerarin by acute treatment
To evaluate whether the reversion of depressive- and anxiogenic-like behavior is induced by puerarin acute treatment, the FST and elevated plus maze test (EPMT) were performed, respectively. The FST was conducted as above. The EPMT is one of the widely utilized assessments for evaluating anxiogenic behavior in rodents. The apparatus consisted of four branching arms (60 × 12 cm) with two open arms and two closed arms with dark walls (40 cm high). The arms were connected by a centre platform (12 × 12 cm), and the maze was 50 cm above the ground. Each rat was placed in the central platform facing the closed arms. Rats were scored as entering an open or closed arm when all four paws passed over the dividing line. The exposure during initial 5 min was taped with a video camera. Time and numbers of entries into open arms were obtained as anxiety indices by an investigator who was blind to treatment conditions of animals. The percentage of time spent in and the entries into the open arms was calculated by dividing the time spent in and the entries into the open arms by the total time spent in and the total arm entries into the both arms, respectively. Both behavioral tests were performed 1 h after drug acute administration. Control animals received 0.9% normal saline.
Statistical analysis
Statistical analysis was performed by GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). All data were presented as the mean ± S.E.M. The statistical significance of experimental observations was determined by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests, as indicated in the results section. Differences in body weight gain were analyzed by two-way repeated measures ANOVA. For all tests, the level of statistical significance was set at p < 0.05.
Results
Effects of puerarin on the CUS-induced behavioral deficits in SPT
The effects of puerarin on CUS rats in SPT were shown in Fig. 2. Sucrose preference was significantly decreased in CUS rats. Consistent with Ser (15 mg/kg, i.g), puerarin (60 and 120 mg/kg, i.g) reversed the decreased sucrose preference (F (5,54) = 14.25, p = 0.0000; Fig. 2) in CUS rats. The results indicated that puerarin ameliorated the CUS-induced behavioral deficits in SPT.
Figure 2.
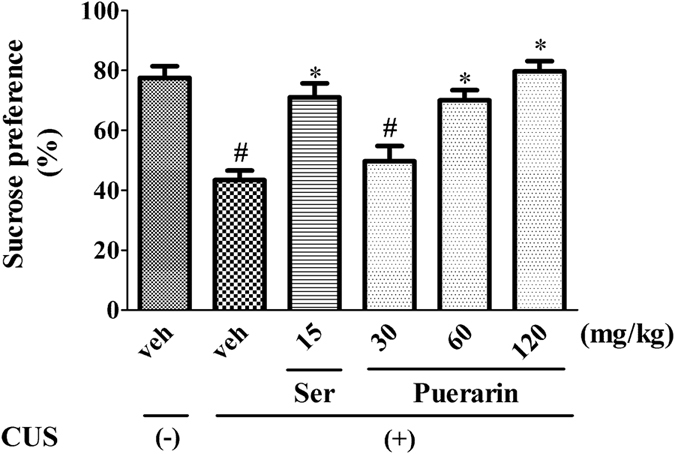
Puerarin attenuated the CUS-induced behavioral deficits in SPT. # p < 0.05 vs. vehicle-treated CUS (−) group; *p < 0.05, **p < 0.01 vs. vehicle-treated CUS (+) group (n = 10 per group).
Effects of puerarin on the CUS-induced behavioral deficits in NSFT
As shown in Fig. 3, the latency to feed was increased significantly in CUS rats. Consistent with Ser (15 mg/kg, i.g), puerarin (60 and 120 mg/kg, i.g) blocked the increase of latency to feed (F (5,54) = 8.889, p = 0.0131; Fig. 3A). Moreover, no differences of in home-cage food consumption were observed among the groups (F (5,54) = 0.5905, p = 0.7072; Fig. 3B). The results indicated that repeated treatment of puerarin ameliorated the CUS-induced behavioral deficits in NSFT.
Figure 3.
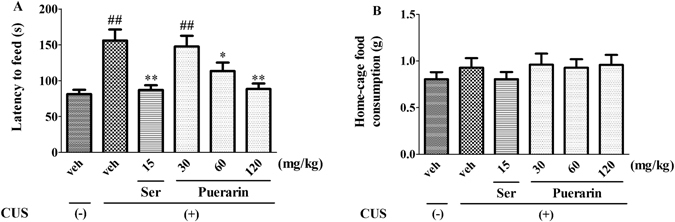
Puerarin attenuated the CUS-induced behavioral deficits in NSFT. The latency to feed was increased by CUS and reversed by puerarin. ## p < 0.01 vs. vehicle-treated CUS (−) group; *p < 0.05, **p < 0.01 vs. vehicle-treated CUS (+) group (n = 10 per group).
Effects of puerarin on the CUS-induced behavioral deficits in FST
The effects of puerarin in FST were shown in Fig. 4. There was no significant effect on the immobility time (F (5,54) = 1.145, p = 0.3483; Fig. 4A) in pretest duration among groups. However, the immobility time was increased significantly in CUS rats in test section. Similar to Ser (15 mg/kg, i.g), puerarin (60 and 120 mg/kg, i.g) produced the antidepressant-like effects, as evidenced by the decreased immobility time (F (5,54) = 4.253, p = 0.0025; Fig. 4B). The results indicated that repeated treatment of puerarin ameliorated the CUS-induced behavioral deficits in FST.
Figure 4.
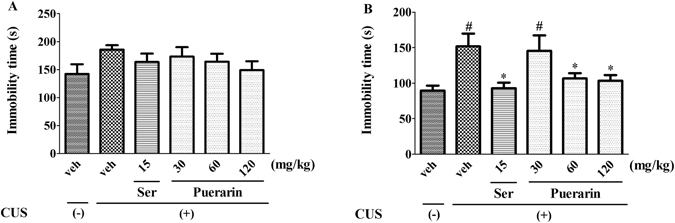
Puerarin attenuated the CUS-induced behavioral deficits in FST. # p < 0.05 vs. vehicle-treated CUS (−) group; * p < 0.05, ** p < 0.01 vs. vehicle-treated CUS (+) group (n = 10 per group).
