Abstract
Validation of biodosimetry assays is normally performed with acute exposures to uniform external photon fields. Realistically, exposure to a radiological dispersal device or reactor leak will include exposure to low dose rates and likely exposure to ingested radionuclides. An improvised nuclear device will likely include a significant neutron component in addition to a mixture of high- and low-dose-rate photons and ingested radionuclides. We present here several novel irradiation systems developed at the Center for High Throughput Minimally Invasive Radiation Biodosimetry to provide more realistic exposures for testing of novel biodosimetric assays. These irradiators provide a wide range of dose rates (from Gy/s to Gy/week) as well as mixed neutron/photon fields mimicking an improvised nuclear device.
INTRODUCTION
The radiation fields normally encountered in radiation protection scenarios are typically complex involving a wide range of dose rates and radiation qualities. Realistically, exposure to a radiological dispersal device (RDD) or reactor leak will include exposure to low-dose rates and likely exposure to ingested radionuclides. An improvised nuclear device (IND) will likely include a significant neutron component in addition to a mixture of high- and low-dose-rate photons and ingested radionuclides.
To effectively respond to these scenarios the U.S. Government is supporting development of medical countermeasures against radiation as well as high-throughput biodosimetry which can be used for identifying exposed individuals who would benefit from them (1). This requires testing of countermeasures and biodosimetry using irradiation fields that mimic realistic exposure scenarios that include neutron exposures, a wide range of dose rates and internal emitters. Despite this, validation of biodosimetry assays is normally performed with acute exposures to uniform external photon fields.
The Radiological Research Accelerator Facility at Columbia University has been developing novel irradiation systems and radiation measuring devices for 50 years. In this article, we describe the irradiation systems developed for modeling realistic radiation exposure scenarios within the context of radiation biodosimetry and discuss their use.
SCENARIOS
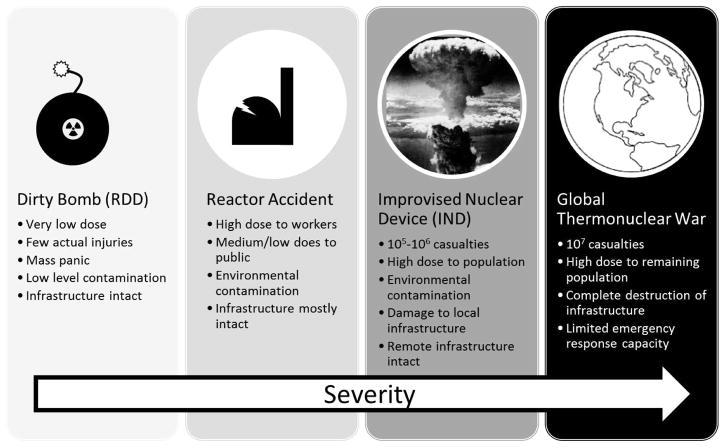
Over the past decades, with changes in technology and global politics, the planning scenarios for radiological events (Fig. 1) have shifted: Through the late 1980s planning revolved around a major exchange of sophisticated nuclear devices between the two superpowers (2, 3). During the 1950s and 1960s it was believed that sheltering in place could provide adequate protection to the population (2). Later planning scenarios (3) assumed that most of the population near potential detonation sites, could be evacuated and sheltered in the days leading up to the attack. This scenario specifically states that “attention should not be given to protection against nuclear blast and fire” near the detonation sites as it would not be feasible to do so. The post attack planning (3, 4) thus concentrated on protecting the evacuees from fallout (by sheltering), surveying fallout levels and transporting food and fuel to the evacuees. It was assumed that all exposures would be “intentional” (i.e. leaving the shelter for some important reason) and accompanied by physical dosimetry.
FIG. 1.
Scenarios involving radiation exposure to the general population.
These approaches were criticized by the medical community as unrealistic (5, 6), stating that the destruction and loss of life (especially within the medical professions) was likely to be much higher than planned and would not allow any type of medical response for treating injured individuals, who were largely ignored in the planning.
With the collapse of the Soviet Bloc, emphasis has shifted dramatically. Recent planning scenarios (7) discuss more limited events for which biodosimetry, triage and medical countermeasures are relevant and therefore are in the planning stages (1).
Improvised Nuclear Device
The main planning scenario being considered [scenario 1 in ref. (7)] involves a single “improvised” device deployed by a terrorist organization. The standard model of such an IND is a 10kT “gun-type” device based on enriched uranium and detonated at ground level, similar to, but somewhat smaller than the Hiroshima bomb, “Little Boy”. This is considered to be the simplest design, based on the most “easily” obtainable nuclear materials.
A notable difference between this IND scenario and the Hiroshima bomb is that an IND is expected to be detonated at ground level, whereas Little Boy was detonated at an altitude of 600 m. The main consequence of this is that buildings will partially shield the photon component but have little effect on the neutron component (8), so that, while total doses would be roughly a third of those at Hiroshima, at the same distance from Ground Zero, the fractional neutron dose, would be significantly higher (9), with a corresponding increase in biological effects.
This type of scenario would therefore consist of the following radiation fields:
Prompt radiation: a mixture of photons and MeV-range neutrons, delivered essentially instantaneously;
Delayed radiation: both due to groundshine and fallout. Here significant doses are delivered over a period of days, until the individual is evacuated; and
Internal emitters: Ingested radionuclides will likely persist in the host for weeks, providing a low level internal irradiation. For example the biological half-life of Cesium-137 in humans is about 100 days (10), unless chelating agents are used.4
Significance of Neutrons
Within an IND scenario, a significant fraction of the prompt dose is delivered in the form of MeV-range neutrons. The radiobiological consequences of these neutrons have been studied since the 1960s and they have been shown to be much more effective in the induction of radiation endpoints than photons [e.g. (12)]. Based on Monte Carlo calculations of radiation transport in an urban environment (9), it is expected that the neutron dose at a survivable distance from an IND detonation, would be on the order of 10–20% of the total dose. Factoring in that these neutrons are 2–6 times more effective than photons in inducing cytogenetic damage (8, 13, 14), roughly half of the biological effect observed will be due to neutrons with the other half due to photons. Hence it is important to assess whether the two radiation types act independently or synergistically in terms of biodosimetric dose reconstruction and in evaluating acute radiation effects. To establish this, it is crucial that biodosimetry assay validation be performed in radiation fields containing a mix of photons and MeV-neutrons.
