Abstract
Embryonic development is an exceptionally dynamic process, requiring a provisional extracellular matrix that is amenable to rapid remodeling, and proteolytic or non-proteolytic mechanisms that can remodel the major components of this matrix. Versican is a chondroitin-sulfate proteoglycan that forms highly hydrated complexes with hyaluronan and is widely distributed in the provisional matrix of mammalian embryos. It has been extensively studied in the context of cardiovascular morphogenesis, neural crest cell migration and skeletal development. Analysis of Vcan transgenic mice has established the requirement for versican in cardiac development and its role in skeletogenesis. The ADAMTS family includes several versican-degrading proteases that are active during remodeling of the embryonic provisional matrix, especially during sculpting of versican-rich tissues. Versican is cleaved at specific peptide bonds by ADAMTS proteases, and the cleavage products are detectable by neo-epitope antibodies. Myocardial compaction, closure of the secondary palate (in which neural crest derived cells participate), endocardial cushion remodeling, myogenesis and interdigital web regression are developmental contexts in which ADAMTS-mediated versican proteolysis has been identified as a crucial requirement. ADAMTS proteases are expressed coordinately and function cooperatively in many of these contexts. In addition to versican clearance, ADAMTS proteases generate a bioactive versican fragment containing the N-terminal G1 domain, which we have named versikine. This review promotes the view that the embryonic extracellular matrix has evolved not only to provide a permissive environment for embryo growth and morphogenesis, but through its dissolution to influence and regulate cellular processes.
Keywords: A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs, ADAMTS, Versican, Embryogenesis, Soft tissue syndactyly, Cardiac jelly, Heart valve, Limb development, Cleft palate, Melanoblast
1. Introduction
The vertebrate embryonic extracellular matrix (ECM) is compositionally, organizationally and mechanically distinct from the extensively specialized and mechanically robust ECMs of adult tissues. It is a provisional or temporary matrix, such as that present during the early stages of wound healing. Because of extensive and rapid cell migration, folding, branching and sculpting of tissues required for embryogenesis, a mature or adult ECM, such as one comprising a well-established collagenous structure, could be an impediment to successful development. Instead, the provisional matrix is enriched in hyaluronan, fibronectin and proteoglycans such as versican, rendering it highly hydrated and malleable. mRNA expression of versican, the focus of this review, is the highest during embryogenesis and is much reduced in most adult tissues (Naso et al., 1995), which are more cellular, contain more collagen, elastin and other ECM components, but, with the exception of the brain, relatively little versican. It could be speculated that the embryonic provisionalmatrix evolved to support morphogenesis, and consequently, required the evolution of specific mechanisms to dissolve it during tissue sculpting to achieve the mature form, or prior to replacement by specialized ECM. This model has a precedent in the requirement for tissue collagenase in rapid collagen remodeling during resorption of the vestigial tail and fin, a defining event in amphibian metamorphosis (Gross and Lapiere, 1962). By analogy, it could be argued that specific proteolytic activities are necessary for digesting the relatively collagen-poor provisional matrix during mammalian embryogenesis. This review will focus on the developmental role of versican and the mechanisms of its clearance during embryogenesis. Indeed, one purpose of this reviewis to emphasize the extraordinarily broad, yet specific role of ADAMTS proteases in versican removal during morphogenesis. Coordinated evolution of provisional matrix with specific proteolysis mechanisms that remodel it, and especially, the existence of optimized temporal and spatial relationships of the protease and substrate (using versican as a model) is a key concept underlying this review.
2. Structure and genetics of versican
Versican (synonyms: PG-M, CSPG2), was identified in the culture medium of metabolically labeled fibroblasts (Coster et al., 1979), initially characterized as a proteoglycan from chick limb buds and first cloned from a human placenta cDNA library (Kimata et al., 1986; Zimmermann and Ruoslahti, 1989). It is a chondroitin sulfate (CS) proteoglycan belonging to a family of hyaluronan (HA) binding proteins (also referred to as hyalectans or lecticans) that includes aggrecan, brevican and neurocan (Yamaguchi, 2000). Versican is found in vertebrates, but not in invertebrate model organisms such as Caenorhabditis elegans and Drosophila melanogaster (Ismat et al., 2013).