Effects of puerarin on the locomotor activity and body weight gain in rats
The effects of puerarin on locomotor activity were shown in Fig. 5. There was no significant effect on the number of line crossings (F (5,54) = 1.922, p = 0.1058, Fig. 5A), rears (F (5,54) = 1.409, p = 0.2359, Fig. 5B), or fecal pallets (F (5,54) = 0.2403, p = 0.9429, Fig. 5C) among groups. In addition, body weight gain (F(5,54) = 1.389, p = 0.2387; Fig. 5D) was monitored throughout the drug treatment duration. In the puerarin-treated group, body weights was increased with time in a manner similar to that of the vehicle-treated group. These results indicated that neither puerarin treatment nor CUS modeling affected locomotor activity or body weight gain in rats.
Figure 5.
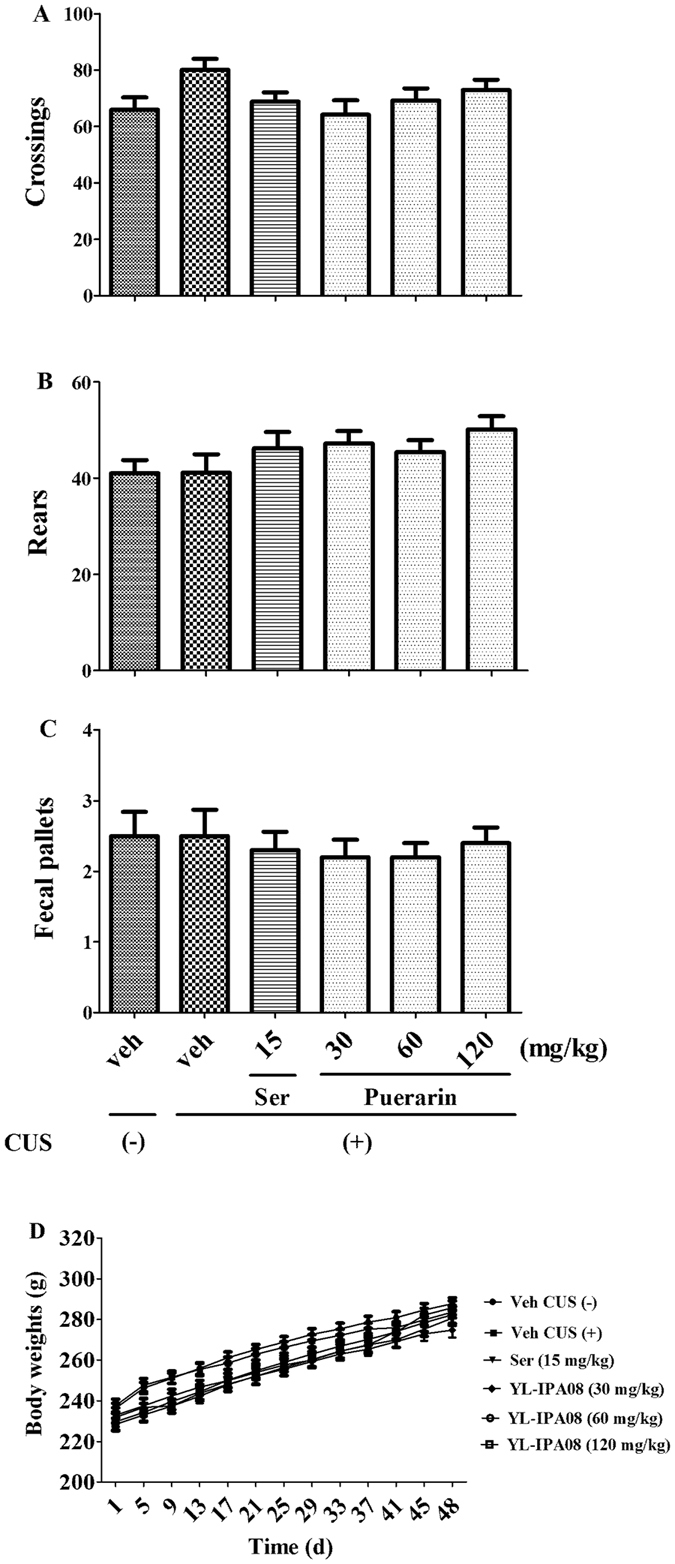
The effects of puerarin on locomotor activity. None of the treatments altered the number of line crossings (A), rears (B), and fecal pallets (C) in OFT. The body weights (D) were not altered by puerarin (n = 10 per group).
Effects of puerarin on the neurosteroid levels in CUS rats
The effects of puerarin on the levels of neurosteroids in CUS rats were shown in Fig. 6. After CUS exposure, levels of progesterone and allopregnanolone in the prefrontal cortex and hippocampus were significantly decreased, respectively. Similar to Ser (15 mg/kg, i.g.), both decreased levels of neurosteroids were significantly reversed by puerarin (60 and 120 mg/kg, i.g.) in the prefrontal cortex (F (5,30) = 4.510, p = 0.0035, for progesterone, Fig. 6A; F (5,30) = 6.102, p = 0.0005, for allopregnanolone, Fig. 6B) and hippocampus (F (5,30) = 4.993, p = 0.0019, for progesterone, Fig. 6C; F (5,30) = 3.377, p = 0.0154, for allopregnanolone, Fig. 6D), respectively. These results indicated that puerarin attenuated the CUS-induced behavioral deficits were associated with the biosynthesis of progesterone and allopregnanolone in brain.
Figure 6.
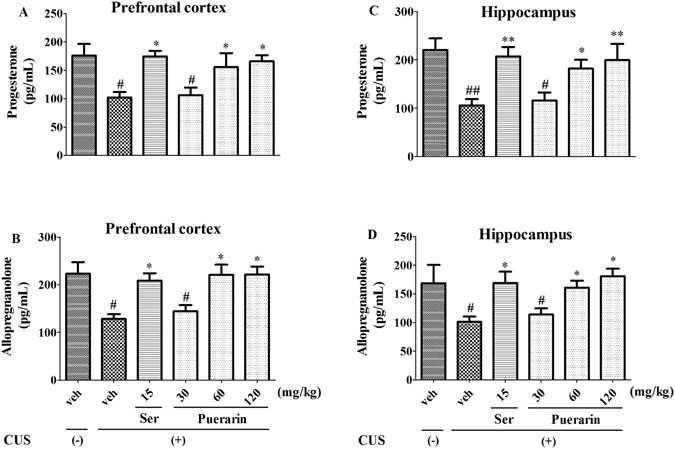
The effects of puerarin on levels of progesterone and allopregnanolone in the prefrontal cortex (A,C) and hippocampus (B,D), respectively. # p < 0.05, ## p < 0.01 vs. vehicle-treated CUS (−) group; * p < 0.05, ** p < 0.01 vs. vehicle-treated CUS (+) group (n = 6 per group).
