Abstract
Oxidative stress caused by reactive oxygen species is considered a major mediator of tissue and cell injuries in various neuronal conditions, including neurological emergencies and neurodegenerative diseases. Molecular hydrogen is well characterized as a scavenger of hydroxyl radicals and peroxynitrite. Recently, the neuroprotective effects of treatment with molecular hydrogen have been reported in both basic and clinical settings. Here, we review the effects of hydrogen therapy in acute neuronal conditions and neurodegenerative diseases. Hydrogen therapy administered in drinking water may be useful for the prevention of neurodegenerative diseases and for reducing the symptoms of acute neuronal conditions.
Keywords: hydrogen, central nervous system, neurodegenerative disease, oxidative stress, neuroinflammation
Introduction
Oxidative stress caused by reactive oxygen species (ROS) is a major mediator of tissue and cellular injuries in various neuronal conditions, including neurological emergencies and neurodegenerative diseases.(1–7) Control of oxidative stress is a major therapeutic strategy for various neuronal conditions.(6,8,9) There are many methods for controlling oxidative stress with the use of free radical scavengers being the most common approach.(6,8) Evidence from animal experiments support the notion that free radical scavengers and antioxidants dramatically reduce cerebral damage.(9) Edaravone (MCI-186), a novel free radical scavenger, was developed to prevent lipid peroxidation in pathological neurological conditions.(8,9) Edaravone is currently the only antioxidant drug approved for treating cerebral infarction that improves the functional outcome of ischemic stroke.(8) Brain hypothermia therapy (targeted temperature management) can also effectively control oxidative stress. Brain hypothermia therapy is effective in patients with various acute neuronal diseases.(6,10,11)
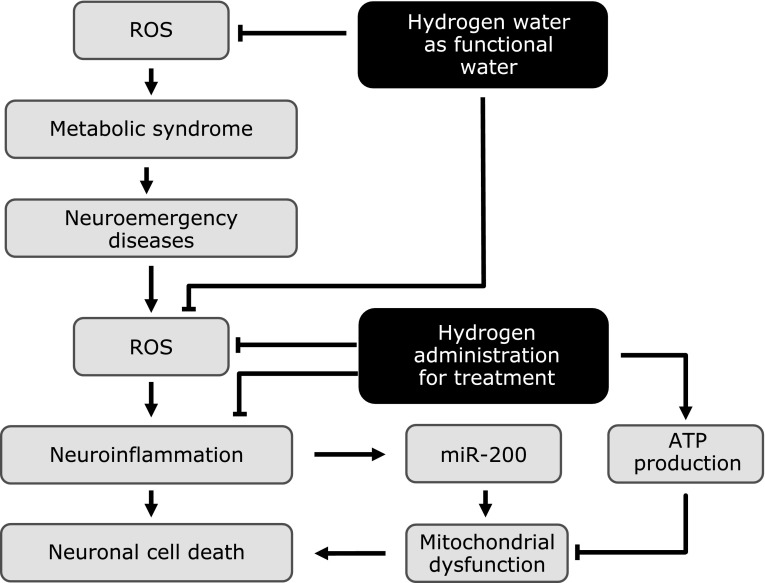
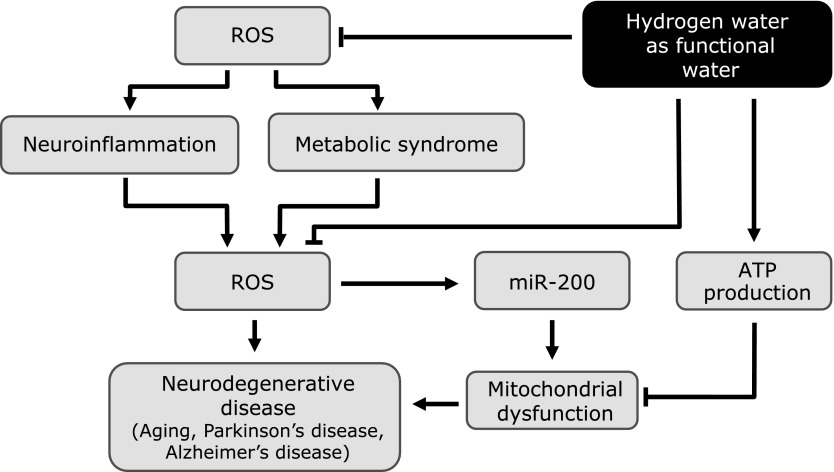
In 2007, Ohsawa et al.(12) reported that molecular hydrogen (H2) can act as an antioxidant to prevent and treat middle cerebral artery occlusion–reperfusion injury in rats. This effect has been supported by additional reports. Recently, the beneficial effect of H2 has been reported in many other organs, including the brain.(13–17) The first major therapeutic effect of H2 was that of an antioxidant, combining with hydroxyl ions to produce water.(12) Recently, other biological mechanisms of H2 (anti-inflammatory, anti-apoptosis, anti-cytokine, DNA expression, and energy metabolism) have been proposed (Fig. 1 and 2).(18) Therefore, the biology of H2 is not simple. In this review, we discuss the role of H2 in various neuronal conditions.
