Abstract
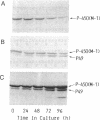
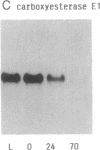
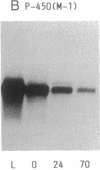
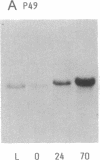
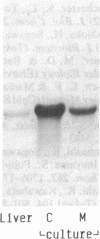
A 49-kDa protein (P49) was discovered in the primary cultures of rat hepatocytes. P49 cross-reacted with the antibodies against purified P450IIC11 [formerly P-450(M-1)]. P49 was located in microsomes and highly induced after plating of isolated hepatocytes on collagen-coated culture dishes. To characterize P49, cDNA clones were screened from a rat liver lambda gt11 expression library. From sequence analysis of the cloned cDNAs, the amino acid sequence of P49 was deduced, and the protein was identified as a previously uncharacterized form of cytochrome P450. P49 consists of 489 amino acids and shows approximately 60% similarity with the members of class IIC subfamily of rat cytochrome P450, such as P450IIC11 and P450IIC12 [formerly P-450(F-1)]. RNA blot analysis indicates that the mRNA translating P49 was induced approximately 20- to 30-fold at 70 hr in the primary cultures compared with the liver of adult rats. Induction of P49 was not affected by density of the plated cells and the presence or absence of several hormones, serum, or antibiotics in the culture medium. On the other hand, lower induction of P49 was seen when the hepatocytes were cultured on Matrigel-coated plates. Expression of P49 mRNA was low in the liver of adult rats and was not detectable in the livers of 1- and 2-week-old male and female rats. P49 is an additional form of cytochrome P450, which is induced in the primary culture of rat hepatocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by pregnenolone-16 alpha-carbonitrile in primary monolayer cultures of adult rat hepatocytes and in a cell-free translation system. J Biol Chem. 1981 Jun 25;256(12):6060–6068. [PubMed] [Google Scholar]
- Emi Y., Omura T. Synthesis of sex-specific forms of cytochrome P-450 in rat liver is transiently suppressed by hepatic monooxygenase inducers. J Biochem. 1988 Jul;104(1):40–43. doi: 10.1093/oxfordjournals.jbchem.a122418. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Marsden E., Thorgeirsson S. S. Regulation of heme metabolism and cytochrome P-450 levels in primary culture of rat hepatocytes in a defined medium. Biochem Pharmacol. 1984 Feb 15;33(4):565–569. doi: 10.1016/0006-2952(84)90308-3. [DOI] [PubMed] [Google Scholar]
- Fahl W. E., Michalopoulos G., Sattler G. L., Jefcoate C. R., Pitot H. C. Characteristcs of microsomal enzyme controls in primary cultures of rat hepatocytes. Arch Biochem Biophys. 1979 Jan;192(1):61–72. doi: 10.1016/0003-9861(79)90071-7. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mizukami Y., Kawajiri K., Sogawa K., Muramatsu M. Primary structure of a cytochrome P-450: coding nucleotide sequence of phenobarbital-inducible cytochrome P-450 cDNA from rat liver. Proc Natl Acad Sci U S A. 1982 May;79(9):2793–2797. doi: 10.1073/pnas.79.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Spray D. C., Choi H., Saez J. C., Watanabe T., Rosenberg L. C., Hertzberg E. L., Reid L. M. Glycosaminoglycans and proteoglycans induce gap junction expression and restore transcription of tissue-specific mRNAs in primary liver cultures. Hepatology. 1987 Jan-Feb;7(1 Suppl):1S–9S. doi: 10.1002/hep.1840070702. [DOI] [PubMed] [Google Scholar]
- Gotoh O., Tagashira Y., Iizuka T., Fujii-Kuriyama Y. Structural characteristics of cytochrome P-450. Possible location of the heme-binding cysteine in determined amino-acid sequences. J Biochem. 1983 Mar;93(3):807–817. doi: 10.1093/jb/93.3.807. [DOI] [PubMed] [Google Scholar]
- Guzelian P. S., Bissell D. M., Meyer U. A. Drug metabolism in adult rat hepatocytes in primary monolayer culture. Gastroenterology. 1977 Jun;72(6):1232–1239. [PubMed] [Google Scholar]