Effects of puerarin on CUS-induced HPA axis changes
The effects of puerarin on Cort, CRH and ACTH levels in rats were shown in Fig. 7. Following CUS exposure, levels of Cort (F (5,30) = 2.882, p = 0.0306; Fig. 7A), CRH (F (5,30) = 7.639, p = 0.0000; Fig. 7B) and ACTH (F (5,30) = 8.872, p = 0.0000; Fig. 7C) in serum were significantly increased. In accordance with Ser (15 mg/kg, i.p), these effects were significantly reversed by treatment with puerarin (60 and 120 mg/kg, i.g), respectively. These results indicated that the effects of repeated puerarin treatment on CUS-induced behavioral deficits were associated with decreased HPA stress hormone (Cort, CRH and ACTH) levels.
Figure 7.
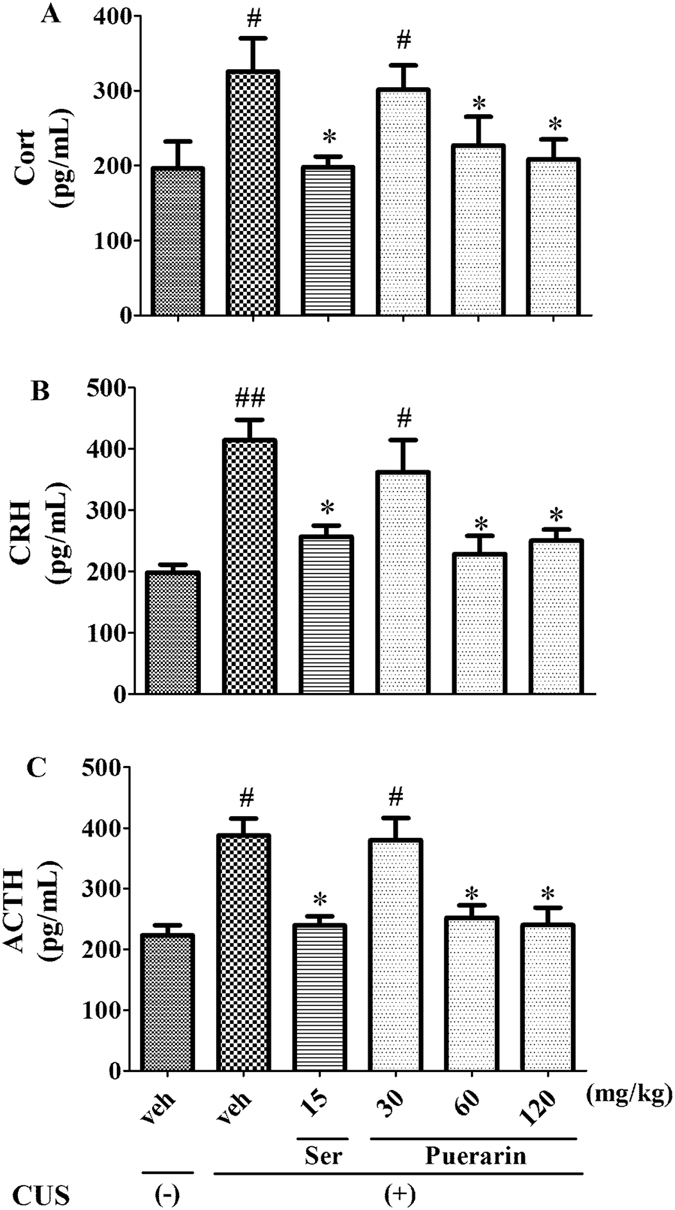
The effects of puerarin on Cort (A), CRH (B), ACTH (C) in serum. # p < 0.05, ## p < 0.01 vs. vehicle-treated CUS (−) group; *p < 0.05, **p < 0.01 vs. vehicle-treated CUS (+) group (n = 6 per group).
Effects of puerarin on the monoamine neurotransmitter levels in CUS rats
The effects of puerarin on the levels of monoamine neurotransmitters in rats were shown in Table (Tables 2 and 3). After CUS exposure, levels of 5-HT and 5-HIAA in the prefrontal cortex and hippocampus were significantly decreased, respectively. Similar to Ser (15 mg/kg, i.g.), the decreased levels of 5-HT (F (5,30) = 3.538, p = 0.0124, for prefrontal cortex, Table 2; F (5,30) = 4.778, p = 0.0025, for hippocampus, Table 3) and 5-HIAA (F (5,30) = 2.919, p = 0.0290, for prefrontal cortex, Table 2; F (5,30) = 4.742, p = 0.0026, for hippocampus, Table 3) were significantly reversed by puerarin (60 and 120 mg/kg, i.g.), respectively.
Table 2.
The effects of puerarin on prefrontal cortex monoamine neurotransmitter levels in CUS rats.
| Groups | 5-HT | 5-HIAA | NE | AD | HVA | DA | DOPAC |
|---|---|---|---|---|---|---|---|
| CUS (−) | 194.5 ± 26.15 | 147.0 ± 23.24 | 118.0 ± 12.12 | 153.7 ± 20.57 | 47.83 ± 4.408 | 43.50 ± 3.888 | 51.50 ± 6.893 |
| CUS (+) | 85.83 ± 9.325## | 83.50 ± 9.929# | 115.2 ± 14.47 | 165.0 ± 22.84 | 49.00 ± 6.787 | 51.83 ± 4.840 | 59.33 ± 5.200 |
| Ser 15 mg/kg, i.g | 163.0 ± 29.14* | 151.8 ± 22.81* | 130.5 ± 17.84 | 162.0 ± 22.89 | 60.50 ± 9.280 | 56.33 ± 6.238 | 52.67 ± 8.531 |
| puerarin 30 mg/kg, i.g | 126.5 ± 18.30 | 113.5 ± 11.30 | 138.2 ± 16.45 | 186.3 ± 21.84 | 60.33 ± 7.953 | 54.67 ± 7.491 | 53.33 ± 5.512 |
| puerarin 60 mg/kg, i.g | 181.5 ± 21.61* | 149.2 ± 10.94* | 134.5 ± 24.30 | 180.0 ± 25.41 | 50.50 ± 8.782 | 55.17 ± 6.740 | 52.83 ± 4.722 |
| puerarin 120 mg/kg, i.g | 164.5 ± 16.87* | 158.5 ± 18.88* | 125.2 ± 10.76 | 148.7 ± 14.97 | 59.33 ± 7.297 | 51.83 ± 5.902 | 55.17 ± 4.578 |
# p < 0.05, ## p < 0.01 vs. vehicle-treated CUS (−) group; *p < 0.05 vs. vehicle-treated CUS (+) group (n = 6).
Table 3.