Fig. 1.
Beneficial effects of molecular hydrogen in pathophysiology of various acute neuronal conditions. ATP, adenosine triphosphate; miR-200, microRNA-200; ROS, reactive oxygen species.
Fig. 2.
Effect of consumption of hydrogen-rich water as functional water in pathophysiology of neurodegenerative diseases. ATP, adenosine triphosphate; miR-200, microRNA-200; ROS, reactive oxygen species.
Neurological Diseases
Ischemic brain injury
It has been reported that H2 prevents ischemic brain damage in animal experiments.(12,19–21) Ohsawa et al.(12) reported that inhalation of 2% H2 gas strongly suppressed infarct volume after middle cerebral artery ischemia–reperfusion in rats. In an electron spin resonance (ESR) study, they showed that H2 had hydroxyl radical scavenging activity. Hydroxynonenal (HNE) and 8-hydroxy-2'-deoxyguanosine (8-OHdG) immunoreactivity was suppressed in the damaged brain after treatment with 2% H2. H2 inhalation reduced ischemic damage and hemorrhagic volume after transient middle crebral artery occlusion (MCAO) ischemia.(19) Free radical generation after ischemia induces matrix metalloproteinase (MMP) expression.(19,20) MMP-9 promotes hemorrhagic infarction by disrupting cerebral vessels.(20) H2 inhalation has been found to reduce MMP-9 expression in an MCAO rat model. H2 also has a neuroprotective effect against global ischemia. Ji et al.(21) reported that H2-rich saline injection [5 ml/kg intra-peritoneal (i.p.) administration] after global ischemia reduced neuronal cell death in hippocampal Cornet d'Ammon 1 (CA1) lesions in rats. Cerebral hypoxia–ischemia and neonatal asphyxia are major causes of brain damage in neonates. H2 gas inhalation and H2-rich saline injection provide early neuroprotection from neonatal neurological damage.(22) Nagatani et al.(23) reported that that an H2-enriched intravenous solution is safe for patients with acute cerebral infarction, including patients treated with tissue plasminogen activator (t-PA) therapy.
Metabolic syndrome is a strong risk factor of stroke. It has been reported that H2 therapy can improve metabolic syndrome in basic and clinical settings.(24–27) H2 therapy may reduce stroke in patients with metabolic syndrome involving diabetes mellitus.
Hemorrhagic stroke
Hemorrhagic stroke involving intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) is a critical neuronal condition, and the mortality rate of hemorrhagic stroke is still high.(28–30) Manaenko et al.(28) reported a neuroprotective effect of H2 gas inhalation using an experimental ICH animal model. H2 gas inhalation suppresses redox stress and blood brain barrier (BBB) disruption by reducing mast cell activation and degranulation. Brain edema and neurological deficits were also suppressed. In SAH, there are several studies demonstrating the neuroprotective effect of H2 treatment.(29–31) A clinical trial has started in patients with SAH (Table 1).(32)
Table 1.