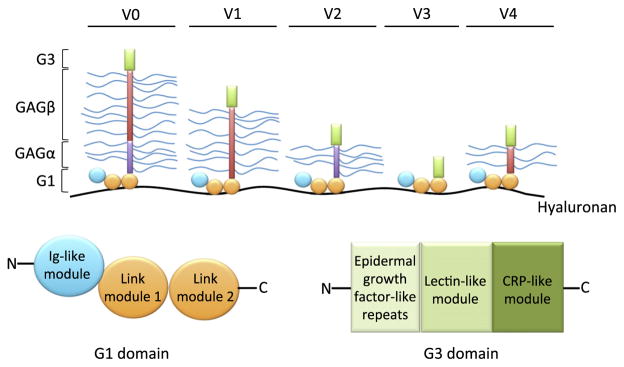
Vcan is located on human chromosome 5q (the mouse gene, Vcan is on chromosome 13) and contains 15 exons (Iozzo et al., 1992). It comprises three major domains with distinct functions: an N-terminal G (globular) 1 domain, which mediates HA binding through two link protein-like modules (link modules, Fig. 1), one or both of two alternatively spliced, extended glycosaminoglycan (GAG) attachment domains (GAGα and GAGβ), and a C-terminal G3 domain (Zimmermann and Ruoslahti, 1989; Wight, 2002). The versican–HA complex may be further stabilized by link protein, but link protein appears not to be essential for formation of the complex. Versican participates in several intermolecular interactions through its G3 domain, such as with fibrillins, fibulins, tenascin-R, fibronectin and integrin β1, as well as with cytokines, selectins, apolipoproteins and CD44 via the GAG-chains as previously reviewed (Wu et al., 2005). Four versican splice variants result from alternate splicing of exons 7 and 8, encoding the GAGα and GAGβ domains, respectively (Fig. 1). Versican V0 contains both GAG attachment exons and is the largest isoform, containing up to 23 CS chains. Versican V1 contains only exon 8 and has up to 15 CS chains, versican V2 contains only exon 7 and has up to 8 GAG attachment sites, whereas versican V3 does not contain either large exon and thus lacks CS chains (Dours-Zimmermann and Zimmermann, 1994; Zako et al., 1995). A V4 variant mRNA, predicting a truncated GAGβ domain from utilization of a cryptic splice site in exon 8, has 5 predicted CS attachment sites, and was recently identified in human breast cancer (Kischel et al., 2010). Versican isoforms V0 and V1 are expressed in the developing heart and limb, vascular smooth muscle cells, fibroblasts, and many non-neural cells, whereas versican V2 and V0 are the predominant isoforms in the nervous system (Schmalfeldt et al., 1998; Evanko et al., 1999; Lemire et al., 1999; Zimmermann and Dours-Zimmermann, 2008).
Fig. 1.
Domain structure of versican splice variants: Each versican variant shown binds to hyaluronan through the G1 domain, and has additional interactions through the G3 domain. As shown, inclusion of the alternatively spliced CS-bearing GAGα (violet) and GAGβ (red) regions defines distinct splice variants. CS-chains are shown as wavy blue lines. The cartoons at the bottom define the subdomains identified within the G1 and G3 domains.
Wagner syndrome (OMIM: 143200) and erosive vitreoretinopathy are allelic autosomal dominant eye disorders caused by Vcan mutations. These mutations cluster around the consensus splice sites flanking exon 8, and are thought to lead to exon skipping. This removes the GAGβ domain, and reduces the proportion of V0 and V1 isoforms, in relation to V2 and V3 isoforms (Kloeckener-Gruissem et al., 2006; Mukhopadhyay et al., 2006; Brezin et al., 2011). Clinically, these disorders result in an optically empty vitreous cavity but appear to have no extra-ocular/systemic effects (Meredith et al., 2007).
3. ADAMTS proteolysis of versican
Most analyses of ECM proteolysis focus on matrix metalloproteinases (MMPs), which are thought to have central roles in ECM turnover. Although MMPs can degrade versican (Perides et al., 1995; Halpert et al., 1996; Barascuk et al., 2013), none of the observed developmental defects in MMP-deficient model systems have been attributed to defective versican proteolysis. In contrast, based on several interesting developmental outcomes of ADAMTS gene mutagenesis, much interest in recent years has focused on ADAMTS proteases. Like MMPs, ADAMTS proteases are zinc-dependent multi-domain proteinases and have an N-terminal domain organization that includes a propeptide and a zinc/calcium metalloprotease domain with a characteristic cysteine signature (Apte, 2009). A hallmark of ADAMTS proteases is their C-terminal ancillary domain, which has a characteristic domain structure containing one or more thrombospondin type 1 motifs. Of the 19 members of the ADAMTS family, 6 have been identified as proteoglycanases and are capable of cleaving versican; these include ADAMTS1, 4, 5, 9, 15, and 20 (Sandy et al., 2001; Somerville et al., 2003; Longpre et al., 2009; Dancevic et al., 2013). ADAMTS9 and ADAMTS20 are highly homologous, and ADAMTS1, -4, -5 and -15 form an evolutionarily related cluster. Investigations into the mechanism of aggrecan proteolysis by ADAMTS proteoglycanases predicted consensus cleavage sites in the versican GAGα and GAGβ domains, i.e., two sites in versican V0; Glu405–Gln406 located in GAGα and Glu1428–Ala1429 in GAGβ (all human sequence enumerations), and the corresponding single sites in the V1 and V2 isoforms, Glu441–Ala442 and Glu405–Gln406 respectively (Sandy et al., 2001; Westling et al., 2004). These were experimentally validated both in vivo and in vitro using neoepitope antibodies to the predicted new C-terminal sequences resulting from cleavage, anti-NIVSFE405 (human V2 sequence enumeration) and anti-DPEAAE441 (human V1 sequence enumeration) (Sandy et al., 2001; Westling et al., 2004). These antibodies, particularly anti-DPEAAE441, have been exceptionally useful in identifying versican processing during embryogenesis. Several other cleavage sites have been predicted using peptide fragments, such as Glu950–Gly951 (in the V2 isoform), and Tyr423–Ile424 (in the V1 isoform) (human sequence enumeration) but are not yet validated as being physiologically relevant (Jonsson-Rylander et al., 2005). The molecular interactions between versican and ADAMTS proteases are not fully understood, but ongoing studies suggest a major role for the CS chains of versican in binding to an ADAMTS protease (Foulcer et al., manuscript in preparation).