The effects of puerarin on hippocampal monoamine neurotransmitter levels in CUS rats.
| Groups | 5-HT | 5-HIAA | NE | AD | HVA | DA | DOPAC |
|---|---|---|---|---|---|---|---|
| CUS (−) | 205.5 ± 33.45 | 145.7 ± 25.66 | 140.7 ± 24.38 | 172.7 ± 24.49 | 47.17 ± 6.358 | 36.50 ± 5.602 | 49.83 ± 5.449 |
| CUS (+) | 99.17 ± 12.04## | 58.83 ± 8.328## | 123.7 ± 16.95 | 190.0 ± 24.24 | 44.33 ± 9.054 | 37.00 ± 6.282 | 45.50 ± 8.269 |
| Ser 15 mg/kg, i.g | 175.0 ± 19.23** | 141.8 ± 17.66** | 150.3 ± 12.39 | 193.5 ± 18.25 | 51.33 ± 6.136 | 30.50 ± 4.890 | 39.50 ± 4.904 |
| puerarin 30 mg/kg, i.g | 104.2 ± 6.194 | 92.33 ± 13.44 | 150.8 ± 20.49 | 176.5 ± 27.14 | 52.17 ± 8.856 | 33.67 ± 6.998 | 48.17 ± 7.748 |
| puerarin 60 mg/kg, i.g | 168.2 ± 12.62* | 128.0 ± 24.85* | 121.3 ± 15.81 | 205.8 ± 33.74 | 48.33 ± 8.617 | 39.33 ± 4.951 | 45.17 ± 4.020 |
| puerarin 120 mg/kg, i.g | 201.3 ± 30.03** | 151.0 ± 28.28** | 126.7 ± 21.50 | 190.5 ± 52.26 | 44.00 ± 4.389 | 38.17 ± 4.881 | 44.00 ± 5.342 |
## p < 0.01 vs. vehicle-treated CUS (−) group; * p < 0.05, ** p < 0.01 vs. vehicle-treated CUS (+) group (n = 6).
However, NE (F (5,30) = 0.3034, p = 0.9069, for prefrontal cortex, Table 2; F (5,30) = 0.4986, p = 0.7748, for hippocampus, Table 3), AD (F (5,30) = 0.4599, p = 0.8028, for prefrontal cortex, Table 2; F (5,30) = 0.1421, p = 0.9809, for hippocampus, Table 3), HVA (F (5,30) = 0.6397, p = 0.6711, for prefrontal cortex, Table 2; F (5,30) = 0.2120, p = 0.2120, for hippocampus, Table 3), DA (F (5,30) = 0.6057, p = 0.6960, for prefrontal cortex, Table 2; F (5,30) = 0.3288, p = 0.8916, for hippocampus, Table 3), DOPAC (F (5,30) = 0.2146, p = 0.9536, for prefrontal cortex, Table 2; F (5,30) = 0.3393, p = 0.8850, for hippocampus, Table 3) in both brain regions were not significantly affected by CUS and puerarin. These results indicated that the effects of repeated puerarin treatment on CUS-induced behavioral deficits were associated with the normalized levels of 5-HT and 5-HIAA in the prefrontal cortex and hippocampus.
The antidepressant- and anxiolytic- like effects of acute puerarin treatment in rats
The antidepressant- and anxiolytic- like effects of acute puerarin treatment in rats were shown in Fig. 8. In FST, there was no significant effect on the immobility time in pretest (F (4,45) = 0.3010, p = 0.8758; Fig. 8A) and test (F (4,45) = 0.1689, p = 0.9531; Fig. 8B) duration among groups. Similar to FST (15 mg/kg, i.g), both Ser and puerarin did not produce the anxiolytic-like effects in EPMT, as evidenced by that the percentage of the time (F (4,45) = 0.3949, p = 0.8112; Fig. 8C)/entries (F (4,45) = 0.5266, p = 0.7167; Fig. 8D) into open arms and total time (F (4,45) = 0.8678, p = 0.4906; Fig. 8E)/entries (F (4,45) = 0.6711, p = 0.6154; Fig. 8F) in arms among groups were not different. The results indicated that acute puerarin treatment did not ameliorate the antidepressant- and anxiolytic- like effects in FST and NSFT, respectively.
Figure 8.
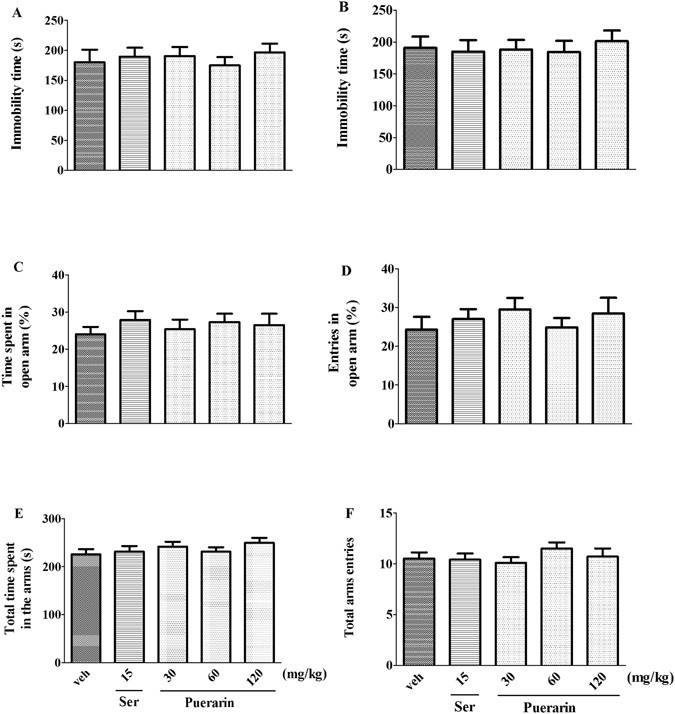
The acute effects of puerarin on FST and EPMT. In FST, there was no significant effect on the immobility time in pretest (A) and test (B) duration among groups. Similar to FST (15 mg/kg, i.g), both drugs did not induce the anxiolytic-like effects in EPMT, as evidenced by that the percentage of the time (C)/entries (D) into open arms and total time (E)/entries (F) in arms among groups were not different. (n = 10 per group).