Clinical trials of molecular hydrogen in central nervous system (CNS) diseases
| Disease | Hydrogen administration | Reference number |
|---|---|---|
| Subarachnoid hemorrhage | Intravenous infusion | (32) |
| Post cardiac arrest encephalopathy | 2% H2 gas inhalation | (none) |
| Parkinson’s disease | water | (49, 50) |
Traumatic brain injury (TBI)
The efficacy of H2 for treating TBI has been investigated in several studies.(18,33,34) Ji et al.(33) reported that in a rat TBI model, H2 gas inhalation has been found to protect BBB permeability and regulate posttraumatic brain edema, thereby inhibiting brain damage. H2 gas inhalation also inhibits the decrease in superoxide dismutase (SOD) activity and catalase (CAT) activity. These are antioxidant enzymes in posttraumatic brains that inhibit the production of malondialdehyde (MDA) and 8-iso-prostaglandin F2α (8-iso-PGF2α). Eckermann et al.(34) reported that in a surgical trauma mouse model involving right frontal lobectomy, H2 gas inhalation has been found to inhibit postoperative brain edema and improve the postoperative neurobehavioral score. The same report also showed that lipid peroxidation and the production of oxidative stress substances were not inhibited by H2 gas inhalation.(34) The therapeutic effect of H2-rich water following TBI and in posttraumatic onset of Alzheimer’s disease (AD) was investigated by Dohi et al. in 2014,(18) who investigated whether the consumption of H2-rich water 24 h prior to trauma can inhibit neuronal damage in a controlled cortical injury model using mice. The authors found that the expression of the phosphorylated tau proteins AT8 and Alz50 in the hippocampus and cortex was blocked in mice that consumed H2-rich water. Moreover, the activity of astrocytes and microglia were inhibited in mice TBI model consuming H2-rich water. The expression of genes induced by TBI, particularly those that are involved in oxidation/carbohydrate metabolism, cytokine release, leukocyte or cell migration, cytokine transport, and adenosine triphosphate (ATP) and nucleotide binding, was inhibited by consuming H2-rich water. Dohi et al.(18) specifically reviewed the role of H2-rich water in neuroinflammation following brain trauma. The consumption of H2-rich water influenced the production of cytokines and chemokines in the damaged brain and inhibited the production of hypoxia inducible factor-1 (HIF-1), MMP-9, and cyclophilin A. However, H2-rich water did not affect the production of amyloid precursor protein (APP), Aβ-40, or Aβ-42. They also investigated the relationship between H2 and ATP production and reported that H2 increased basal respiration, reserve capacity, and nonmitochondrial respiration but did not increase aerobic ATP production. It has thus been demonstrated that the inhibitory effects of H2 on nerve damage are not solely due to its simple function as a free radical scavenger (Fig. 1 and 2).
Spinal cord injury
Chen et al.(35) reviewed the effects of H2-rich saline administration (i.p.) in a rat traumatic spinal cord injury model. They found that posttraumatic neurological symptoms were improved by H2-rich saline treatment. Furthermore, H2-rich saline treatment has been found to reduce inflammatory cell infiltration, TdT-mediated dUTP nick and labeling (TUNEL)-positive cells, and hemorrhage. In addition, oxidative stress was inhibited and the expression of brain derived neurotrophic factor (BDNF) was increased. The effects of H2 administration on spinal cord ischemia have also been reported.(36,37) Huang et al.(36) investigated the effects of H2 gas inhalation in a rabbit spinal cord ischemia–reperfusion model. They reviewed the effects of H2 inhalation with different concentrations (1, 2, and 4%) and reported that H2 gas inhalation at concentrations of 2% and 4% inhibited neuronal death. However, they did not observe significant differences between the two groups in terms of effects with 2% and 4% being equally effective.(36) It has been reported that the inhalation of 2% H2 gas inhibits apoptosis following spinal cord injury caused by ischemia–reperfusion. In addition, H2 gas inhalation regulates caspase-3 activity, the production of inflammatory cytokines, oxidative stress, and the decrease in endogenous antioxidant substances. Zhou et al.(37) also reported that H2-rich saline administration (i.p.) has beneficial effects on spinal cord ischemia–reperfusion injury in rabbits.
Other acute neurological conditions
In recent years, research has shown that there is a high incidence of comorbid central nervous system symptoms in sepsis cases.(38) Using a mice cecal ligation and puncture (CLP) model, Liu et al.(39) reported that H2 gas inhalation improves septic encephalopathy. They reported that 2% H2 gas inhalation inhibited post-CLP apoptosis, brain edema, BBB permeability, cytokine production, and oxidative stress in the CA1 hippocampus region as well as improves cognitive function. Nakano et al.(40) reported that maternal administration of H2 has a suppressive effect on fetal brain injury caused by intrauterine inflammation with maternal intraperitoneal injection of lipopolysaccharide (LPS).
The treatment of carbon monoxide (CO) poisoning encephalopathy, which is a common gas poisoning, is yet to be established.(41,42) Sun et al.(42) and Shen et al.(41) investigated the effects of H2-rich saline. They reported that in a CO poisoning model, the administration of H2-rich saline decreased glial activation, cytokine production, oxidative stress, and caspase 3 and 9 production as well as inhibited nerve cell death.