Significance of Dose Rate
For sparsely ionizing radiation (photons), dose rate is one of the principle factors determining the biological consequences of radiation. For example, below about 1.5 Gy/h, the characteristic time between photon traversals in a cell is longer than the typical time for rejoining (or mis-rejoining) of DNA double-strand breaks (15). Thus at very low-dose rates, sublethal damage is repaired as fast as it is formed. Conversely, at higher dose rates, there is an increased possibility of multiple reparable lesions interacting to form a complex, irreparable lesion. This would indicate that radiation effects from fallout-type exposures, where the dose is delivered over days and weeks would be qualitatively and quantitatively different from similar exposures delivered in minutes. Furthermore, direct experiments (16), and recent measurements of fast repair times for double strand breaks (17) strongly suggest that there will be increased effects from a dose delivered in ~1 s, compared with the same dose delivered in ~1 min. Nevertheless, the bulk of exposures used in biodosimetry and countermeasure testing utilize dose rates of about 1 Gy/min.
Other Radiological Devices
Radiological Dispersal or Exposure Devices (RDD/RED) are easier to construct than an IND. In an RDD, a quantity of radioactive material is mixed with explosive and detonated, thus the radioactive material is aerosolized and dispersed in the environment. Typical scenarios talk of such a device made from 137Cs, 60Co, 90Sr or 241Am, which are all used industrially and are potentially available in significant quantities. An RED is essentially the same thing but without the explosive – a large gamma emitter is placed in a public location with the hopes of exposing as many people as possible.
In either case the public health consequences are small. While there would probably be mass panic, there would be very few individuals who would actually require medical intervention. A good model for this scenario is the Goiania accident (18), where a 1375 Ci 137Cs teletherapy source was accidentally dispersed. In a city of 1 million people, 249 individuals were contaminated (of them 151 contaminated internally), 49 individuals required hospitalization, 28 suffered radiation burns and five died (8).
It is therefore likely that an RDD/RED would result in a few hundreds or thousands of individuals contaminated (internally or externally), receiving low-dose-rate irradiations over a period of days to weeks with a much smaller number of acutely irradiated individuals (who will likely also be injured by the blast). In the specific case of an RDD based on an alpha emitter (e.g. 241Am or 210Po), identifying internally exposed individuals without using a biological assay is near impossible due to the short range of the alpha particles, on the other hand, these individuals are those who would need treatment most, as even a low dose of high-LET alpha particles would have significant biological consequences.
Radiological Accident
Presently, more than 400 nuclear power plants are in use globally (19). These reactors are in risk of accidental release of radiation due to human error [as in the case of Chernobyl (20) or Three Mile Island] or natural disaster (as occurred at Fukushima). In such a scenario, the affected individuals can be divided into two groups, radiation workers and general population. The radiation workers include individuals within the plant during the disaster as well as the cleanup workers and are potentially exposed to high doses of radiation. These individuals typically would have physical dosimetry and would not necessarily require bioassays for triage. The general population, however, would likely be exposed to low-dose rates of radiation, either externally or via radionuclides (in particular 137Cs, 134Cs, 90Sr and 131I) entering the food chain (21, 22) and would potentially require the use of bioassays.
What arises from these scenarios is the need for both triage and countermeasures in mixed irradiation scenarios that may contain a mixture of neutrons and photons and/or a broad range of exposure timescales, from fractions of a second to days and weeks. It is therefore critical to have available irradiators that can provide such irradiation fields in a controlled, reproducible manner. At the Radiological Research Accelerator Facility, we have developed (and are continuing to develop) several such facilities, allowing both ex-vivo irradiation of blood samples and in vivo irradiation of small animals.
Model Systems
With the exception of patients undergoing therapeutic irradiation (23, 24) and the rare accidental exposure (25), it is not feasible to validate biological dosimetry assays in in vivo irradiated humans. To perform these studies under controlled irradiation conditions, one is therefore limited to irradiation of animal model systems or blood from “healthy” human volunteers. While the latter is extremely useful for testing blood-based biodosimetry assays, one or more animal models are required for testing and FDA approval of both biodosimetry devices and medical countermeasures (26).
The bulk of in vivo studies in biodosimetry and medical countermeasure development are performed in mice. A wide variety of mice strains, with varying degrees of radiation sensitivity (27, 28), are commercially available and can be used for exploratory studies. Once an appropriate candidate drug or biomarker is identified, it can be further validated in non-human primate (NHP) experiments which are significantly more expensive and complicated to perform.
Due to the larger size of NHPs, they require significantly larger radiation fields, and more penetrating radiations – while mice studies can be easily performed with X rays from a standard orthovoltage machine operating at 250–320 kVp, NHP studies are better performed with more penetrating gamma rays from a 137Cs- or 60Co-based irradiator and even then are typically performed by either irradiating the animal twice (front and back), or rotating the animal during irradiation, to achieve a homogenous exposure.
The irradiation systems described below are aimed at initial stage studies, using ex vivo irradiated human blood and in vivo irradiated mice.
IRRADIATION FACILITIES
Neutrons
Two types of neutron irradiation systems are generally available for radiobiology studies, reactor based and accelerator based.
In a reactor based system [e.g. (29–31)], neutrons are generated via 235U fission, with a wide range of energies peaked around about 1 MeV. Depending on the type of reactor used, unmoderated fission neutrons can be obtained directly (32) or by using a 235U converter to transform thermal neutrons from a small research reactor to fission neutrons (29). Samples can be either inserted into the reactor core or exposed to neutrons extracted via a window into an experimental room. The latter allows exposing large specimens, such as NHP.