Discussion
In the present study, we preliminarily evaluate the effects of puerarin on CUS-induced behavioral deficits via the CUS model and its possible mechanism. The CUS-induced behavioral deficits were significantly ameliorated by puerarin without affecting locomotor activity in rats. In addition, together with the results of neurosteroids biosynthesis, serotonergic system and hormones of HPA axis, we found that the effects of repeated puerarin treatment on CUS-induced behavioral deficits were associated with biosynthesis of neurosteroids, normalization of serotonergic system and preventing HPA axis dysfunction.
Depressive-like behavior is a serious mental health problem associated with psychiatric morbidity and has been increased worldwide from past several decades1, 2. The CUS model mimics the depressive-like symptoms and is widely used in the preclinical antidepressants evaluation which offers a closer resemblance to chronic stress24, 38. CUS induced behavioral changes that resemble clinical depressive-like behavior, such as reduced sucrose intake and responsiveness to rewarding stimuli24, 39. In present study, CUS induced a significant reduction of sucrose preference in SPT (Fig. 2) and the increased immobility time in FST (Fig. 4B), two indicators of the core symptoms of depressive-like behavior. Furthermore, it has been documented that the CUS model may also induce anxiogenic-like symptoms, which is evidenced by the increased latency to feed (Fig. 3). The NSFT is initially used to examine the effects of anxiolytic agents, and also sensitive to chronic treatment with antidepressants40, 41. These findings were further supported by the successful experimental protocol of the CUS procedure.
The CUS-induced behavioral parameters can be reversed by the chronic administration of antidepressants7, 24. Our results showed that similar to Ser (15 mg/kg i.g.), puerarin (60 and 120 mg/kg i.g.) reversed the decreased sucrose preference to normal levels in SPT (Fig. 2) and the increased immobility time in FST (Fig. 4B). Moreover, the increased latency to feed in NSFT was blocked by puerarin (60 and 120 mg/kg i.g.) (Fig. 3A) without affecting home-cage food consumption (Fig. 3B), suggesting that puerarin attenuated the CUS-induced behavioral deficits in rats. The effective doses of puerarin (60 and 120 mg/kg i.g.) were almost confirmed among SPT (Fig. 2), FST (Fig. 4B) and NSFT (Fig. 3A) and in line with depressive-like behavioral deficits that were reversed by puerarin with the similar doses16. Moreover, the OFT was also performed to evaluate whether the reversion of chronic stress behavioral deficits by puerarin was dependent on locomotor activity. Consistent with the previous findings and Ser (15 mg/kg i.g.)28, the present study also showed that the locomotor activity (e.g line crossings, rears and fecal pallets) was not affected by puerarin in OFT (Fig. 5). In addition, these findings were also consistent with our observations found that the anxiolytic activities of puerarin determined by EPMT which the total time (Fig. 8E) and entries (Fig. 8F) in arms were not significantly altered by puerarin. The data indicated that the effects of puerarin on the CUS-induced behavioral deficits were not mediated by affecting the locomotor activity in rats.
Ser was delivered 1 h before behavioral experiments and exerted the anti-stress activities with its chronic treatment27, 42. Therefore, puerarin had the similar the treatment schedule with Ser and showed that both drugs elicited the antidepressant-like effects via behavioral tests in present study. The study showed that the behavioral experiments were performed after the drug treatment at least 1 week (chronic/subchronic treatment). Moreover, our studies also found that the single treatment of puerarin 1 h before the behavioral experiments in rodents did not produce the anxiolytic- and antidepressant- like effects (Fig. 8). The findings were supported by the antidepressant-like effects of puerarin with repeated treatment16. Thus, it indicated that the effects of puerarin might be induced by its chronic treatment effect.
It was reported that dysfunction of the prefrontal cortex or hippocampus is implicated in the pathogenesis of depressive-like behavior34, 43. Both brain regions play an important role in fear conditioning, emotional processing and explicit memory that are relevant to anxiogenic- and depressive-like behavior43–45. Thus, to confirm the role of neurosteroids in the effects of puerarin on the CUS-induced behavioral deficits, we then measured levels of endogenous neurosteroids and monoamine neurotransmitters in the above brain regions.
Although the pathological factor of depressive-like behavior have been widely researched, the exact factors involved are still not discovered. More evidences demonstrate that the biosynthesis of neurosteroids (e.g. progesterone and allopregnanolone) has been implicated as one of the possible contributors in the development of depressive-like behavior46, 47. The present findings showed that similar to Ser, both decreased levels of neurosteroids were significantly reversed by puerarin in the prefrontal cortex (Fig. 6A and B) and hippocampus (Fig. 6C and D), respectively. The results indicated that the effects of puerarin on the CUS-induced behavioral deficits were associated with the biosynthesis of progesterone and allopregnanolone in brain. Our present study was also confirmed by the finding that altered levels of progesterone affected the levels of metabolite steroids, such as allopregnanolone, and progesterone withdrawal in animals also dramatically reduces levels of allopregnanolone in brain48.