4. Major developmental contexts and functions for versican
4.1. Versican and cardiac development
The microenvironment for myocardial growth and trabeculation is provided by cardiac jelly, which is enriched in hyaluronan and versican, and located between the endocardium and developing myocardium (Stankunas et al., 2008; Gittenberger-de Groot et al., 2013). The cardiac developmental role of Vcan was first elucidated in the Vcanhdf (hdf, heart defect)mutant, in which transgene insertion disrupted Vcan, resulting in loss of all versican splice variants, i.e., a null allele (Yamamura et al., 1997; Mjaatvedt et al., 1998). Vcan expression via a LacZ reporter in this transgene showed expression from E8.0 in the heart field region that generates the right ventricle and conus cordis. Vcanhdf/hdf embryos did not survive past 10.5 days of gestation (E10.5), were small, with defects of the anterior segment of the primordial heart, and the primitive right ventricle was abnormal and lacked an outflow track (Yamamura et al., 1997; Mjaatvedt et al., 1998). In addition to the myocardium, Vcan is expressed during formation of the endocardial cushions that are the precursors of the heart valve leaflets (Henderson and Copp, 1998). In Vcanhdf/hdf embryos, these cardiac jelly and mesenchyme-containing swellings between the endocardium and the myocardium do not form (Markwald et al., 1981; Mjaatvedt et al., 1998). Since explants of valve primordia of Vcanhdf/hdf embryos can form endothelium-transformed mesenchyme, a defect in matrix expansion of the cardiac cushions, which are enriched in versican, was implicated. Indeed, versican, HA and link protein are each expressed in the expanding cushions, suggesting they form a functional complex required for cushion expansion. Hyaluronan synthase 2 (Has2) null embryos have a similar cardiac phenotype as Vcanhdf/hdf embryos, suggesting that a hyaluronan–versican composite matrix is crucial during heart development in mice (Camenisch et al., 2000).
That this crucial composite ECM likely takes the form of a HA–versican complex was bolstered by a gene-targeted allele (VcanΔ3), from which a mutant versican with reduced HA binding was produced (Hatano et al., 2012). VcanΔ3/Δ3 mice in the congenic C57BL/6 background died around E10.5, like Vcanhdf/hdf embryos mice, with defective endocardial cushion development, whereas some VcanΔ3/Δ3 embryos survived longer in a mixed genetic background, and had a ventricular septal defect. This suggests that disruption of the HA–versican interaction, albeit concomitant with reduction of Vcan expression, could lead to heart defects.
In zebrafish, versican (Cspg2) is expressed in the AV canal by both endocardial and myocardial cells of the valve primordia (Walsh and Stainier, 2001; Hurlstone et al., 2003). Treatment of zebrafish embryos with Amiodarone, an anti-arrhythmia drug, caused a massive ectopic upregulation of versican in the atrium, the ventricle and the AV canal and resulted in a loss of endocardial cushion formation in developing embryos that was restored by morpholino-mediated knockdown of versican (Chen et al., 2012). This suggested that tight regulation of versican expression was crucial for normal heart development. A similar outcome arose from miR-138 knockdown in zebrafish embryos (Morton et al., 2008). miR-138 is critical for the chamber-specific expression of versican, and restricts versican expression to the AV canal region via Raldh2-mediated retinoic acid signaling, while repressing the Cspg2 transcript in the ventricles. The mouse versican 3′ UTR contains a miR-138 responsive binding site and Vcan transcript and protein levels are reduced when miR-138 is introduced in NIH 3T3 cells (Morton et al., 2008).
During in-vitro differentiation of human embryonic stem cells into cardiomyocytes, dynamic HA synthesis and versican expression were reported (Chan et al., 2010). Ventricular myocardial cells underwent terminal differentiation concurrently with versican downregulation during development, implicating versican in regulating myocardial cell proliferation and differentiation (Henderson and Copp, 1998). Collectively these studies demonstrate that versican is essential for normal heart development, regulating heart tube segmentation, endocardial cushion formation, ventricular septation, and myocardial differentiation. The literature thus clearly indicates that tight temporal and site-specific regulation of versican expression is required for normal heart development.
4.2. Versican in neural crest cell migration
Upon closure of the neural tube, streams of neural crest cells (NCCs) migrate from the ectoderm toward the peripheral target sites via specific migratory routes (Mayor and Theveneau, 2013). For example, melanoblasts take a dorso-lateral path to their eventual destination in the skin. It is believed that versican-rich areas are non-permissive tracts/barriers for NCC migration whereas fibronectin and laminin provide pro-migratory substrates for these cells (Landolt et al., 1995; Dutt et al., 2006). However, there is also evidence from chick embryos, using versican V0/V1 immobilized on transplantable micromembranes, that some migratory NCCs may be attracted toward versican-rich matrices (Perissinotto et al., 2000).
One of the three pathways of NCC migration is directly through developing sclerotomes. In chick embryos, versican V0/V1 isoforms are present at a higher density in the caudal halves of each sclerotome (Dutt et al., 2006), whereas NCC migrates through the rostral sclerotome (Serbedzija et al., 1989). A similar rostral sclerotome migratory path has been demonstrated during mouse embryogenesis (Serbedzija et al., 1990), suggesting that versican present in the caudal sclerotome may lead to preferred rostral sclerotome migration by NCCs. Direct evidence for versican as a barrier for NCC migration came from in-vitro stripe-choice assays, utilizing stripes coated with different substrates. Preferential migration of NCC through stripes composed of fibronectin and laminin was observed in these assays and may reflect expression of pro-migratory adhesion molecules such as integrins, which are vital for NCC migration (Lallier and Bronner-Fraser, 1993; Kil et al., 1996). A recent study shows that peripheral axon guidance during chick hind limb development is also dependent on an inhibitory role by versican V0/V1 isoforms, similar to NCC migration (Dutt et al., 2011).