For example, the Petten reactor (29), used for key in vivo neutron RBE studies (for hematopoietic and GI death) (33, 34), is a small research reactor using a standard 235U fuel plate as a converter resulting in a highly uniform flux of energetic neutrons allowing irradiation of mice or NHP at a dose rate of up to 6 Gy/h.
Accelerator based systems use ion beams, typically protons or deuterons, impinging on a low-Z target (for example beryllium or tritium-impregnated titanium). Neutrons are formed via nuclear reactions with different energy neutrons emitted at different angles with respect to the beam direction. In this case, by adjusting the beam type and energy, target type and angle to the sample, quasi-monoenergetic neutron beams (energy spread of up to ±15%) can be generated. Due to the angular dependence of the neutron energies, such a facility is limited to irradiation of small samples, which subtend an angle of about 10°, as seen from the target. Larger samples will suffer increased inhomogeneity in both neutron energy and flux.
For example, the neutron facility at the Radiological Research Accelerator Facility (RARAF)5 has had 50 years of experience performing neutron irradiations of small animals (35, 36) and of cells (37–39) using monoenergetic neutrons having energies from 0.22 to 15 MeV (12).
IND-Spectrum Neutron Irradiator
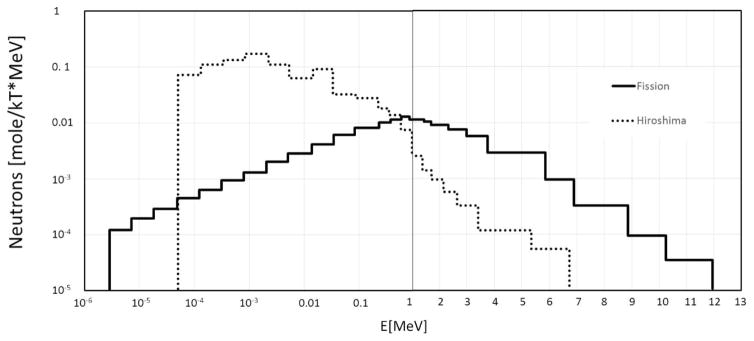
However, neither monoenergetic nor fission spectra are appropriate for studying IND-type exposures. While the initial neutrons with a fission spectrum are formed in the bomb, the spectrum changes dramatically as the neutrons are transported through the bomb casing and a kilometer or more of air. For example, Fig. 2 contrasts the bare fission spectrum and the Hiroshima source term (40). As can be seen the bare fission spectrum is much harder than the one from an actual bomb.
FIG. 2.
Comparison of a pure fission spectrum (solid line) and the spectrum from “Little Boy”. Data reproduced from ref. (40).
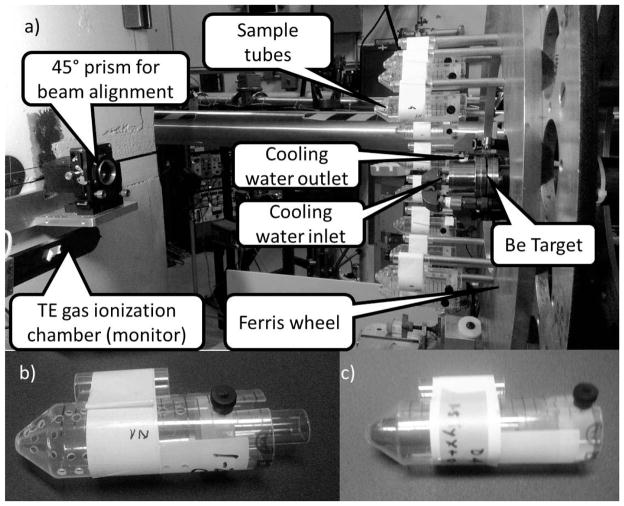
For generating neutron fields, mimicking exposure from an IND, we have developed an accelerator based neutron source [Fig 3a, (13)], providing neutrons having the same energy spectrum as those modeled for Hiroshima (41, 42) at 1 km from the epicenter [where we would expect a survivable neutron dose of about 0.25 Gy (9)]. The facility is designed for performing irradiations of mice (unpublished data) and of blood (13).
FIG. 3.
Panel a: The neutron irradiation facility [Reproduced with permission from (13)]. Panel b: Sample tube for irradiating mice. Panel c: Sample tube, containing human blood for ex vivo irradiations.
Briefly, samples are placed in modified 50 ml conical tubes (Fig. 3b and c) placed on a Ferris wheel, rotating at 30 revolutions per hour around the beryllium target. A mixed beam of roughly 15% protons, 30% deuterons and 55% molecular ions is accelerated to 5 MeV and bombarded on a thick beryllium target generating a spectrum of neutrons with a dose rate of 0.085 [Gy/h/μA of beam], at the sample location, with an additional 17% photon dose, delivered simultaneously. In order to allow measuring other photon/neutron mixes, a highly filtered 250kVp X-ray machine is available.
Results
Using this source, we have tested a variety of cytogenetic and transcriptomic endpoints:
For micronuclei, for example, we have seen that these neutrons are roughly 4 times more efficient than photons (13) and have preliminary data demonstrating that, micronucleus formation by neutrons and photons is additive. We are also investigating dicentrics and inter-chromosome translocations (43) as possible biomarkers. The latter is known to be much more sensitive to densely ionizing radiation (such as neutrons). Reconstruction of the fraction of dose due to neutrons vs. photons would therefore possible when using this a translocation assay in conjunction with a second assay such as dicentrics or micronuclei.
In parallel transcriptomic studies (unpublished data), we have identified genes that are sensitive to radiation quality as well as genes that were insensitive to it. This will allow developing transcriptomic signatures that can identify both the total dose received and the fraction of neutrons.
Furthermore, metabolomic and lipidomic analyses of easily accessible biofluids from mice irradiated using these neutrons have shown a distinct dysregulation of metabolism as well as alterations of basic metabolic functions such as energy metabolism through fatty acid beta oxidation.