Progesterone serves as the main precursor molecule for 3β-pregnane neuroactive steroids which possessed antidepressant-like activities49. The beneficial effect of progesterone may come after its conversion to allopregnanolone and through that metabolite’s agonistic action at GABA (γ-aminobutyric acid) A receptors47. Allopregnanolone is the brain neurosteroid that acts GABAA receptors and is the selective positive endogenous modulator of the action of GABAA at GABAA receptors in brain49, 50. More clinical studies have shown that depressive-like behavior is closely associated with the biosynthesis of neurosteroids. For instance, decreased allopregnanolone in peripheral blood or cerebrospinal fluid (CSF) is found to relevant to MDD, anxiety disorders, premenstrual dysphoric disorders, negative symptoms in schizophrenia, or impulsive aggression51. These might be related to the effects of levels of allopregnanolone on the regulation of GABAA receptor function, which plays a role in the pathophysiology of depressive-like behavior. The GABAA agonist modulator interacted on the brain by changing the expression of GABAA receptor subunit to produce the neuroprotective effects on the depressive-like behavior52.
Moreover, the hyperactivity of the HPA axis, which is commonly seen in patients with depressive-like symptoms, is the most commonly observed neuroendocrine abnormality in MDD53. Here, we found that the increased levels of Cort (Fig. 7A), CRH (Fig. 7B) and ACTH (Fig. 7C) in serum of post-CUS rats. The findings were supported by that the increased levels of CRH, Cort and ACTH in menopause depressive ovariectomized rats under chronic unpredictable mild stress54. The results were also consistent with the previous study that the role of allopregnanolone as an endogenous negative regulator of HPA axis activity, and plasma Cort was elevated concomitantly with decreased levels of allopregnanolone in chronically stressed rats55. Interestingly, study also demonstrated that the hormones of HPA axis above in post-CUS rats were reversed by puerarin (Fig. 7), which indicated that the normalization of brain neurosteroid levels and the subsequent prevention HPA axis dysfunction may underlie the activities of puerarin on the CUS-induced behavioral deficits.
In mammals, the HPA axis and the monoamines system closely interact in central nervous system (CNS) (particularly in prefrontal cortex and hippocampus) and are greatly involved in stress-related disorders56, 57. Based on these evidences, the role of monoamines in the effects of puerarin on the CUS-induced behavioral deficits were evaluated. Our findings demonstrated after CUS exposure, levels of 5-HT and 5-HIAA in both brain regions were significantly decreased (Tables 2 and 3). The results were confirmed with decreased serotonin levels (e.g 5-HT and 5-HIAA) in a CUS animal model7. As the pathophysiological theory of depressive-like behavior, the monoamine hypothesis holds that lowered levels of 5-HT in the CNS are closely associated with depressive-like behavior35. However, similar to Ser, the decreased levels of 5-HT and 5-HIAA were significantly reversed by puerarin (Tables 2 and 3), indicating that the effects of puerarin on the CUS-induced behavioral deficits were associated with the normalized levels of 5-HT and 5-HIAA in brain. Substantial evidences indicated that depressive-like behavior was due to an absence of brain monoaminergic activity7, 35, and the levels of monoamine neurotransmitters (e.g 5-HT) in brain were increased in the experimental groups after the antidepressant treatments58.
In neurological study, reports showed that puerarin elicited the potential effects on attenuating memory and learning disorders, including Parkinson’s disease, Alzheimer’s disease, ischaemic stroke et al.59–61. However, not many studies on the antidepressant-like effect of puerarin and its possible mechanism. It is possible that puerarin normalizes the depressive-like behavior by other alternative mechanisms, such as activation of brain-derived neurotrophic factor (BDNF) and glutamate receptor. Recently, study showed puerarin ameliorates depressive-like behaviors and chronic pain in mice with SNI which markedly promoted the activation of CAMP-response element binding protein (CREB) pathway and induced BDNF expression16. BDNF plays an important role on neuronal survival, neurogenesis, differentiation of neurons and synapses, learning and memory62. Interestingly, BDNF was decreased in hippocampus and prefrontal cortex of patients with depressive-like symptoms and other psychiatric disorders63. Increased BDNF expression could largely improve depressive disorders64. Thus, BDNF was recently suggested as an important biomarker for successful treatment of depressive-like behaviors. Puerarin profoundly induced the phosphorylation of CREB and expression of BDNF16. Therefore, it is possible that puerarin promotes the cross-talks between BDNF-CREB signaling pathways.
Moreover, the glutamate receptors have also been examined as potential therapeutic targets for depressive-like behavior65. Puerarin ameliorated learning and memory deficits in mice through normalizing the glutamatergic/GABAergic system and causing synaptic structural modifications in the hippocampus66. Previous study showed that neuroprotective effect of puerarin was potentially mediated through the inhibition of glutamate-induced activation of mitochondrial-dependent signaling pathway and calmodulin-dependent protein kinase II (CaMKII)-dependent apoptosis signal-regulating kinase 1(ASK-1)/c-Jun N-terminal kinase (JNK)/p38 signaling pathway67, 68.
Taken together, the present study showed that puerarin exerted the ameliorative activities via the CUS-induced stress and various behavioral tests. The possible mechanism may be associated with biosynthesis of neurosteroids, normalization of serotonergic system and preventing HPA axis dysfunction, which may account for the molecular and cellular mechanism underlying the effects of puerarin on the CUS-induced behavioral deficits. Based on the previous and present study, it indicates that not only advance our knowledge of the theories of MDD, but also have clinical implications for puerarin that may serve as a novel compound for the therapeutics of depressive-like behavior. Although we preliminarily evaluated the effects of puerarin on CUS-induced behavioral deficits and its possible mechanism, the effect of chronic puerarin on behavior and molecular readouts was not fully performed. Thus, it is possible that partial activity may not be specific to CUS. More work should be conducted the chronic puerarin in rats to provide more evidences for the chronic activity of puerarin on CUS-induced behavioral deficits and molecular pathways/targets. In addition, the pharmacodynamics of puerarin in CNS towards the clinical translation are also needed.
Acknowledgements
This study was supported by funds: Natural Science Foundation of Guangdong Province, China (No. 2014A030310103), Traditional Chinese Medicine Bureau of Guangdong Province, China (No. 2014A030310103) and Educational Commission of Guangdong Province, China (No. 2016KQNCX086).