It is known that stress causes nerve cell impairments.(43) The consumption of H2-rich water inhibits oxidative stress and thereby inhibits the onset of stress-induced brain damage.(43)
Hypoxic brain injury caused by asphyxiation, hypoxic ischemic encephalopathy, neonatal asphyxia, and other similar hypoxia-mediated event is a common clinical condition in medical emergencies. H2 treatment has been found to inhibit cell death in an in vitro hypoxia/reoxygenation model using immortalized mouse hippocampal (HT-22) cells. H2 treatment increased phosphorylated Akt (p-Akt) and B-cell leukemia/lymphoma-2 (BCL-2), while it decreased Bax and cleaved caspase-3.(44) In recent years, it has been found that the microRNA-200 (miR-200) family regulates oxidative stress.(44) The inhibition of miR-200 suppresses H/R-induced cell death, reducing ROS production and MMP. H2 treatment suppressed H/R-induced expression of miR-200. In Japan, a double blind randomized controlled trial for post cardiac arrest syndrome has started from 2017 (Table 1).
Neurodegenerative Diseases
Parkinson’s disease (PD)
PD is a disorder that presents with extrapyramidal symptoms caused by the degeneration and loss of dopamine-producing cells in substantia nigra. Oxidative stress is known to be involved in the clinical condition of PD.(7) Moreover, the involvement of mitochondrial dysfunction in PD has been reported.(45) The effects of H2 on PD have been reported in animal models of PD as well as in clinical studies.(46–48) In 2009, Fujita et al.(47) and Fu et al.(48) reported that consuming H2-rich water inhibits oxidative stress on the nigrostriatal pathway and prevents the loss of dopamine cells in a PD animal model. With the consumption of H2-rich-water-drinking, oxidative stress in the nigrostriatal pathway was inhibited and loss of dopamine cells was decreased. These results suggest that consuming H2-rich water could affect the onset of PD. In recent years, the results of a clinical trial on the effects of consuming H2-rich water for PD have been reported.(49) A randomized double-blind study showed that consuming H2-rich water (1,000 ml/day) for 48 weeks significantly improved the total Unified Parkinson’s Disease Rating Scale (UPDRS) score of PD patients treated with levodopa. A double-blind multi-center trial of H2 water is currently underway (Table 1).(50)
Alzheimer’s disease (AD)
AD, an age-related neurodegenerative disease, is the most common cause of dementia.(1,51) Pathologically, it is characterized by the deposition of Aβ protein outside nerve cells and the accumulation of phosphorylated tau protein inside nerve cells. There is also a marked loss of nervous cells in the cerebral cortex.(52) In recent years, oxidative stress and neuroinflammation have been reported to be involved in AD.(1,5) To date, reports have centered on the involvement of oxidative stress in brain parenchyma.(1,51,53) The accumulation of Aβ protein is strongly associated with the failure of Aβ clearance that is closely related to the pathogenesis of AD.(5) It is known that low-density lipoprotein receptor-related protein 1 (LRP1) is involved in Aβ protein elimination. LRP dysfunction caused by oxidative stress and neuroinflammation is involved in the onset of AD.(5) The regulation of oxidative stress and neuroinflammation may prevent the onset or progression of AD. A number of reports have investigated the effects of H2 for the prevention of AD onset.(51,53) In a rat AD model, it has been reported that the administration of H2-rich saline (5 ml/kg, i.p., daily) inhibited oxidative stress, cytokine production, and nuclear factor-κB (NF-κB) production in the hippocampus and cerebral cortex, and improved impaired memory.(51,53) It has also been reported that consuming H2-rich water inhibits age-related brain alterations and spatial memory decline.(54)
Method and Route of Administration in H2 Therapy
As a small (2 Da), uncharged molecule H2, would be expected to readily distribute throughout the body, including being able to easily penetrate cell membranes, However we are unable to determine the distribution of H2 among organs and its concentrations in each organ and serum based on the administration methods and dosage. This problem was investigated in 2014.(55) A comparative review was conducted on the consumption of H2-rich water, i.p. or intravenous administration of H2-rich saline, and inhalation of H2 gas. The results showed that the highest concentrations are reached 1 min after intravenous administration and 5 min after oral administration. The highest concentration was reached 30 min after the inhalation of H2 gas and was maintained for some time. Although H2 concentrations in the brain tend to be high after either intravenous administration or inhalation, no significant differences have been observed in comparison with the concentrations after the consumption of H2-rich water and i.p. administration of H2-rich saline. Thus, although there have been variations based on the administration method, all methods have been found to result in the presence of H2 in the serum and brain tissue. Liu et al.(39) measured H2 levels in the arteries, veins, and brain tissues after the inhalation of 2% H2 gas. They found that arterial H2 peaked at 30 min after administration, whereas venous and brain tissue H2 peaked at 45 min after administration. They reported that H2 levels were similar in arteries and brain tissues. This demonstrated that H2 migrates to the brain tissue regardless of the method of administration. These results suggest that the consumption of H2-rich water prevents neurodegenerative disease and that H2-rich drinking water could be used to treat acute brain disorders (Fig. 1 and 2).