High-Dose-Rate Irradiator
Standard X-ray irradiators typically provide dose rates of about 1 Gy/min. This can be somewhat increased by reducing filtering and placing samples close to the X-ray tube, but in such a case, beam quality (spectrum and beam homogeneity) may suffer. Industrial gamma irradiators, providing high dose rates on the order of multiple Gy per second are available but they typically have a high minimal dose, due to the time required to insert/retract the sample into the irradiator, and thus do not allow irradiations to doses relevant for biodosimetry. Medical linear accelerators, on the other hand, provide both high dose rates and very low minimal delivered dose. Both are required for rapid and efficient stereotactic radiosurgery or IMRT, where the prescribed dose is split into hundreds of radiation pulses delivered from different directions and using differing collimation patterns (44).
The prevalence of these devices [over 11,000 deployed worldwide (45)] makes them an ideal candidate for use in radiobiology studies. We are currently investigating the use of one such device (the Varian TruBeam) for performing high-dose-rate whole-body irradiation of mice and blood. Preliminary measurements using the TrueBeam available at Columbia University Medical Center demonstrated that dose rates of 2 Gy/s over a 6 × 6 cm area are easily achievable using the machine in its standard configuration. A dose rate of about 5 Gy/s, would be attainable with the flattening filter removed. While this does not yet match the dose rate from an IND, which would be on the order of Gy/μs, it does allow irradiations which would be instantaneous on the time scale of biological DNA damage processing.
Low-Dose-Rate Irradiations
For performing studies of dose rate effects in the range of Gy/min to Gy/day, a standard X-ray machine may be used. At our facility, we have modified an X-Rad 320 (Precision X Ray Inc., North Branford, CT) to support long term irradiations of mice and blood (46, 47). An important requirement in beam preparation is that the radiation quality be the same in both high-dose-rate (HDR) and low-dose-rate (LDR) modes. This is typically not the case when using shielding to reduce dose rate as the added shielding may filter the X Rays to some extent. We therefore designed a custom Thoreaus filter (1.25 mm tin, 0.25 mm copper, 1.5 mm aluminum HVL: 4.9 mm copper) to allow a wider range of dose rates than that available with the standard filters provided with the X-Rad. Using this filter, we were able to provide 4 Gy/day at a source to surface distance (SSD) of 90 cm and 0.1 mA and 1 Gy/min at 40 cm SSD and 12.5 mA, with a variation of about ±6% across a 25 × 25 cm field.
Ex Vivo Irradiations
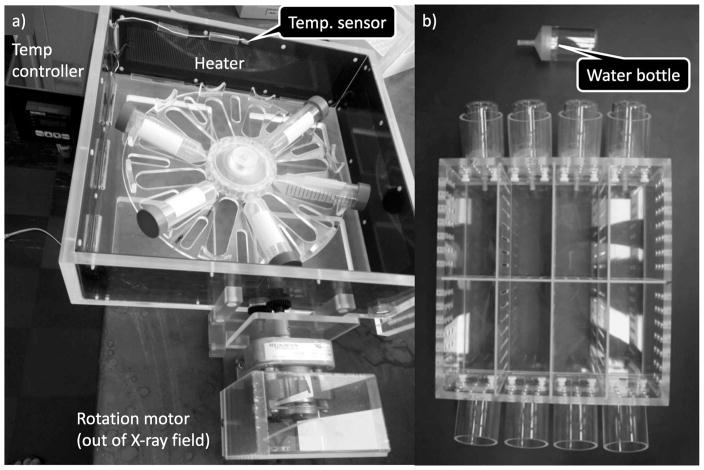
For ex vivo irradiations, low-dose-rate experiments require storage of blood samples for many hours under controlled environmental conditions, mimicking those found in an incubator (37°C, 5% CO2, 80% humidity). To fulfill this requirement, we built a custom incubator (47–49) (Fig. 4a) mainly made of plastic to minimize scattered radiation. Temperature is controlled through solid-state heaters on a feedback loop attached to the walls of the incubator to distribute the heat evenly. The CO2 concentration, humidity and temperature within the incubator were monitored using data loggers. Blood is exposed in 50 ml conical tubes angled to increase surface area and therefore gas exchange and to keep the samples within a 20 cm diameter and minimize planar dose variation. The tube holder is rotated at three rotations per hour to further minimize any dose inhomogeneity.
FIG. 4.
Low-dose-rate irradiation chamber for (panel a) blood and (panel b) mice.
Tests have shown that the temperature within our custom incubator fluctuates approximately 0.5–1.5°C, over a 24 h time period, somewhat higher than that in a commercial incubator. We, therefore investigated whether this temperature variation was likely to confound assays of radiation response by measuring expression of two heat shock (HSPA1L and HSPH1) and 1 cold shock (CIRBP) genes using quantitative real-time RT-PCR. With the temperature maintained at a nominal 37° C, there was no difference in gene expression between our custom incubator and a commercial one.
In Vivo Irradiations
For in vivo irradiations of mice, the situation is different, as mice need to be maintained at lower temperatures than those achieved within an enclosed X-ray machine operated continuously for 24 h. We have designed and constructed a “mouse air conditioner” consisting of a CPU cooling fan, a water pump with cooling element and an ice bath. The CPU cooling fan is a double fan with a radiator between the two fans. Cold water circulates through the radiator, cooling the blown air. Measurements show that this design adequately cools both the inside of the irradiator and the inside of the mouse housing maintaining a temperature of 22 ± 0.5°C.
In addition to temperature control, mice require a 12 h day/night cycle and adequate space. When irradiating mice using X rays, it is important that the mice are not able to huddle, and shield each other from radiation (this is less of an issue using a 137Cs irradiator, which has more penetrating radiation). We have therefore built custom mouse housing (Fig. 4b) that can hold up to 8 mice, in individual compartments [6 cm (w) × 12.5 cm (l) × 12 cm (h)]. Each compartment was supplied with food, bedding and water (via an all-plastic water bottle). Ten air volume changes are provided per hour, satisfying animal care requirements.