Additional evidence supporting versican as a negative regulator for NCC migration in mice came from the Pax3 Splotch mutant (Pax3Sp). Pax3Sp/Sp embryos have upregulated Vcan mRNA and exhibit anomalies such as pigmentation defects, cardiac outflow tract septation defects, and absent dorsal root ganglia, all indicative of NCC compromise (Tremblay et al., 1995; Conway et al., 1997a,b; Henderson et al., 1997). Pax3Sp/Sp NCCs migrate normally when surrounded by wild type ECM (Mansouri et al., 2001), supporting the possibility that Vcan upregulation may in part be responsible for defective NCC migration observed in these embryos. Pax3 and Vcan show mutually exclusive expression domains, but when Pax3 is lost, the Vcan expression domain expands along the NCC migratory path, suggesting that Pax3 directly or indirectly regulates Vcan expression (Henderson et al., 1997).
In Xenopus laevis, the versican V0 isoform is highly restricted to the dorsal organizer and the neural plate cells during development (Casini et al., 2008), potentially playing important developmental roles in these tissues during gastrulation and neurulation, respectively. Vcan expression is intimately associated with hair follicle development (Kishimoto et al., 1999) and may have a role in formation and maintenance of these and other integumentary structures, in addition to melanoblast colonization of hair follicles.
4.3. Versican in limb development
The appendicular skeleton is formed from cartilage anlagen that are preceded by mesenchymal condensations. Versican V0 and V1 are expressed in early limb bud mesenchyme, and upregulated in prechondrogenic mesenchymal condensations (Kamiya et al., 2006; Shepard et al., 2008). Chondrogenesis is severely compromised in micromass cultures established from Vcanhdf/hdf limb bud mesenchyme (Williams et al., 2005). Following chondrogenesis, versican persists in the perichondrium around the cartilage anlagen, which now strongly upregulate aggrecan expression, and in the prospective joint interzones (Shibata et al., 2001; Capehart, 2010; Nagchowdhuri et al., 2012). Mice with conditional Vcan deletion in limb mesenchyme developed distal limb anomalies, including tilted joints, clefts in proximal phalanges, delayed chondrocyte differentiation in distal skeletal elements and abnormal nests of hypertrophic chondrocytes (Choocheep et al., 2010). The joint interzones did not bind TGFβ in the absence of versican, suggesting that versican enabled proper localization of TGFβ in the limb mesenchyme.
5. Developmental significance of versican processing
The developmental significance of versican processing by ADAMTS proteases has been hitherto uncovered at the sites of the most dramatic sculpting of the provisional matrix, namely regression of interdigital webs, sculpting, redirection and migration of the secondary palate shelves prior to their midline fusion, resorption of cardiac jelly during myocardial compaction, and remodeling of endocardial cushions to form mature heart valve leaflets. These findings complement the developmental contexts described above in which versican is functionally significant, and correlate with high expression levels of one or more ADAMTS proteases. These studies elucidate the developmental significance of ADAMTS proteases and illustrate how proteolysis of versican deposited in the early embryo could be a regulator of morphogenetic processes during subsequent development, upon expression of these proteases.
5.1. ADAMTS proteases are essential for versican processing in cardiac development
During myocardial growth and trabeculation, endocardial ADAMTS1 expression is repressed by a transcriptional complex, which includes Brg1, a chromatin-remodeling protein (Stankunas et al., 2008). Once myocardial growth is achieved, ADAMTS1 is de-repressed to stifle further growth and achieve compaction of the developing myocardium. In mice with Brg1 inactivation, ADAMTS1 was upregulated and implicated in premature resorption of versican in the cardiac jelly, leading to an abnormally early termination of myocardial trabeculation (Stankunas et al., 2008). E13.5 Adamts1−/− embryos had greater trabeculation than wild-type littermates, further supporting a physiological role for ADAMTS1 in myocardial compaction. Subsequent work showed that in mice lacking fibulin-1, an ADAMTS cofactor, versican proteolysis was reduced, leading to increased trabecular cardiomyocyte proliferation (Lee et al., 2005; McCulloch et al., 2009b; Cooley et al., 2012).
Versican cleavage occurs sub-endocardially as the endocardial cells transform to form the cushion mesenchyme, and is co-localized with ADAMTS1 and fibulin-1 (Kern et al., 2006). Later during development, endocardial cushions are rapidly remodeled (sculpted) to achieve their mature structure, and cleaved versican is broadly distributed around cushion mesenchyme cells. Valve anomalies associated with accumulation of versican were seen in both Adamts9+/− mice and Adamts5−/− mice (Kern et al., 2007, 2010). In particular, Adamts5−/− mice have unsculpted pulmonic valves and adult Adamts5−/− mice have myxomatous mitral valves, suggestive of failed remodeling of the matured valve. Versican remodeling has also been observed as the myocardium regresses around the outflow tract and is replaced by smooth muscle cells (Kern et al., 2007). Altered TGFβ and BMP signaling have been noted in association with reduced versican processing, although the detailed mechanism underlying this effect is presently unclear (Kern et al., 2010; Dupuis et al., 2011, 2013).