Author Contributions
All authors participated in the preparation of the manuscript, and read and approved the final manuscript. Zhi-Kun Qiu and Jia-Li He conceived the idea, directed the work and designed the experiments; Zhi-Kun Qiu, Guan-Hua Zhang and Xu Liu performed the experiments and paper writing; The data analyses were performed by Zhi-Kun Qiu, Da-Lian Wei and Xu Liu; Ji-sheng Chen and De-Sheng Zhong provided comments and technical support.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Zhi-Kun Qiu, Guan-Hua Zhang and De-Sheng Zhong contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia-Li He, Email: doctor_carriehe@163.com.
Ji-Sheng Chen, Email: cjslym@163.com.
Da-Nian Wei, Email: weidanian@163.com.
References
- 1.Deumic E, et al. Sexual Functioning in Adolescents With Major Depressive Disorder. J Clin Psychiatry. 2016;77:957–962. doi: 10.4088/JCP.15m09840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev. 2016;69:313–32. doi: 10.1016/j.neubiorev.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26:397–410. doi: 10.1016/j.euroneuro.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Y, Zhu H, Leung SW. Comparative efficacy of selective serotonin reuptake inhibitors (SSRI) in treating major depressive disorder: a protocol for network meta-analysis of randomised controlled trials. BMJ Open. 2016;6:e010142. doi: 10.1136/bmjopen-2015-010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David DJ, Gourion D. Antidepressant and tolerance: Determinants and management of major side effects. Encephale. 2016;42:553–561. doi: 10.1016/j.encep.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Pugh CM, et al. Selective serotonin reuptake inhibitor (SSRI) toxicosis in cats: 33 cases (2004–2010) J Vet Emerg Crit Care (San Antonio). 2013;23:565–570. doi: 10.1111/vec.12091. [DOI] [PubMed] [Google Scholar]
- 7.Wang YL, et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol. 2016;179:9–15. doi: 10.1016/j.jep.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. 2014;12:407–414. doi: 10.1016/S1875-5364(14)60064-9. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, et al. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res. 2013;47:55–63. doi: 10.3109/10715762.2012.742518. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, et al. Puerarin attenuates severe burn-induced acute myocardial injury in rats. Burns. 2015;41:1748–1757. doi: 10.1016/j.burns.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Overstreet DH, et al. NPI-031G (puerarin) reduces anxiogenic effects of alcohol withdrawal or benzodiazepine inverse or 5-HT2C agonists. Pharmacol Biochem Behav. 2003;75:619–625. doi: 10.1016/S0091-3057(03)00114-X. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, et al. Puerarin protects against CCl4-induced liver fibrosis in mice: possible role of PARP-1 inhibition. Int Immunopharmacol. 2016;38:238–245. doi: 10.1016/j.intimp.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Yao XJ, et al. Puerarin exerts antipyretic effect on lipopolysaccharide-induced fever in rats involving inhibition of pyrogen production from macrophages. J Ethnopharmacol. 2012;141:322–330. doi: 10.1016/j.jep.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Yuan SY, et al. Puerarin prevents bone loss in ovariectomized mice and inhibits osteoclast formation in vitro. Chin J Nat Med. 2016;14:265–269. doi: 10.1016/S1875-5364(16)30026-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhao SS, Yang WN, Jin H, Ma KG, Feng GF. Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice. Neurotoxicology. 2015;51:166–171. doi: 10.1016/j.neuro.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Luo D, Liang Z, Lao L, Rong J. Plant Natural Product Puerarin Ameliorates Depressive Behaviors and Chronic Pain in Mice with Spared Nerve Injury (SNI) Mol Neurobiol. 2017;54:2801–2812. doi: 10.1007/s12035-016-9870-x. [DOI] [PubMed] [Google Scholar]
- 17.Ancelin ML, et al. Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology. 2016;77:90–94. doi: 10.1016/j.psyneuen.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama K, et al. Relationship between hypothalamic-pituitary-adrenal axis dysregulation and insulin resistance in elderly patients with depression. Psychiatry Res. 2015;226:494–498. doi: 10.1016/j.psychres.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Chang HS, Won E, Lee HY, Ham BJ, Lee MS. Association analysis for corticotropin releasing hormone polymorphisms with the risk of major depressive disorder and the response to antidepressants. Behav Brain Res. 2015;292:116–124. doi: 10.1016/j.bbr.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Franco AJ, et al. Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology. 2016;157:2346–2355. doi: 10.1210/en.2015-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereiro N, Moyano R, Blanco A, Lafuente A. Regulation of corticosterone secretion is modified by PFOS exposure at different levels of the hypothalamic-pituitary-adrenal axis in adult male rats. Toxicol Lett. 2014;230:252–262. doi: 10.1016/j.toxlet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Blier P. Neurobiology of depression and mechanism of action of depression treatments. J Clin Psychiatry. 2016;77:e319. doi: 10.4088/JCP.13097tx3c. [DOI] [PubMed] [Google Scholar]
- 23.Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69:147–153. doi: 10.1159/000358838. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LM, et al. Antidepressant-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in chronically stressed rats. Neuropharmacology. 2017;113:567–575. doi: 10.1016/j.neuropharm.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Devall AJ, et al. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol. 2015;25:113–123. doi: 10.1016/j.euroneuro.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl). 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 27.Qiu ZK, et al. Repeated administration of AC-5216, a ligand for the 18 kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog. Neuropsychopharmacol Biol Psychiatry. 2013;45:40–46. doi: 10.1016/j.pnpbp.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LM, et al. Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid Based Complement Alternat Med. 2012;2012:623753. doi: 10.1155/2012/623753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurhe Y, Mahesh R. Pioglitazone, a PPARγ agonist rescues depression associated with obesity using chronic unpredictable mild stress model in experimental mice. Neurobiol Stress. 2016;3:114–121. doi: 10.1016/j.ynstr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- 31.Li D, et al. Wuling powder prevents the depression-like behavior in learned helplessness mice model through improving the TSPO mediated-mitophagy. J Ethnopharmacol. 2016;186:181–188. doi: 10.1016/j.jep.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 32.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 33.Porsolt RD, Le, Pichon. M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- 35.Xue R, et al. Antidepressant-like effects of 071031B, a novel serotonin and norepinephrine reuptake inhibitor. Eur Neuropsychopharmacol. 2013;23:728–741. doi: 10.1016/j.euroneuro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Deligiannidis KM, et al. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 2016;70:98–107. doi: 10.1016/j.psyneuen.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin ZL, et al. Anxiolytic effects of GLYX-13 in animal models of posttraumatic stress disorder-like behavior. J Psychopharmacol. 2016;30:913–921. doi: 10.1177/0269881116645298. [DOI] [PubMed] [Google Scholar]