Results
Using this low-dose-rate irradiator we have studied a variety of cytogenetic (46, 47), transcriptomic (48, 50) and metabolomic (49) endpoints. We have seen that both HDR and LDR perturb the same general metabolic pathways but that individual metabolites may be used to discriminate between high- and low-dose rates (49). Similarly gene expression patterns in both mouse (50) and ex vivo irradiated blood (48) showed gene signatures that were independent of dose rate as well as signatures that were dose rate dependent. As expected, micronuclei (47) showed a linear dose response for LDR and a quadratic dose response for HDR, indicating repair of sublethal damage in the former.
Ultra-Low-Dose Rate
It is likely that 137Cs is the most biologically important fission product from many IND (51), RDD (7) or nuclear accident (52) scenario. Thus, further research regarding the effects of 137Cs, both from internal or external exposure, is much needed (53). To model this in animal studies, we and others have previously used an injection of soluble 137CsCl (54–56). In these studies, the amount of activity of the solution can be varied to produce a specific absorbed cumulative dose by specific time points with the time dependence of the dose determined by the biokinetics of Cs within the animal under study (Fig. 5). This type of experiment is complicated to do – resulting in radioactive excreta and biofluids, which require dedicated “hot” equipment for analysis and, secondly, disposal is expensive.
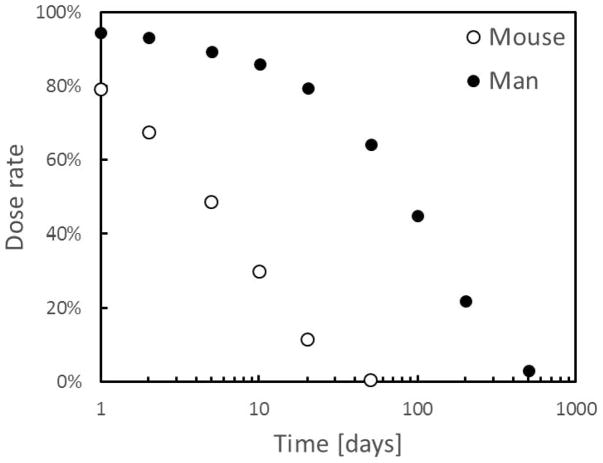
FIG. 5.
Biokinetics of cesium in mouse and man. The full circles represent the time dependent dose rate from ingested 137Cs, as per the model described in (10). Open circles are from our own data based on total body counting in mice (49).
An alternate approach is to use a low-dose-rate, external 137Cs source that can be adjusted to provide a variable dose rate. Due to the high energy of the 137Cs gamma rays, the physical dose distribution will be the same for the internal vs. external exposures.
The advantages of such a system are clear: it allows for simpler experiments and moreover decouples the time dependence of the dose delivered from the biokinetics in the animal model used. This allows exposing mouse models to the temporal dose profile that would be experience by a human that experiences a different biokinetics (e.g., Fig. 5) or even to a constant ultra-low dose rate.
It should be noted that modeling internal irradiations with external ones is only valid for deeply penetrating ionizations such as 137Cs gamma rays. In order to study the effects of (and develop bioassays for) internal exposure to alpha particles, for example from an 241Am RDD, an injection or inhalation based study is the only valid course of action.
Attenuated 137Cs Irradiator
The Howell group have demonstrated the validity of this approach using a cabinet type 137Cs irradiator coupled to a computer controlled mercury attenuator (57, 58). In their system a mercury reservoir is placed between an 18 Ci 137Cs source and one or more cages holding four mice each. The amount of mercury in the reservoir can be modified attenuating the gamma-ray flux achieving dose rates between approximately 10−4 and 0.25 Gy/h (57). This setup has been routinely used for calibration studies for internal emitter biomarker studies (59–61).
Variable Dose-Rate External Irradiator (VADER)
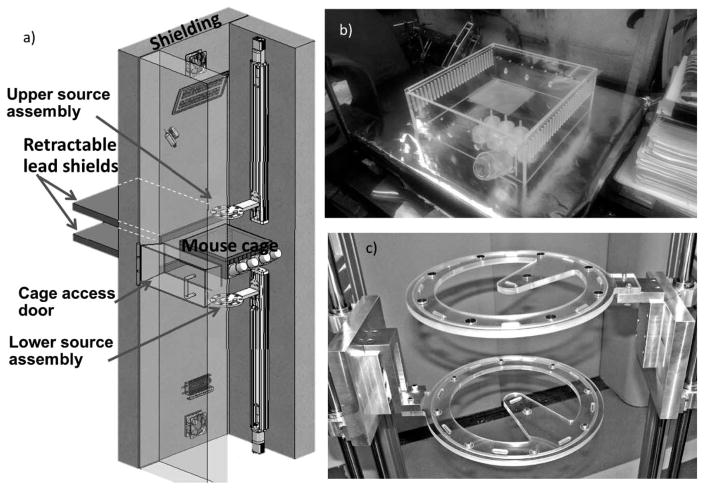
The system under development at our center uses continuously retracting “recycled” low-activity 137Cs brachytherapy seeds. These seeds were much used starting in the 1980s to treat cervical cancer at low dose rate (62), but are no longer in use and many of these 137Cs seeds are in long-term storage, making them readily available. For example, at Columbia University we have available 30 such sources, each in the ~20 mCi range.
The design of the system is shown in Fig. 6a: A plastic cage (Fig. 6b), holding up to 18 mice, is placed between two source assemblies each containing ten 20 mCi 137Cs seeds positioned in a circular pattern, one above and one below the mouse cage. This configuration can provide a dose rate of between 0.05 and 1.5 Gy/day depending on the vertical position of the sources.
FIG. 6.
Ultra-low-dose-rate irradiation system under construction.
During irradiation, the source assemblies (Fig. 6c) are slowly retracted under computer control away from the mouse cage. As the sources are retracted over time-scales of days to weeks, the mice are exposed to a decreasing dose rate that can mimic the dose-rate/time pattern shown in Fig. 5, or any other desired dose-rate/time pattern, such as the much slower 137Cs retention kinetics in man (10), or a constant low dose rate.
For these long-term irradiations the mice are free to move around, eat and drink ad libitum. Within the irradiator, temperature, humidity, air flow and lighting are fully controlled to the required animal care standards. Mouse handling is possible at any time by retracting the sources, inserting retractable lead shields (Fig. 6a), opening the interlocked lead cage access door, and extracting the cage.