5.2. Cooperation of ADAMTS9 and ADAMTS20 in closure of the secondary palate
The secondary palate separates the oral and nasal cavities and forms by the midline fusion of two palatal shelves that arise from the maxilla, migrate to the midline and fuse. Adamts20 mutant mice (Adamts20bt/bt, see below) have a low incidence of cleft palate, but Adamts9 haploinsufficiency in Adamts20bt/bt mice resulted in fully penetrant cleft palate in mice owing to reduced shelf elevation, sculpting and growth (Enomoto et al., 2010). Adamts20 was expressed in the palate mesenchyme, which is of NCC lineage, whereas Adamts9 expression was seen exclusively in mesoderm-derived microvascular endothelial cells (Enomoto et al., 2010), implying coordination between these two different lineages. Reduced sculpting of the shelves and decreased growth were accompanied by accumulation of ECM and reduced cell density, with decreased cell proliferation in palate mesenchyme of the Adamts9+/− and Adamts20bt/bt embryos (Enomoto et al., 2010). Furthermore, the palates of these embryos showed a clear reduction of processed versican as evident from reduced anti-DPEAAE staining. As in the syndactyly mutants described below, Vcan haploinsufficiency in the Adamts20bt/bt, background also led to cleft palate, implying that versican was a necessary partner of ADAMTS proteases during palate closure, possibly by providing a bioactive fragment (Enomoto et al., 2010). However, local administration of the G1-DPEAAE441 N-terminal ADAMTS-generated versican fragment, named versikine, did not rescue the proliferation defect, suggesting that another mechanism or a different versican fragment may be operational in this context. Interestingly, the G3 domain of versican, which contains EGF-like repeats, was previously implicated in regulation of cell proliferation (Zhang et al., 1999).
5.3. ADAMTS proteolysis of versican in interdigital web regression
Interdigital webs are present in birds that swim, such as ducks and geese. In bats the forelimbs have webs that increase the surface area for flight. Webs do not develop as new structures in these species, but rather, represent the persistence of interdigital tissue that is formed by default during development of the distal limb (autopod). During embryogenesis, the autopod is the last limb segment to develop, and in most mammals, retains interdigital webs until the specification of the digits is completed. Once patterning is complete, the webs regress with massive apoptosis of the interdigit mesenchyme. The webs contain a provisional ECM whose fate and resorption mechanism was until recently, unknown and largely ignored. Although interdigital webs may seem trivial, the dexterity afforded by freed digits is crucial for survival in the wild, and web regression in terrestrial mammals is a key evolutionary development. Persistence of one or more interdigital webs, termed soft-tissue syndactyly, is a common birth defect in humans.
Immediately prior to apoptosis, ADAMTS1, ADAMTS5, ADAMTS9 and ADAMTS20 are upregulated in the webs (Thai and Iruela-Arispe, 2002; McCulloch et al., 2009a,b). Although single ADAMTS mutants, with the exception of Adamts9 (Dubail et al., manuscript in revision) have a low incidence of soft-tissue syndactyly, combinatorial mutants of ADAMTS5, ADAMTS9 and ADAMTS20 showed fully penetrant soft tissue syndactyly (McCulloch et al., 2009b). Additional genetic evidence established that the ADAMTS co-factor fibulin-1 (Lee et al., 2005), and versican itself, were essential for web regression and that versikine was causally implicated in induction of apoptosis (McCulloch et al., 2009b). Specifically, beads soaked in versikine could induce apoptosis in ADAMTS-deficient interdigital tissues. Versikine activity was shown to be independent of and parallel to the established FGF–BMP pathway governing apoptosis during interdigital tissue regression, indicating that cell and ECM turnover are coordinated and interdependent during web regression.
5.4. ADAMTS proteolysis of versican in other developmental contexts
The Adamts20 mutant Belted (Adamts20bt), is a recessive allele with unpigmented regions (white spotting) in the lumbar torso of mutant mice (Rao et al., 2003). Melanoblasts, which are of NCC origin, migrate normally in this mutant, but fail to colonize the hair follicles, where they eventually reside. Increased melanoblast apoptosis in Adamts20Bt/Bt embryos was associated with reduced versican processing (Silver et al., 2008). Interestingly, throughout melanoblast migration and colonization of hair follicles, Adamts20 is not expressed by melanoblasts, but by adjacent dermal mesenchymal cells (Rao et al., 2003). This suggests that versican proteolysis provides environmental cues or survival signals for melanoblasts. Moreover, Adamts20Bt/Bt melanoblasts have reduced sensitivity to soluble Kit-ligand, a crucial signal for melanoblast survival, suggesting a link between versican processing and Kit-signaling that is presently unclear (Silver et al., 2008). Adamts9 haploinsufficiency in Adamts20Bt/Bt mice increased the extent of depigmented hair follicles (Silver et al., 2008), identifying a crucial cooperative role for these highly homologous proteases in addition to web regression and palatogenesis. However, it is not known if the function of ADAMTS9 in melanoblast development is temporally and spatially identical to that of ADAMTS20.
Skeletal muscle arises fromthe fusion of myoblasts to form multinucleated myotubes, a process that is accompanied by versican processing (Stupka et al., 2013). Adamts5 and Adamts15 are expressed during myoblast fusion in developing embryonic muscle and differentiating C2C12 cells. Adamts5 knockdown in vitro was shown to impair myoblast fusion, associated with expansion of a HA and versican-rich matrix, and could be rescued with catalytically active but not inactive ADAMTS5 or ADAMTS15. Instead, inactive ADAMTS5, ADAMTS15, or full-length V1 versican impaired myoblast fusion (Stupka et al., 2013). However, a myopathy has not yet been observed in ADAMTS deficient animals, suggesting that specific combinatorial mutants, such as of Adamts5 and Adamts15, may be required to elucidate it.
