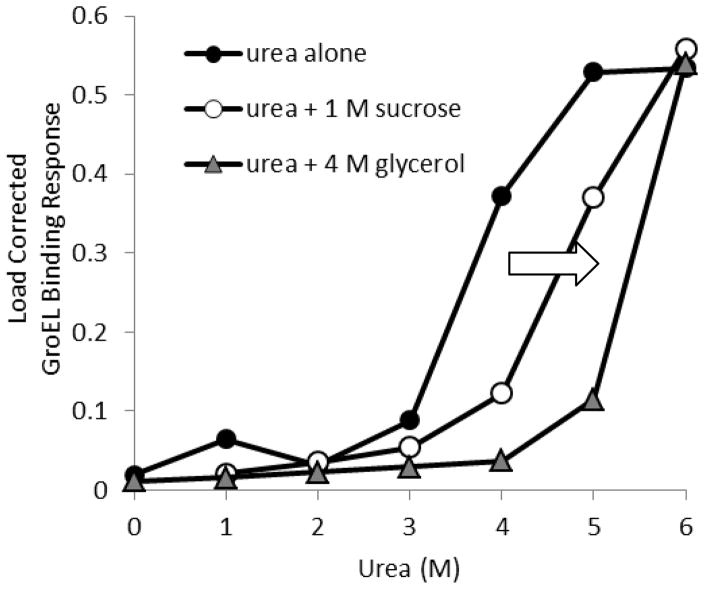
Figure 2.
Kinetic stabilization of MalZ in presence of different osmolytes. The MalZ protein was loaded onto Ni-NTA biosensor tips at 1 μM, and loading was consistent throughout the titration experiment. Each MalZ tip was exposed to various concentrations of urea, and GroEL binding amplitude was measured after 5 min. A separate set of MalZ attached tips were exposed to both urea and sucrose or glycerol. This figure denotes the GroEL binding response as a function of urea concentration with or without osmolytes. Diminished GroEL binding amplitudes were observed at each urea titration point upon the addition of the stabilizing osmolytes (1 M sucrose or 4 M glycerol), indicating a delay in the kinetically controlled denaturation isotherms, which results in an apparent stabilization of MalZ. A clear rightward shift in the GroEL binding amplitude response (denoted by arrow).