- 38.Kumar. S, Mondal AC. Neuroprotective, Neurotrophic and Anti-oxidative Role of Bacopa monnieri on CUS Induced Model of Depression in Rat. Neurochem Res. 2016;41:3083–3094. doi: 10.1007/s11064-016-2029-3. [DOI] [PubMed] [Google Scholar]
- 39.Jiang LH, et al. Pinching spine: A potential treatment for depression. Chin J Integr Med. 2014;20:272–279. doi: 10.1007/s11655-012-1028-8. [DOI] [PubMed] [Google Scholar]
- 40.Yi LT, et al. BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:135–141. doi: 10.1016/j.pnpbp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K, et al. Analysis of main constituents and mechanisms underlying antidepressant-like effects of Xiaochaihutang in mice. J Ethnopharmacol. 2015;175:48–57. doi: 10.1016/j.jep.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Zhang LM, et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2014;17:1659–1669. doi: 10.1017/S1461145714000479. [DOI] [PubMed] [Google Scholar]
- 43.Kong L, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38:417–422. doi: 10.1503/jpn.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melas PA, Mannervik M, Mathé AA, Lavebratt C. Neuropeptide, Y: identification of a novel rat mRNA splice-variant that is downregulated in the hippocampus and the prefrontal cortex of a depression-like model. Peptides. 2012;35:49–55. doi: 10.1016/j.peptides.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Zhong T, et al. Swimming exercise ameliorates neurocognitive impairment induced by neonatal exposure to isoflurane and enhances hippocampal histone acetylation in mice. Neuroscience. 2016;316:378–388. doi: 10.1016/j.neuroscience.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 46.Gunn BG, et al. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015;36:28–48. doi: 10.1016/j.yfrne.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Beckley EH, Finn DA. Inhibition of progesterone metabolism mimics the effect of progesterone withdrawal on forced swim test immobility. Pharmacol Biochem Behav. 2007;87:412–419. doi: 10.1016/j.pbb.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Raaby KF, Sánchez C, Gulinello M. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res. 2013;256:520–528. doi: 10.1016/j.bbr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Hedström H, et al. Women with polycystic ovary syndrome have elevated serum concentrations of and altered GABA(A) receptor sensitivity to allopregnanolone. Clin Endocrinol (Oxf). 2015;83:643–650. doi: 10.1111/cen.12809. [DOI] [PubMed] [Google Scholar]
- 51.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2012;24:102–116. doi: 10.1111/j.1365-2826.2011.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kosari-Nasab M, Babri S, Fatehi-Gharehlar L, Doosti MH, Pakzad S. Involvement of GABAergic system in regulation of the anxiolytic- and antidepressant-like effects of Scrophularia striata extract in rats. Pharm Biol. 2013;51:581–588. doi: 10.3109/13880209.2012.749924. [DOI] [PubMed] [Google Scholar]
- 53.Salvat-Pujol N, et al. Hypothalamic-pituitary-adrenal axis activity and cognition in major depression: The role of remission status. Psychoneuroendocrinology. 2017;76:38–48. doi: 10.1016/j.psyneuen.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, et al. Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int J Clin Exp Med. 2015;8:5103–5011. [PMC free article] [PubMed] [Google Scholar]
- 55.Biggio G, Pisu MG, Biggio F, Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology (Berl). 2014;231:3437–3444. doi: 10.1007/s00213-014-3521-6. [DOI] [PubMed] [Google Scholar]
- 56.Grafe LA, et al. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience. 2017;348:313–323. doi: 10.1016/j.neuroscience.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Xian YF, Fan D, Ip SP, Mao QQ, Lin ZX. Antidepressant-Like Effect of Isorhynchophylline in Mice. Neurochem Res. 2017;42:678–685. doi: 10.1007/s11064-016-2124-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhu G, et al. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine induced Parkinson’s disease model in mice. Phytother Res. 2014;28:179–186. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, et al. Puerarin protects Alzheimer’s disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways. Exp Gerontol. 2011;46:30–37. doi: 10.1016/j.exger.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Wu M, et al. Effects of delayed puerarin treatment in long-term neurological outcomes of focal ischemic stroke in rats. Indian J Pharmacol. 2014;46:157–160. doi: 10.4103/0253-7613.129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minami. A, et al. Rapid regulation of sialidase activity in response to neural activity and sialic acid removal during memory processing in rat hippocampus. J Biol Chem. 2017;292:5645–5654. doi: 10.1074/jbc.M116.764357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu. Y, et al. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol Nutr Food Res. 2015;59:1130–1142. doi: 10.1002/mnfr.201400753. [DOI] [PubMed] [Google Scholar]
- 64.Reinhart V, et al. Evaluation of TrkB and BDNF transcripts in prefrontal cortex, hippocampus, and striatum from subjects with schizophrenia, bipolar disorder, and major depressive disorder. Neurobiol Dis. 2015;77:220–227. doi: 10.1016/j.nbd.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 65.Pick JE, Khatri L, Sathler MF, Ziff EB. mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum. EMBO J. 2017;36:232–244. doi: 10.15252/embj.201694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu XH, Zheng XX, Zhou Q, Li H. Inhibition of excitatory amino acid efflux contributes to protective effects of puerarin against cerebral ischemia in rats. Biomed Environ Sci. 2007;20:336–342. [PubMed] [Google Scholar]
- 67.Hwang YP, et al. Puerarin activates endothelial nitric oxide synthase through estrogen receptordependent PI3-kinase and calcium-dependent AMP-activated protein kinase. Toxicol Appl Pharmacol. 2011;257:48–58. doi: 10.1016/j.taap.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 68.Wang PP, et al. Puerarin stimulates osteoblasts differentiation and bone formation through estrogen receptor, p38 MAPK, and Wnt/β-catenin pathways. J Asian Nat Prod Res. 2012;14:897–905. doi: 10.1080/10286020.2012.702757. [DOI] [PubMed] [Google Scholar]