The VADER device and shielding were designed based on a Monte Carlo transport simulation of the entire system, allowing a design with a predicted spatially uniform dose distribution across the mouse cage, at source-cage separations of 10 cm and greater. Spatial dose homogeneity at the location of the mouse cage will be verified using Gafchromic film, with absolute dosimetry based on a NIST-traceable ion chamber. Our calculations have also shown that possible dose variations due to mutual shielding (e.g. by mice huddling) are small. Nevertheless we will also verify individual dosimetry on a mouse-by-mouse basis by subcutaneously injecting into each an encapsulated high-sensitivity “pin-worm” LiF:Mg,Cu,P miniature TLD rod. These TLDs (diameter 0.6 mm, length 6 mm,) are designed for insertion into needles/catheters for in vivo application and provide a linear dose response up to 10 Gy, and better than 2% reproducibility. Following sacrifice, the TLD will be removed and read, giving the cumulative skin dose received by that mouse.
CONCLUSION
Within a realistic exposure scenario, such as the ones used for national planning purposes (7) it is expected that the population will be exposed to a wider range of dose rates and possibly mixed neutron/photon fields. Nevertheless, the bulk of studies on radiation biodosimetry and radiation mitigators focus on acute doses of photons. Over the past years we have developed several irradiation facilities allowing systematic studies of low dose rates and of mixed neutron/photon exposures using both ex vivo irradiated blood and mouse models. These systems have been used for testing various biological endpoints and are also available for mitigator studies in mice. We are currently working on expanding the palette of irradiation systems available to provide a much wider range of dose rates more suited to modeling exposures from the prompt and protracted exposure expected from a nuclear detonation and for modelling the dose profile from ingested 137Cs.
Acknowledgments
The authors would like to acknowledge the continued support of Gary W. Johnson and the staff of the Design and Instrument Shop at the Center for Radiological Research who were integral in the design and construction of most irradiation systems described in this manuscript. This work was supported by grant number U19-AI067773 to the Center for High-Throughput Minimally Invasive Radiation Biodosimetry, from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or NIH.
Footnotes
For example, Prussian Blue reduces the biological half-life of 137Cs to 30–50 days (11).
See also “50 Years of the Radiological Research Accelerator Facility (RARAF)”, by Stephen A. Marino. (Found in this issue.)
References
- 1.Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, et al. Rapid Radiation Dose Assessment for Radiological Public Health Emergencies: Role of NIAID and BARDA. Health Phys. 2010;98(2):172–78. doi: 10.1097/01.HP.0000348001.60905.c0. [DOI] [PubMed] [Google Scholar]
- 2.Biological and Environmental Effects of Nuclear War, Summary-Analysis of Hearings. Joint Committee on Atomic Energy. Place Government Printing Office: Government Printing Office; 1959. [Google Scholar]
- 3.Survival of the Relocated Population of the U.S. after a Nuclear Attack. Oak Ridge, TN: Oak Ridge National Laboratory; 1976. Report No. ORNL-5041. ( http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA026362) [Google Scholar]
- 4.Subcommittee on Health and Scientific Research of the Committee on Labor and Human Resources, United States Senate Place U.S. Government Printing Office. U.S. Government Printing Office; 1980. Short- and Long-Term Health Effects of the Surviving Population of a Nuclear War. [Google Scholar]
- 5.Sidel VW, Geiger HJ, Lown B. The physician’s role in the postattack period. New Eng J Med. 1962;266(22):1137–45. doi: 10.1056/NEJM196205312662205. [DOI] [PubMed] [Google Scholar]
- 6.Abrams HL. Medical resources after nuclear war: Availability v need. JAMA. 1984;252(5):653–58. [PubMed] [Google Scholar]
- 7.National planning scenarios (final version 21.3) Washington DC: Homeland Security Council; 2006. p. 9. https://publicintelligence.net/national-planning-scenarios-version-21-3-2006-final-draft/) [Google Scholar]
- 8.Wuttke K, Müller W-U, Streffer C. The sensitivity of the in vitro cytokinesis-blocked micronucleus assay in lymphocytes for different and combined radiation qualities. Strahlentherapie und Onkologie. 1998;174(5):262–68. doi: 10.1007/BF03038719. [DOI] [PubMed] [Google Scholar]
- 9.Monte Carlo Modeling of the Initial Radiation Emitted by an Improvised Nuclear Device in the National Capital Region (Revision 1) 2016 Report No. DTRA-TR-13-045 (R1) ( https://www.hsdl.org/?view&did=756467)
- 10.Leggett RW. Biokinetic models for radiocaesium and its progeny. J Radiol Prot. 2013;33(1):123–40. doi: 10.1088/0952-4746/33/1/123. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DF, Church CO. Prussian blue for treatment of radiocesium poisoning. Pharmacotherapy. 2001;21(11):1364–67. doi: 10.1592/phco.21.17.1364.34426. [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ, Rossi HH, Kellerer AM, Goodman L, Marino S. Radiobiological studies with monoenergetic neutrons. Radiat Res. 1973;54(3):431–43. [PubMed] [Google Scholar]
- 13.Xu Y, Randers-Pehrson G, Turner HC, Marino SA, Geard CR, Brenner DJ, et al. Accelerator-based biological irradiation facility simulating neutron exposure from an improvised nuclear device. Radiat Res. 2015;184(4):404–10. doi: 10.1667/RR14036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber R, Schraube H, Nahrstedt U, Braselmann H, Bauchinger M. Dose-response relationships of micronuclei in human lymphocytes induced by fission neutrons and by low LET radiations. Mutat Res. 1994;306(2):135–41. doi: 10.1016/0027-5107(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 15.Purrott RJ, Reeder E. The effect of changes in dose rate on the yield of chromosome aberrations in human lymphocytes exposed to gamma radiation. Mutat Res. 1976;35(3):437–44. doi: 10.1016/0027-5107(76)90206-2. [DOI] [PubMed] [Google Scholar]