Conclusions
We have examined the effects of H2 treatment on acute central nervous system diseases and on chronic neurodegenerative diseases. We have also examined the various mechanism by which H2 exerts its neuroprotective effects H2 acts as a scavenger for OH− and ONOO−, affects neuroinflammation, preserves mitochondrial energy production, and possesses neuroprotective properties. Unlike more conventional drugs, H2 treatment, particularly the consumption of H2-rich water, has no known serious side effects and is effective for preventing the onset of neurodegenerative disease and aggravation of acute neuronal conditions.
Acknowledgments
Many People have made contributions to this review. We thank for their contributions. First, we wish to thank the member of our laboratory members, and we also wish to thank the Society of Free Radical Research Japan for their thoughtful suggestions and contributions. This work was supported by JSPS KAKENHI Grant Numbers JP 23592683, JP26462769.
Abbreviations
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ATP
adenosine triphosphate
- BBB
blood brain barrier
- CA1
Cornet d'Armon 1
- CLP
cecal ligation and puncture
- CO
carbon monoxide
- ICH
intracerebral hemorrhage
- LRP
lipoprotein receptor-related protein
- MCAO
middle cerebral artery occlusion
- miR-200
microRNA-200
- MMP
matrix metalloproteinase
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- TBI
traumatic brain injury
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4:519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohi K, Ohtaki H, Nakamachi T, et al. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 4.Gaetani P, Pasqualin A, Rodriguez y Baena R, Borasio E, Marzatico F. Oxidative stress in the human brain after subarachnoid hemorrhage. J Neurosurg. 1998;89:748–754. doi: 10.3171/jns.1998.89.5.0748. [DOI] [PubMed] [Google Scholar]
- 5.Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19:121–130. doi: 10.1159/000330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohi K, Miyamoto K, Fukuda K, et al. Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxid Med Cell Longev. 2013;2013:562429. doi: 10.1155/2013/562429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohi K, Satoh K, Mihara Y, et al. Alkoxyl radical-scavenging activity of edaravone in patients with traumatic brain injury. J Neurotrauma. 2006;23:1591–1599. doi: 10.1089/neu.2006.23.1591. [DOI] [PubMed] [Google Scholar]
- 9.Dohi K, Satoh K, Nakamachi T, et al. Does edaravone (MCI-186) act as an antioxidant and a neuroprotector in experimental traumatic brain injury? Antioxid Redox Signal. 2007;9:281–287. doi: 10.1089/ars.2007.9.281. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko T, Kasaoka S, Nakahara T, et al. Effectiveness of lower target temperature therapeutic hypothermia in post-cardiac arrest syndrome patients with a resuscitation interval of ≤30 min. J Intensive Care. 2015;3:28. doi: 10.1186/s40560-015-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silveira RC, Procianoy RS. Hypothermia therapy for newborns with hypoxic ischemic encephalopathy. J Pediatr (Rio J) 2015;91 (6 Suppl 1):S78–S83. doi: 10.1016/j.jped.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 13.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Terasaki Y, Ohsawa I, Terasaki M, et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011;301:L415–L426. doi: 10.1152/ajplung.00008.2011. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Li B, Liu C, et al. Hydrogen-rich saline protects immunocytes from radiation-induced apoptosis. Med Sci Monit. 2012;18:BR144–BR148. doi: 10.12659/MSM.882616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng K, Huang H, Jiang XQ, Chen XJ, Huang W. Protective effects of hydrogen on renal ischemia/reperfusion injury in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:39–42. (in Chinese) [PubMed] [Google Scholar]
- 17.Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohi K, Kraemer BC, Erickson MA, et al. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PLoS One. 2014;9:e108034. doi: 10.1371/journal.pone.0108034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CH, Manaenko A, Zhan Y, et al. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 21.Ji Q, Hui K, Zhang L, Sun X, Li W, Duan M. The effect of hydrogen-rich saline on the brain of rats with transient ischemia. J Surg Res. 2011;168:e95–e101. doi: 10.1016/j.jss.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Domoki F, Oláh O, Zimmermann A, et al. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr Res. 2010;68:387–392. doi: 10.1203/PDR.0b013e3181f2e81c. [DOI] [PubMed] [Google Scholar]