6. Conclusions and future directions
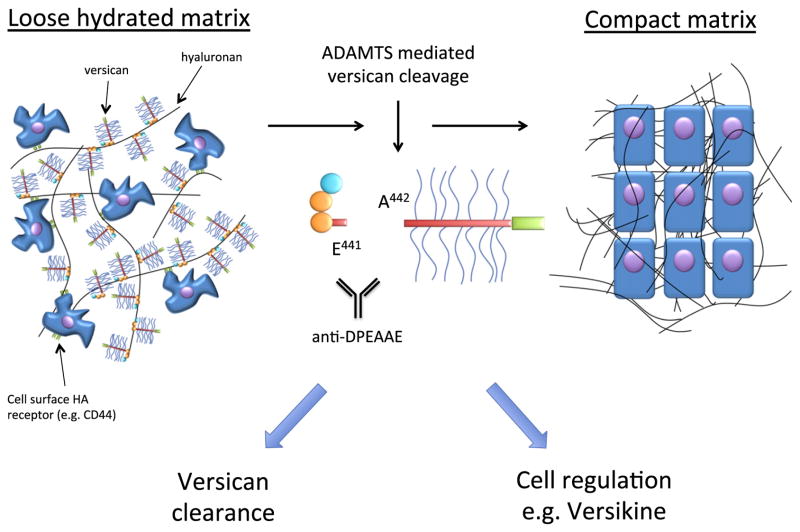
In summary, versican is a crucial component of the provisional matrix that is required for tissue expansion and regulates morphogenetic cell movements in the embryo. Versican accumulation occurs primarily during the embryonic periods from 8 to 14 days of gestation in the mouse, and once organogenesis and morphogenesis are sufficiently advanced, versican content of tissues is drastically reduced by a combination of decreased gene expression (Naso et al., 1995) and increased proteolysis, much of it, apparently, mediated by ADAMTS proteases (this review). The literature reviewed here supports the concept that specific proteolytic mechanisms evolved to clear away versican once its embryonic functions were met. However, the specific mechanisms that evolved for embryonic versican proteolysis (i.e., ADAMTS proteases), may do more than simply clear the versican, i.e. they may provide a bioactive molecule, such as versikine (Fig. 2). Indeed, the process of versican breakdown is spatially and temporally highly regulated in developmental contexts, with versican deposition typically preceding upregulation of ADAMTS protease mRNA, which shows a high degree of temporal and spatial specificity in the various developmental contexts cited here.
Fig. 2.
Dual function of versican and versican proteolysis in provisional ECM: During early development, the HA–versican composite forms a loose hydrated matrix (left) that is permissive form orphogenesis and amenable to rapid remodeling. Specific ADAMTS proteases cleave versican, leading to compaction of this matrix (right) concurrent with removal of other components of the provisional matrix, such as hyaluronan by other mechanisms, and elaboration of specialized matrix components (black fibers). Versican proteolysis by ADAMTS proteases occurs at a specific peptide bond (center). In addition to versican clearance and ECM remodeling occurring via the ADAMTS activity, versican fragments, such as versikine, may influence cell proliferation and apoptosis locally.
The investigations to date raise several intriguing questions: Since versican is a widespread component of embryonic matrices, are there other organ sites where versicanolysis is crucial, such as the brain, or organs that arise from epithelial–mesenchyme interactions, such as the lung and kidney? In light of several intriguing in vitro effects of versican and versican domains on cells (Yang et al., 1999; Wight, 2002; Yee et al., 2007), does the versican-rich provisional matrix promote specific cell behaviors, and are these abrogated or reversed by ADAMTS proteolysis? An unexplored and potentially important context for versican turnover is the fetal–maternal axis, including extra-embryonic tissues such as the umbilical cord, which contains a provisional matrix rich in HA and versican called Wharton’s jelly. What proteolytic mechanisms remodel Wharton’s jelly and enable umbilical cord growth? Since versican guides NCC migration, does versican proteolysis participate in regulating NCC dispersal to target sites, and in the brain, for establishing neural projections and networks? Are bioactive fragments other than versikine generated from versican? How does versikine regulate and interact with cells? Does it have a cellular receptor that transduces a signal, or does it act by disruption of an ECM network followed by cellular sensing of that disruption? What co-factors other than fibulin-1 regulate versican proteolysis? DoMMPs and ADAMTS proteases collaborate specifically in versican processing? Is versican proteolysis coordinated with turnover of other provisional matrix components such as fibronectin and HA, and does it influence their turnover? These intriguing questions deserve to be the focus of future investigations.
Acknowledgments
The authors thank Tim Mead and the reviewers for critical reading of the manuscript.
Abbreviations
- ADAMTS
A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs
- ECM
extracellularmatrix
- GAG
glycosaminoglycan
- HA
hyaluronan
- CS
chondroitin sulfate
Footnotes
Grant support: This work was supported by a National Institutes of Health Programs of Excellence in Glycosciences award (NIH PO1 HL107147) and by NIH RO1 HD069747 to S. A.