- 16.Ling CC, Spiro IJ, Stickler R. Dose-rate effect between 1 and 10 Gy/min in mammalian cell culture. Br J Radiol. 1984;57(680):723–8. doi: 10.1259/0007-1285-57-680-723. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds P, Anderson JA, Harper JV, Hill MA, Botchway SW, Parker AW, et al. The dynamics of Ku70/80 and DNA-PKcs at DSBs induced by ionizing radiation is dependent on the complexity of damage. Nucleic Acids Res. 2012;40(21):10821–31. doi: 10.1093/nar/gks879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Atomic Energy Agency. The Radiological accident in Goiânia. Vienna: IAEA; 1988. [Google Scholar]
- 19.Hasegawa A, Ohira T, Maeda M, Yasumura S, Tanigawa K. Emergency responses and health consequences after the Fukushima accident; evacuation and relocation. Clin Oncol. 2016;28(4):237–44. doi: 10.1016/j.clon.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Saenko V, Ivanov V, Tsyb A, Bogdanova T, Tronko M, Demidchik Y, et al. The Chernobyl accident and its consequences. Clin Oncol. 2011;23(4):234–43. doi: 10.1016/j.clon.2011.01.502. [DOI] [PubMed] [Google Scholar]
- 21.Merz S, Shozugawa K, Steinhauser G. Analysis of Japanese radionuclide monitoring data of food before and after the Fukushima nuclear accident. Enviro Sci Tech. 2015;49(5):2875–85. doi: 10.1021/es5057648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshi M, Yamamoto M, Kawamura H, Shinohara K, Shibata Y, Kozlenko MT, et al. Fallout radioactivity in soil and food samples in the Ukraine: measurements of iodine, plutonium, cesium, and strontium isotopes. Health Phys. 1994;67(2):187–91. doi: 10.1097/00004032-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Laiakis EC, Mak TD, Anizan S, Amundson SA, Barker CA, Wolden SL, et al. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res. 2014;181(4):350–61. doi: 10.1667/RR13567.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Templin T, Paul S, Amundson SA, Young EF, Barker CA, Wolden SL, et al. Radiation-Induced micro-rna expression changes in peripheral blood cells of radiotherapy patients. Int J Radiat Oncol Biol Phys. 80(2):549–57. doi: 10.1016/j.ijrobp.2010.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo A, Yamaguchi Y. Analysis of dose distribution for heavily exposed workers in the first critically accident in Japan. Radiat Res. 2003;159(4):535–42. doi: 10.1667/0033-7587(2003)159[0535:aoddfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Radiation Biodosimetry Medical Countermeasure Devices. Guidance for Industry and Food and Drug Administration Staff. U.S. Department of Health and Human Services, Food and Drug Administration; 2016. ( http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidaneDocuments/UCM427866.pdf) [Google Scholar]
- 27.Storer JB. Acute Responses to ionizing radiation. In: Green EL, editor. Biology of the Laboratory Mouse. New York: McGraw-Hill; 1966. [Google Scholar]
- 28.Snyder SL, Walden TL, Patchen ML, MacVittie TJ, Fuchs P. Radioprotective properties of detoxified lipid A from Salmonella minnesota R595. Radiat Res. 1986;107(1):107–14. [PubMed] [Google Scholar]
- 29.Davids JAG, Mos APJ, Oude Ad. Fast-neutron facility for biological exposures in an Argonaut reactor : design, tissue dosimetry and neutron spectrometry. Phys Med Biol. 1969;14(4):573. doi: 10.1088/0031-9155/14/4/305. [DOI] [PubMed] [Google Scholar]
- 30.Zeman GH, Dooley M, Eagleson DM, Goodman LJ, Schwartz RB, Eisenhauer CM, et al. Intercomparison of Neutron Dosimetry Techniques at the AFFRI Triga Reactor. Radiat Prot Dosimet. 1988;23(1–4):317–20. [Google Scholar]
- 31.The TRIGA Reactor Facility at the Armed Forces Radiobiology Research Institute. Report No. AFRRI TR-86-1. Bethesda, MD: Armed Forces Radiological Research Institute; 1986. ( http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA168238) [Google Scholar]
- 32.Auxier JA. The health physics research reactor. Health Phys. 1965;11(2):89–93. doi: 10.1097/00004032-196502000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Broerse JJ. Review of RBE values of 15 MeV neutrons for effects on normal tissues. Eur J Cancer. 1974;10(4):225–30. [PubMed] [Google Scholar]
- 34.Davids JA. RBE of fission neutrons for acute radiation effects in CBA-mice. Int J Radiat Biol. 1967;13:377–8. [Google Scholar]
- 35.Carsten AL, Bond VP, Thompson K. The r.b.e. of different energy neutrons as measured by the heamatopoietic spleen-colony technique. Int J Radiat Biol Relat Stud Phys Chem Med. 1976;29(1):65–70. doi: 10.1080/09553007614551561. [DOI] [PubMed] [Google Scholar]
- 36.Worgul BV, Medvedovsky C, Huang Y, Marino SA, Randers-Pehrson G, Brenner DJ. Quantitative assessment of the cataractogenic potential of very low doses of neutrons. Radiat Res. 1996;145(3):343–9. [PubMed] [Google Scholar]
- 37.Borek C, Hall EJ, Rossi HH. Malignant transformation in cultured hamster embryo cells produced by X-rays, 460-keV monoenergetic neutrons, and heavy ions. Cancer Res. 1978;38(9):2997–3005. [PubMed] [Google Scholar]
- 38.Freyer GA, Palmer DA, Yu Y, Miller RC, Pandita TK. Neoplastic transformation of mouse C3H10T1/2 cells following exposure to neutrons does not involve mutation of ras gene as analyzed by SSCP and cycle sequencing. Mutat Res. 1996;357(1–2):237–44. doi: 10.1016/0027-5107(96)00130-3. [DOI] [PubMed] [Google Scholar]
- 39.Miller RC, Marino SA, Napoli J, Shah H, Hall EJ, Geard CR, et al. Oncogenic transformation in C3H10T1/2 cells by low-energy neutrons. Int J Radiat Biol. 2000;76(3):327–33. doi: 10.1080/095530000138664. [DOI] [PubMed] [Google Scholar]
