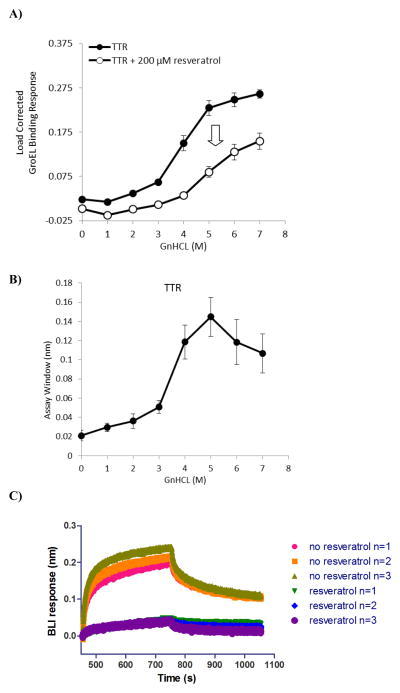
Figure 4.
Detection of resveratrol stabilization effect on TTR: Kinetically controlled denaturation of His-tagged TTR in the absence (closed circles) and presence (open circles) of a known TTR stabilizer, resveratrol. Experiments were performed on the Octet, with each data point comprising 3 separate measurements and error bars representing standard deviation. A reduced GroEL binding signal was observed at each GnHCl titration point when TTR was pretreated with resveratrol, indicating the compound’s stabilizing effect on the protein delays the unfolding of TTR. B) The differences between the wild type and resveratrol stabilized kinetic isotherms were generated to determine an optimal assay window. The optimal GnHCl concentration to readily observe stabilizer effects was in the 4–5 M GnHCl pulse range. C) Aligned (aligned to zero) sensorgram traces for three sample automated runs showing association (GroEL binding to TTR that had been pretreated with or without resveratrol) and dissociation steps after a 4 M GnHCl pulse.