References
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barascuk N, Genovese F, Larsen L, Byrjalsen I, Zheng Q, Sun S, Hosbond S, Poulsen TS, Diederichsen A, Jensen JM, Mickley H, Register TC, Rasmussen LM, Leeming DJ, Christiansen C, Karsdal MA. A MMP derived versican neoepitope is elevated in plasma from patients with atherosclerotic heart disease. Int J Clin Exp Med. 2013;6:174–184. [PMC free article] [PubMed] [Google Scholar]
- Brezin AP, Nedelec B, Barjol A, Rothschild PR, Delpech M, Valleix S. A new VCAN/versican splice acceptor site mutation in a French Wagner family associated with vascular and inflammatory ocular features. Mol Vis. 2011;17:1669–1678. [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capehart AA. Proteolytic cleavage of versican during limb joint development. Anat Rec. 2010;293:208–214. doi: 10.1002/ar.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini P, Ori M, Avenoso A, D’Ascola A, Traina P, Mattina W, Perris R, Campo GM, Calatroni A, Nardi I, Campo S. Identification and gene expression of versican during early development of Xenopus. Int J Dev Biol. 2008;52:993–998. doi: 10.1387/ijdb.082582pc. [DOI] [PubMed] [Google Scholar]
- Chan CK, Rolle MW, Potter-Perigo S, Braun KR, Van Biber BP, Laflamme MA, Murry CE, Wight TN. Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J Cell Biochem. 2010;111:585–596. doi: 10.1002/jcb.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Lee HC, Hsu RJ, Chen TY, Huang YK, Lo HC, Hu SC, Harn HJ, Jeng JR, Sun CK, Lin SZ, Tsai HJ. The toxic effect of Amiodarone on valve formation in the developing heart of zebrafish embryos. Reprod Toxicol. 2012;33:233–244. doi: 10.1016/j.reprotox.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Choocheep K, Hatano S, Takagi H, Watanabe H, Kimata K, Kongtawelert P. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J Biol Chem. 2010;285:21114–21125. doi: 10.1074/jbc.M109.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997a;124:505–514. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 1997b;36:163–173. doi: 10.1016/s0008-6363(97)00172-7. [DOI] [PubMed] [Google Scholar]
- Cooley MA, Fresco VM, Dorlon ME, Twal WO, Lee NV, Barth JL, Kern CB, Iruela-Arispe ML, Argraves WS. Fibulin-1 is required during cardiac ventricular morphogenesis for versican cleavage, suppression of ErbB2 and Erk1/2 activation, and to attenuate trabecular cardiomyocyte proliferation. Dev Dyn. 2012;241:303–314. doi: 10.1002/dvdy.23716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster L, Carlstedt I, Malmstrom A. Isolation of 35S- and 3H-labelled proteoglycans from cultures of human embryonic skin fibroblasts. Biochem J. 1979;183:669–681. doi: 10.1042/bj1830669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancevic CM, Fraser FW, Smith AD, Stupka N, Ward AC, McCulloch DR. Biosynthesis and expression of a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats-15: a novel versican-cleaving proteoglycanase. J Biol Chem. 2013;288:37267–37276. doi: 10.1074/jbc.M112.418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994;269:32992–32998. [PubMed] [Google Scholar]
- Dupuis LE, McCulloch DR, McGarity JD, Bahan A, Wessels A, Weber D, Diminich AM, Nelson CM, Apte SS, Kern CB. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev Biol. 2011;357:152–164. doi: 10.1016/j.ydbio.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis LE, Osinska H, Weinstein MB, Hinton RB, Kern CB. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J Mol Cell Cardiol. 2013;60:50–59. doi: 10.1016/j.yjmcc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Dutt S, Cassoly E, Dours-Zimmermann MT, Matasci M, Stoeckli ET, Zimmermann DR. Versican V0 and V1 direct the growth of peripheral axons in the developing chick hindlimb. J Neurosci. 2011;31:5262–5270. doi: 10.1523/JNEUROSCI.4897-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Nelson C, Somerville RPT, Mielke K, Dixon L, Powell K, Apte SS. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development. 2010;137:4029–4038. doi: 10.1242/dev.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Bartelings MM, Poelmann RE, Haak MC, Jongbloed MR. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Semin Fetal Neonatal Med. 2013;18:237–244. doi: 10.1016/j.siny.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, Shinomura T, Watanabe H. Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology. 2012;22:1268–1277. doi: 10.1093/glycob/cws095. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Ybot-Gonzalez P, Copp AJ. Over-expression of the chondroitin sulphate proteoglycan versican is associated with defective neural crest migration in the Pax3 mutant mouse (splotch) Mech Dev. 1997;69:39–51. doi: 10.1016/s0925-4773(97)00151-2. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Naso MF, Cannizzaro LA, Wasmuth JJ, McPherson JD. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12–5q14) Genomics. 1992;14:845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- Ismat A, Cheshire AM, Andrew DJ. The secreted Adam TS-A metalloprotease is required for collective cell migration. Development. 2013;140:1981–1993. doi: 10.1242/dev.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, Hammarstrom A, Behrendt M, Andersson JO, Lindgren K, Andersson AK, Wallbrandt P, Rosengren B, Brodin P, Thelin A, Westin A, Hurt-Camejo E, Lee-Sogaard CH. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol. 2005;25:180–185. doi: 10.1161/01.ATV.0000150045.27127.37. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Watanabe H, Habuchi H, Takagi H, Shinomura T, Shimizu K, Kimata K. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281:2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235:2238–2247. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev Dyn. 2007;236:671–683. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29:304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil SH, Lallier T, Bronner-Fraser M. Inhibition of cranial neural crest adhesion in vitro and migration in vivo using integrin antisense oligonucleotides. Dev Biol. 1996;179:91–101. doi: 10.1006/dbio.1996.0243. [DOI] [PubMed] [Google Scholar]
- Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, Suzuki S. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem. 1986;261:13517–13525. [PubMed] [Google Scholar]
- Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer. 2010;126:640–650. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial–mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Bartholdi D, Abdou MT, Zimmermann DR, Berger W. Identification of the genetic defect in the original Wagner syndrome family. Mol Vis. 2006;12:350–355. [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. Inhibition of neural crest cell attachment by integrin antisense oligonucleotides. Science. 1993;259:692–695. doi: 10.1126/science.8430321. [DOI] [PubMed] [Google Scholar]
- Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- Lee NV, Rodriguez-Manzaneque JC, Thai SN, Twal WO, Luque A, Lyons KM, Argraves WS, Iruela-Arispe ML. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J Biol Chem. 2005;280:34796–34804. doi: 10.1074/jbc.M506980200. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Braun KR, Maurel P, Kaplan ED, Schwartz SM, Wight TN. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1630–1639. doi: 10.1161/01.atv.19.7.1630. [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Pla P, Larue L, Gruss P. Pax3 acts cell autonomously in the neural tube and somites by controlling cell surface properties. Development. 2001;128:1995–2005. doi: 10.1242/dev.128.11.1995. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Krook JM, Kitten GT, Runyan RB. Endocardial cushion tissue development: structural analyses on the attachment of extracellular matrix to migrating mesenchymal cell surfaces. Scan Electron Microsc. 1981;(Pt 2):261–274. [PubMed] [Google Scholar]
- Mayor R, Theveneau E. The neural crest. Development. 2013;140:2247–2251. doi: 10.1242/dev.091751. [DOI] [PubMed] [Google Scholar]
- McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009a;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009b;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SP, Richards AJ, Flanagan DW, Scott JD, Poulson AV, Snead MP. Clinical characterisation and molecular analysis of Wagner syndrome. Br J Ophthalmol. 2007;91:655–659. doi: 10.1136/bjo.2006.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Morton SU, Scherz PJ, Cordes KR, Ivey KN, Stainier DY, Srivastava D. microRNA-138 modulates cardiac patterning during embryonic development. Proc Natl Acad Sci U S A. 2008;105:17830–17835. doi: 10.1073/pnas.0804673105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Nikopoulos K, Maugeri A, de Brouwer AP, van Nouhuys CE, Boon CJ, Perveen R, Zegers HA, Wittebol-Post D, van den Biesen PR, van der Velde-Visser SD, Brunner HG, Black GC, Hoyng CB, Cremers FP. Erosive vitreoretinopathy and Wagner disease are caused by intronic mutations in CSPG2/Versican that result in an imbalance of splice variants. Invest Ophthalmol Vis Sci. 2006;47:3565–3572. doi: 10.1167/iovs.06-0141. [DOI] [PubMed] [Google Scholar]
- Nagchowdhuri PS, Andrews KN, Robart S, Capehart AA. Versican knockdown reduces interzone area during early stages of chick synovial joint development. Anat Rec. 2012;295:397–409. doi: 10.1002/ar.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso MF, Morgan JL, Buchberg AM, Siracusa LD, Iozzo RV. Expression pattern and mapping of the murine versican gene (Cspg2) to chromosome 13. Genomics. 1995;29:297–300. doi: 10.1006/geno.1995.1251. [DOI] [PubMed] [Google Scholar]
- Perides G, Asher RA, Lark MW, Lane WS, Robinson RA, Bignami A. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312:377–384. doi: 10.1042/bj3120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Morgelin M, Lofberg J, Perris R. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- Rao C, Foernzler D, Loftus SK, Liu S, McPherson JD, Jungers KA, Apte SS, Pavan WJ, Beier DR. A defect in a novel ADAMTS family member is the cause of the belted white-spotting mutation. Development. 2003;130:4665–4672. doi: 10.1242/dev.00668. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441–Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extracellular matrix component of the mature bovine brain. J Biol Chem. 1998;273:15758–15764. doi: 10.1074/jbc.273.25.15758. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Shepard JB, Gliga DA, Morrow AP, Hoffman S, Capehart AA. Versican knockdown compromises chondrogenesis in the embryonic chick limb. Anat Rec. 2008;291:19–27. doi: 10.1002/ar.20627. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. Histochemical localisation of versican, aggrecan and hyaluronan in the developing condylar cartilage of the fetal rat mandible. J Anat. 2001;198:129–135. doi: 10.1046/j.1469-7580.2001.19820129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, Hou L, Somerville R, Young ME, Apte SS, Pavan WJ. The secreted metalloprotease ADAMTS20 is required for melanoblast survival. PLoS Genet. 2008;4:1–15. doi: 10.1371/journal.pgen.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupka N, Kintakas C, White JD, Fraser FW, Hanciu M, Aramaki-Hattori N, Martin S, Coles C, Collier F, Ward AC, Apte SS, McCulloch DR. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and –15 facilitates myoblast fusion. J Biol Chem. 2013;288:1907–1917. doi: 10.1074/jbc.M112.429647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai SN, Iruela-Arispe ML. Expression of ADAMTS1 during murine development. Mech Dev. 2002;115:181–185. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Kessel M, Gruss P. A transgenic neuroanatomical marker identifies cranial neural crest deficiencies associated with the Pax3 mutant Splotch. Dev Biol. 1995;171:317–329. doi: 10.1006/dbio.1995.1284. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405–Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377:787–795. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Williams DR, Jr, Presar AR, Richmond AT, Mjaatvedt CH, Hoffman S, Capehart AA. Limb chondrogenesis is compromised in the versican deficient hdf mouse. Biochem Biophys Res Commun. 2005;334:960–966. doi: 10.1016/j.bbrc.2005.06.189. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Yee AJ, Akens M, Yang BL, Finkelstein J, Zheng PS, Deng Z, Yang B. The effect of versican G3 domain on local breast cancer invasiveness and bony metastasis. Breast Cancer Res. 2007;9:R47. doi: 10.1186/bcr1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zako M, Shinomura T, Ujita M, Ito K, Kimata K. Expression of PGM( V3), an alternatively spliced form of PG-M without a chondroitin sulfate attachment in region in mouse and human tissues. J Biol Chem. 1995;270:3914–3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]