- 40.Report No. LA-UR–94-0153. Los Alamos national Laboratory; 1994. Source and Replica Calculations. Retrieved from: http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/25/043/25043699.pdf. [Google Scholar]
- 41.Egbert SD, Kerr GD, Cullings HM. DS02 fluence spectra for neutrons and gamma rays at Hiroshima and Nagasaki with fluence-to-kerma coefficients and transmission factors for sample measurements. Radiat Environ Biophys. 2007;46(4):311–25. doi: 10.1007/s00411-007-0120-5. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Randers-Pehrson G, Marino SA, Garty G, Harken A, Brenner DJ. Broad energy range neutron spectroscopy using a liquid scintillator and a proportional counter: application to a neutron spectrum similar to that from an improvised nuclear device. Nucl Instr Meth A. 2015;794:234–39. doi: 10.1016/j.nima.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell CR, Azizova TV, Hande MP, Burak LE, Tsakok JM, Khokhryakov VF, et al. Stable intrachromosomal biomarkers of past exposure to densely ionizing radiation in several chromosomes of exposed individuals. Radiat Res. 2004;162(3):257–63. doi: 10.1667/rr3231. [DOI] [PubMed] [Google Scholar]
- 44.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Physics. 2008;35(1):310–17. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 45.DIRAC (DIrectory of RAdiotherapy Centres) Place IAEA. IAEA; ( https://www.iaea.org/resources/databases/dirac) [Google Scholar]
- 46.Turner HC, Shuryak I, Taveras M, Bertucci A, Perrier JR, Chen C, et al. Effect of dose rate on residual gamma-H2AX levels and frequency of micronuclei in X-irradiated mouse lymphocytes. Radiat Res. 2015;183(3):315–24. doi: 10.1667/RR13860.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertucci A, Smilenov LB, Turner HC, Amundson SA, Brenner DJ. In vitro RABiT measurement of dose rate effects on radiation induction of micronuclei in human peripheral blood lymphocytes. Radiation and Environmental Biophysics. 2016:1–7. doi: 10.1007/s00411-015-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghandhi SA, Smilenov LB, Elliston CD, Chowdhury M, Amundson SA. Radiation dose-rate effects on gene expression for human biodosimetry. BMC Medical Genomics. 2015;8:22. doi: 10.1186/s12920-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goudarzi M, Mak TD, Chen C, Smilenov LB, Brenner DJ, Fornace AJ. The effect of low dose rate on metabolomic response to radiation in mice. Radiat Environ Biophysics. 2014;53(4):645–57. doi: 10.1007/s00411-014-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul S, Smilenov LB, Elliston CD, Amundson SA. Radiation dose-rate effects on gene expression in a mouse biodosimetry model. Radiat Res. 2015;184(1):24–32. doi: 10.1667/RR14044.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon SL, Bouville A, Beck HL. The geographic distribution of radionuclide deposition across the continental US from atmospheric nuclear testing. J Environ Radioact. 2004;74(1–3):91–105. doi: 10.1016/j.jenvrad.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 52.Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S, Yasunari T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Natl Acad Sci. 2011;108(49):19530–34. doi: 10.1073/pnas.1112058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong H, Park M, Hwang W, Kim E, Han M. Radiological risk assessment caused by RDD terrorism in an urban area. Appl Radiat Isot. 2013;79:1–4. doi: 10.1016/j.apradiso.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Goudarzi M, Weber W, Mak TD, Chung J, Doyle-Eisele M, Melo D, et al. Development of urinary biomarkers for internal exposure by cesium-137 using a metabolomics approach in mice. Radiat Res. 2014;181(1):54–64. doi: 10.1667/RR13479.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul S, Ghandhi SA, Weber W, Doyle-Eisele M, Melo D, Guilmette R, et al. Gene expression response of mice after a single dose of (137)Cs as an internal emitter. Radiat Res. 2014;182(4):380–9. doi: 10.1667/RR13466.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikula KJ, Muggenburg BA, Chang IY, Griffith WC, Hahn FF, Boecker BB. Biological effects of 137CsCl injected in beagle dogs. Radiat Res. 1995;142(3):347–61. [PubMed] [Google Scholar]
- 57.Howell RW, Goddu SM, Rao DV. Design and performance characteristics of an experimental cesium-137 irradiator to simulate internal radionuclide dose rate patterns. J Nucl Med. 1997;38(5):727–31. [PubMed] [Google Scholar]
- 58.Pasternack JB, Howell RW. RadNuc: A graphical user interface to deliver dose rate patterns encountered in nuclear medicine with a (137)Cs irradiator. Nucl Med Biol. 2013;40(2) doi: 10.1016/j.nuc-medbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Toledo SM, Asaad N, Venkatachalam P, Li L, Howell RW, Spitz DR, et al. Adaptive responses to low-dose/low-dose-rate gamma rays in normal human fibroblasts: the role of growth architecture and oxidative metabolism. Radiat Res. 2006;166(6):849–57. doi: 10.1667/RR0640.1. [DOI] [PubMed] [Google Scholar]
- 60.Bishayee A, Rao DV, Srivastava SC, Bouchet LG, Bolch WE, Howell RW. Marrow-sparing effects of 117mSn(4+)diethylene-triaminepentaacetic acid for radionuclide therapy of bone cancer. J Nucl Med. 2000;41(12):2043–50. [PubMed] [Google Scholar]
- 61.Lenarczyk M, Goddu SM, Rao DV, Howell RW. Biologic dosimetry of bone marrow: induction of micronuclei in reticulocytes after exposure to 32P and 90Y. J Nucl Med. 2001;42(1):162–9. [PubMed] [Google Scholar]
- 62.Bateman TJ, Davy TJ, Skeggs DB. Five years hospital experience with the Amersham caesium 137 manual after loading system. Br J Radiol. 1983;56(666):401–7. doi: 10.1259/0007-1285-56-666-401. [DOI] [PubMed] [Google Scholar]