- 23.Nagatani K, Nawashiro H, Takeuchi S, et al. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med Gas Res. 2013;3:13. doi: 10.1186/2045-9912-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song G, Li M, Sang H, et al. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res. 2013;54:1884–1893. doi: 10.1194/jlr.M036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajiyama S, Hasegawa G, Asano M, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome—an open label pilot study. J Clin Biochem Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto M, Katakura M, Nabika T, et al. Effects of hydrogen-rich water on abnormalities in a SHR.Cg-Leprcp/NDmcr rat - a metabolic syndrome rat model. Med Gas Res. 2011;1:26. doi: 10.1186/2045-9912-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J. Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Crit Care Med. 2013;41:1266–1275. doi: 10.1097/CCM.0b013e31827711c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang Z, Zhou ML, You WC, et al. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012;13:47. doi: 10.1186/1471-2202-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang Z, Sun XJ, Zhang X, et al. Nuclear factor-κB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. doi: 10.1002/jnr.23281. [DOI] [PubMed] [Google Scholar]
- 31.Hong Y, Shao A, Wang J, et al. Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3β signaling pathway. PLoS One. 2014;9:e96212. doi: 10.1371/journal.pone.0096212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi S, Mori K, Arimoto H, et al. Effects of intravenous infusion of hydrogen-rich fluid combined with intra-cisternal infusion of magnesium sulfate in severe aneurysmal subarachnoid hemorrhage: study protocol for a randomized controlled trial. BMC Neurol. 2014;14:176. doi: 10.1186/s12883-014-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji X, Liu W, Xie K, et al. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2010;1354:196–205. doi: 10.1016/j.brainres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Eckermann JM, Chen W, Jadhav V, et al. Hydrogen is neuroprotective against surgically induced brain injury. Med Gas Res. 2011;1:7. doi: 10.1186/2045-9912-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Chen Q, Mao Y, et al. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem Res. 2010;35:1111–1118. doi: 10.1007/s11064-010-0162-y. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Xie K, Li J, et al. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2011;1378:125–136. doi: 10.1016/j.brainres.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Wang X, Xue W, et al. Beneficial effects of hydrogen-rich saline against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2013;1517:150–160. doi: 10.1016/j.brainres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Xie K, Chen H, et al. Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Res. 2014;1589:78–92. doi: 10.1016/j.brainres.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Nakano T, Kotani T, Mano Y, et al. Maternal molecular hydrogen administration on lipopolysaccharide-induced mouse fetal brain injury. J Clin Biochem Nutr. 2015;57:178–182. doi: 10.3164/jcbn.15-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen MH, Cai JM, Sun Q, et al. Neuroprotective effect of hydrogen-rich saline in acute carbon monoxide poisoning. CNS Neurosci Ther. 2013;19:361–363. doi: 10.1111/cns.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Q, Cai J, Zhou J, et al. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. Crit Care Med. 2011;39:765–769. doi: 10.1097/CCM.0b013e318206bf44. [DOI] [PubMed] [Google Scholar]
- 43.Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009;34:501–508. doi: 10.1038/npp.2008.95. [DOI] [PubMed] [Google Scholar]
- 44.Wei R, Zhang R, Xie Y, Shen L, Chen F. Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress. Cell Physiol Biochem. 2015;36:585–598. doi: 10.1159/000430122. [DOI] [PubMed] [Google Scholar]
- 45.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 46.Ito M, Hirayama M, Yamai K, et al. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med Gas Res. 2012;2:15. doi: 10.1186/2045-9912-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita K, Seike T, Yutsudo N, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS One. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu Y, Ito M, Fujita Y, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–839. doi: 10.1002/mds.25375. [DOI] [PubMed] [Google Scholar]
- 50.Yoritaka A, Abe T, Ohtsuka C, et al. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: protocol and baseline characteristics. BMC Neurol. 2016;16:66. doi: 10.1186/s12883-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Li J, Liu Q, et al. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci Lett. 2011;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 54.Gu Y, Huang CS, Inoue T, et al. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J Clin Biochem Nutr. 2010;46:269–276. doi: 10.3164/jcbn.10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Kurokawa R, Fujino M, Hirano S, Sato B, Li XK. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep. 2014;4:5485. doi: 10.1038/srep05485. [DOI] [PMC free article] [PubMed] [Google Scholar]