- Guzelian P. S., Li D., Schuetz E. G., Thomas P., Levin W., Mode A., Gustafsson J. A. Sex change in cytochrome P-450 phenotype by growth hormone treatment of adult rat hepatocytes maintained in a culture system on matrigel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9783–9787. doi: 10.1073/pnas.85.24.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Emi Y., Kawabata S., Omura T. Purification and characterization of three male-specific and one female-specific forms of cytochrome P-450 from rat liver microsomes. J Biochem. 1986 Nov;100(5):1359–1371. doi: 10.1093/oxfordjournals.jbchem.a121842. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Yoshioka H., Sogawa K., Fujii-Kuriyama Y., Omura T. Induction of mRNA coding for phenobarbital-inducible form of microsomal cytochrome P-450 in rat liver by administration of 1,1-Di(p-chlorophenyl)-2,2-dichloroethylene and phenobarbital. J Biochem. 1984 Apr;95(4):949–957. doi: 10.1093/oxfordjournals.jbchem.a134722. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Shinno H., Ichihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J Biol Chem. 1980 Aug 25;255(16):7533–7535. [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nebert D. W., Gielen J. E. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. II. Effects of actinomycin D and cycloheximide on induction processes by phenobarbital or polycyclic hydrocarbons. J Biol Chem. 1971 Sep 10;246(17):5199–5206. [PubMed] [Google Scholar]
- Nielsen P. J., Manchester K. L., Towbin H., Gordon J., Thomas G. The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem. 1982 Oct 25;257(20):12316–12321. [PubMed] [Google Scholar]
- Ozols J., Heinemann F. S., Johnson E. F. The complete amino acid sequence of a constitutive form of liver microsomal cytochrome P-450. J Biol Chem. 1985 May 10;260(9):5427–5434. [PubMed] [Google Scholar]
- Paine A. J., Legg R. F. Apparent lack of correlation between the loss of cytochrome P-450 in hepatic parenchymal cell culture and the stimulation of haem oxygenase activity. Biochem Biophys Res Commun. 1978 Mar 30;81(2):672–679. doi: 10.1016/0006-291x(78)91589-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. A short amino-terminal segment of microsomal cytochrome P-450 functions both as an insertion signal and as a stop-transfer sequence. EMBO J. 1987 Aug;6(8):2425–2431. doi: 10.1002/j.1460-2075.1987.tb02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Sakaguchi M., Mihara K., Omura T. The amino-terminal structures that determine topological orientation of cytochrome P-450 in microsomal membrane. EMBO J. 1990 Aug;9(8):2391–2397. doi: 10.1002/j.1460-2075.1990.tb07414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz E. G., Li D., Omiecinski C. J., Muller-Eberhard U., Kleinman H. K., Elswick B., Guzelian P. S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988 Mar;134(3):309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Song B. J., Gelboin H. V., Park S. S., Yang C. S., Gonzalez F. J. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986 Dec 15;261(35):16689–16697. [PubMed] [Google Scholar]
- Takagi Y., Morohashi K., Kawabata S., Go M., Omura T. Molecular cloning and nucleotide sequence of cDNA of microsomal carboxyesterase E1 of rat liver. J Biochem. 1988 Nov;104(5):801–806. doi: 10.1093/oxfordjournals.jbchem.a122553. [DOI] [PubMed] [Google Scholar]
- Yabusaki Y., Shimizu M., Murakami H., Nakamura K., Oeda K., Ohkawa H. Nucleotide sequence of a full-length cDNA coding for 3-methylcholanthrene-induced rat liver cytochrome P-450MC. Nucleic Acids Res. 1984 Mar 26;12(6):2929–2938. doi: 10.1093/nar/12.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Morohashi K., Sogawa K., Miyata T., Kawajiri K., Hirose T., Inayama S., Fujii-Kuriyama Y., Omura T. Structural analysis and specific expression of microsomal cytochrome P-450(M-1) mRNA in male rat livers. J Biol Chem. 1987 Feb 5;262(4):1706–1711. [PubMed] [Google Scholar]